0022-538X/82/050742-06$02.00/0
Transformation
by Avian Sarcoma Viruses Leads to
Phosphorylation
of
Multiple
Cellular Proteins on
Tyrosine
Residues
KARENBEEMON,t* THOMAS RYDEN,ANDE. ANN McNELLY TumorVirologyLaboratory, The Salk Institute, San Diego, California 92138
Received 8 October1981/Accepted 14 December 1981
Phosphoamino acidcompositionsweredetermined for 10 size classes ofcellular proteins, separated by electrophoresis through one-dimensional sodium dodecyl sulfate-polyacrylamide gels. Phosphotyrosine-containing proteins were observed in uninfected chicken embryo fibroblasts in every size class analyzed, ranging from approximately 20,000 to greater than 200,000 daltons. Transformation of chickenembryo fibroblasts by RoussarcomavirusorPRC II aviansarcomavirus led toincreases inphosphorylation of proteins attyrosine residues in all ofthese sizeclasses.A
large
fraction of thephosphotyrosine-containing protein
molecules observedin Rous sarcomavirus-transformed cellswaslarger than 100,000daltons with a second broad peak in the 35,000- to60,000-dalton
range. This study suggeststhatthereare anumberof substrates of viralorcellulartyrosine-specific protein kinases, which havenotyetbeen identified by other methods.The src gene of Rous sarcoma virus
(RSV)
encodes a
protein
pp6Osrc
that isresponsible
for neoplastic transformation (4). Purified prepara-tions of pp6Osrc possessprotein
kinase activity specific fortyrosine residues (6, 7, 10,15, 16, 20,
21). In uninfected chicken embryo fibroblasts labeled with
32p
under steady-stateconditions,
only about0.03% ofthe acid-stable
phosphory-lated amino acids in protein are phosphotyro-sines;however,this leveloftyrosine phosphor-ylation of cellular protein was observed to be 6- to10-foldhigher after transformation by RSV (15, 28). Studies with cells infected by tempera-ture-sensitive mutants of RSV show that this phosphorylation is readily reversibleupon
shift-ing
the temperature, suggesting that at least someof these additional proteinmolecules phos-phorylated at tyrosine residues are directlyphosphorylated
bypp6Osrc
(28).Althoughnot alltransforming viruses have been shown to induce elevated levels of tyrosine phosphorylation of proteinsnor to encode proteins which either are themselvesor are associated with tyrosine-spe-cific protein kinases (28), several retroviruses appear to be very similar to RSV in having these two properties. This class of viruses includes Abelsonmurine leukemia virus, Snyder-Theilen and Gardner-Amstein feline sarcoma
viruses,
and the defective avian sarcoma viruses (ASV) whichhave oncogenes distinct from the RSV src gene: Fujinami sarcoma virus, PRC II, and Y73 (1, 2, 13, 18, 22, 23, 29-31).
t Present address: Department of Biology, The Johns Hop-kins University, Baltimore, MD 21218.
To further our understanding of the interac-tions between these viruses and their host cells resulting in transformed
phenotypes,
it is impor-tant toidentify
the cellular target protein or proteins which arephosphorylated by
thesety-rosine-specific
protein
kinases. Sincephospho-tyrosine
isaveryrareprotein
modificationevenin transformed cells, it originally seemed that veryfewdifferent
proteins
might
be phosphory-lated at tyrosine residues,making
it easy toidentify
potential
substrates of theprotein
ki-nase activity of pp6Osrc. Phosphotyrosine-con-taining proteins labeledin vivohavepreviously been identifiedby
two-dimensionalgel
electro-phoresis (8, 9, 25, 26) andby
analysis
ofspecifi-callyimmunoprecipitated
proteins
(27). In addi-tion,severalproteins have beenphosphorylated at tyrosine residues in vitro in the presence ofpp6OSrc
(5, 7); however, in some cases theseproteins are not similarly modified in RSV-transformed cells (27).
