Spatial analysis of carbon isotopes reveals seagrass contribution to fishery food web
Full text
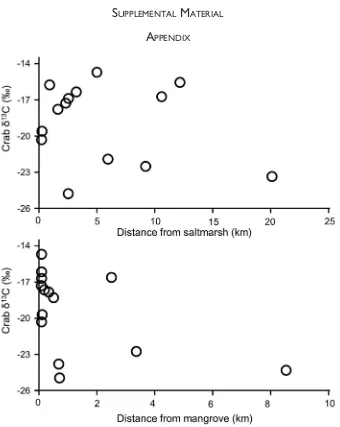
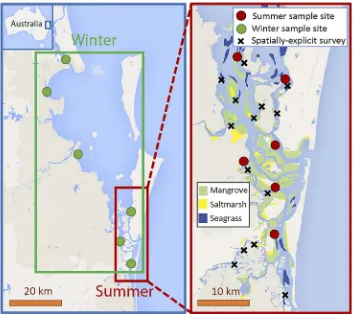
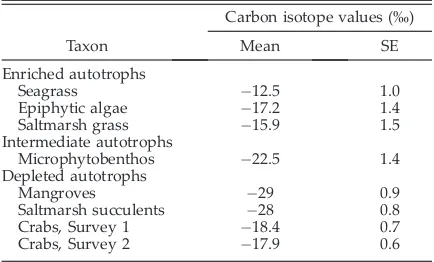
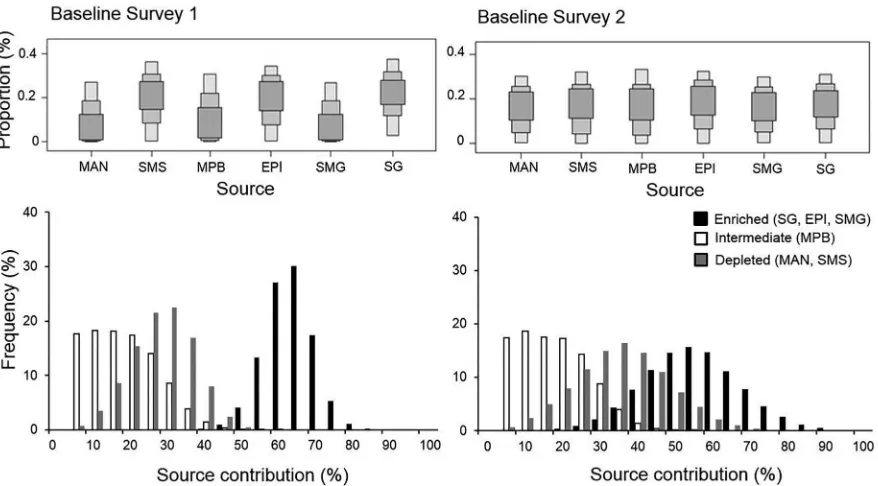
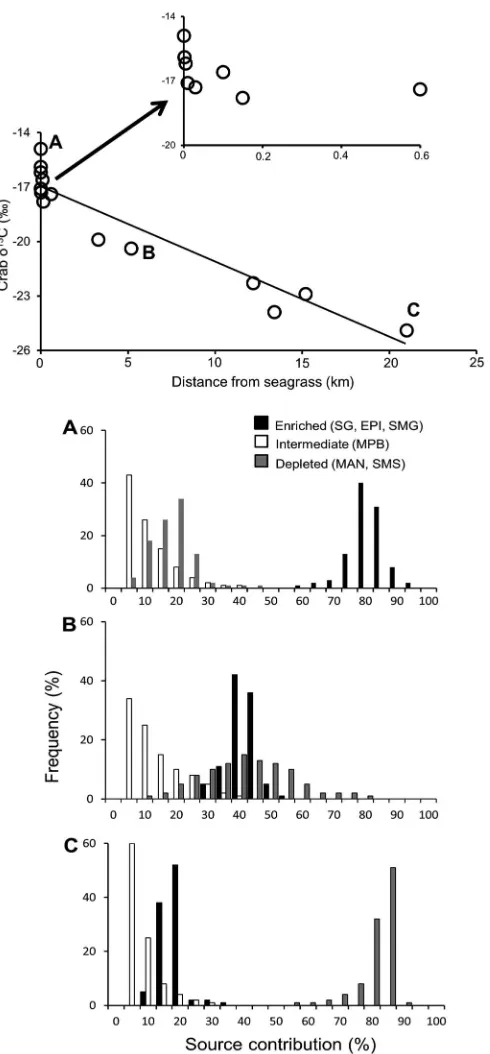
Figure
Related documents
In other words, there are lots of very small droplets in polluted clouds, which cannot easily grow into larger drops of precipitation size during the lifetime of the cloud (figure
Through above interview content, we can get a brief glimpse of activities of strategic human resource management of Vietnamese companies in Vietnam such as training
State Local Health services Federal Operative teams Alto TB México Committee Evaluation Scheme. More than 11,000
• Widespread Internet censorship and data retention practices of Asia/Pacific governments make public cloud computing services of all flavors less useable, reliable and
Read this chapter to set RAID level, Hot spare disk, Host interface ID; expand an existing RAID group; or set a disk array system password.Connect- ing the RS-232 Cable” on page
At the same time that mobile devices are becoming ubiquitous, data center security appliances are failing to keep up with the huge demand for information and application access.. As
TOMMASI, CAPUTO: THE MORE YOU KNOW, THE LESS YOU LEARN (unrelated categories, Section 3.2 ); (b) it knows few categories that are very similar to the new one (related
que: (a) os prefixos relacionais continuam ativos em temas verbais, nominais e posposicionais da língua Kaiowá; (b) o Kaiowá mantém os quatro prefixos relacionais cognatos dos