In this study the phosphotyrosine-containing proteinspresent in cells were labeled with
32p;
in vivo and then quantitatively analyzed after frac-tionation into size classes by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophore-sis. Although this technique affords much less resolution of individual proteins than does two-dimensionalgel analysis, it is more quantitative andcircumventsproblems due to specific losses of proteins or to lack of detection of some proteins. Specific issues which are addressed include: (i)the minimumnumberof phosphoty-rosine-containing proteins in uninfectedASV-742
on November 10, 2019 by guest
http://jvi.asm.org/
VOL.42,1982
transformed cells, (ii) the approximate size of the most abundant phosphotyrosine-containing proteins in transformed cells, (iii) the relative abundance of previously identified potential substrates of ASV protein kinases, and (iv) a comparison ofsubstrate specificities in vivo of tyrosine-specificprotein kinases present in unin-fected cells with those in cells transformed by RSVand byPRC II ASV.
Since phosphotyrosine represents a very small fractionof thephosphorylatedamino acids inprotein, it was first necessarytooptimize the amountof
32Pi
resultinginphosphotyrosine after in vivo labeling. Thiswasaccomplishedby two modifications of published procedures (15). It hadpreviouslybeenshown that altering the time of radiolabeling of the cells from 18 to 2 h resultedinafourfoldgreater fraction of the total label in phosphoamino acids derived from pro-teinpresent asphosphotyrosine(17),apparently owingto afasterturnoverrateforphosphate on tyrosine than for thatonphosphoserineor phos-phothreonine.Wefound that labelingcellsfor 4 hledtosignificantly moreincorporationof32p,
while maintainingthe higher relativeabundance
of
label in phosphotyrosine. The secondmodifi-cation
was to change the time of incubation ofproteins with 6 N
HCl
at 110°C. Since the tyrosine-phosphatebond is somewhat more la-bile in acid than that of serine-phosphate or threonine-phosphate (24), prolonged acid treat-mentleadstopoorerrelativerecoveries ofphos-photyrosine.
Wefound that1 hof acidhydroly-sis led to a higher relative abundance of
phosphotyrosine
recovered, although
1hofhy-drolysis
ledto poorertotal levels ofphospho-aminoacidrecoverythandid2hof
hydrolysis.
Phosphoproteins
present in chicken embryofibroblasts infected with
Schmidt-Ruppin,
sub-group A(SR-A)
RSV wereinitially
compared
with those inuninfected cells by
SDS-polyacryl-amide
gel
electrophoresis.
Both cultures werelabeled with
32p;,
solubilized
in radioimmuneprecipitation buffer
(RIPA) (2),
clarifiedby
cen-trifugation, and the
supernatants wereelectro-phoresed
ondiscontinuous
SDS-polyacrylamide
gels.
Theinsoluble
pellet
was notanalyzed,
although it
haspreviously
been shown that theRIPAsupernatant contains about thesame frac-tion of
phosphotyrosine
as does the whole cell(28).
Therewas nodifference in thephosphopro-teins present in the two cell types that was
discernible
by
this type ofanalysis (data
notshown);
thus,
RSV didnotappeartoperturb
thegross
pattern
of cellularphosphorylation.
This is not toosurprising,
since the RSV-codedprotein
kinase
activity
isspecific
fortyrosine,
and this is a very rare modification even in RSV-trans-formed cells.Toobserve
specific
effects ofpp60Src
andanyNOTES 743
other tyrosine-specifickinasesactivatedby RSV transformation,
32P-labeled
lysates were first electrophoresed through SDS-polyacrylamide gels, and the gels were then sectioned into 10 size classes. Proteins were eluted and their phosphorylatedamino acids were analyzed after partial acid hydrolysis by a two-dimensional electrophoretic separation as previously de-scribed (15, 28). The acid-stable phosphoamino acids were identifiedby theircomigration withinternal
markers. Afterquantitation ofradioac-tivity recovered comigrating with each of the phosphoamino acids, the percentage of phos-photyrosine recovered in each size class of proteinwas determined and is plotted in Fig. 1 for uninfected cells and for cells infected with SR-A RSVorwith PRCIIASV. Differences in the efficiency ofrecovery of proteins from one size class to the next would not affect these results since each gel fraction was analyzed independently.
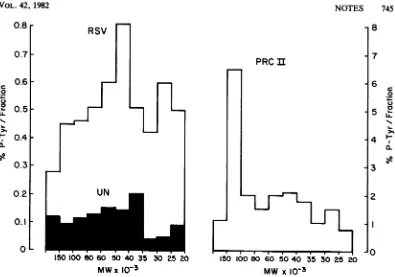
Intheuninfected
cells,
phosphotyrosine-con-taining proteins were observed in every size class analyzed, ranging from approximately 20,000 to greater than 200,000 daltons. The greatestrelative abundance ofphosphotyrosine was present in proteins having sizes ranging from about35,000
to60,000 daltons (greater than 0.15% perfraction). The lowest relative abun-dance of phosphotyrosine was in proteins of 20,000to35,000 daltons (< 0.10%perfraction). Becauseof thesmall
amountofphosphotyrosine in the uninfected cells, quantitation was most difficult here.Acomparisonbetween these results and those ofa parallel experiment using chicken embryo
fibroblasts
transformed by SR-A RSV is alsodisplayed in Fig. 1. Again, every gel fraction analyzed contained protein
phosphorylated
attyrosine;
however, suchproteins
were presentin greater relative abundance here than in the
corresponding
protein
size class derived fromuninfected cells. More than 0.5% ofthe total
phosphoamino
acids recovered werephospho-tyrosines
in fractionscontaining
proteins
of25,000to30,000 and35,000to
80,000
daltons. Iniis
experiment,
thehighest
relative abundanceof
phosphotyrosine
was found inproteins
of40,000to
50,000
daltons.However,
somevaria-tion was
observed
from oneexperiment
toan-other. Three
different
experiments
arecom-pared, all
using
SR-ARSV-transformed
chicken embryo fibroblasts(Table 1).
In the first twoexperiments,
labeling
wasfor4handtreatmentwithHCIwasfor 1 h.However,in
experiment 3,
cells
were labeled for 16 h andproteins
weretreatedwith HCI for 2 h.Differencesinboththe absolute and relativelevels of
phosphotyrosine
recovered per fraction wereobserved
in theseexperiments.
This may bedue eithertoon November 10, 2019 by guest
http://jvi.asm.org/
TABLE 1. Relative abundanceofphosphotyrosine
in individualsize classesofprotein from RSV-transformedcells
Protein size %Phosphotyrosine per gel fraction (xlO-3daltons) Expt 1 Expt 2 Expt 3
>150 0.28 0.48 NDa
100-150 0.45 0.26 0.19
80-100 0.46 0.43 0.17
60-80 0.51 0.41 0.37
50-60 0.60 0.40 0.39
40-50 0.82 0.67 0.65
35-40 0.51 0.55 0.65
30-35 0.42 0.26 0.35
25-30 0.60 0.84 0.47
20-25 0.50 0.22 0.10
aND, Not
determined.
mental variability, for example, differences in theslicing of fractions from geltogel,oritmay representactualheterogeneity existingbetween
differentpopulations of RSV-transformed cells. Inall of theseexperiments, the highestrelative amounts of phosphotyrosine per gel fraction
appeared in the middle of the gel region
ana-lyzed, encompassing proteins with sizes of
ap-proximately 35,000 to 60,000 daltons.
Interest-ingly, the uninfected chicken embryo fibroblasts also had the highest levels ofphosphotyrosine
perfraction in these size classes, although the
overall profiles are not identical for uninfected and RSV-infected cells.
Inchicken embryofibroblasts transformed by PRCIIASV,therelativephosphotyrosinelevels
were highestin the samefractions asfor RSV,
with theexceptionthat the 100,000-to 150,000-dalton fraction from PRC II-transformed cells contained amuch greater amount of phospho-tyrosine (Fig. 1). It is likely that at least a
largefractionof thephosphotyrosine in this size class is duetothe PRC II-encoded polyprotein P105gagfps, which has been showntobe heavily phosphorylated in vivo attyrosine residues (2,
22; unpublisheddata). P105 was also themajor
phosphotyrosine-containing protein detected in
PRC II-infected cells by two-dimensional gel
electrophoresis (9).
Analysis of Snyder-Theilen feline sarcoma
virus-transformedmink lungcellsbythis
proce-duregaveresults ratherlike those with PRCII,
despite the difference incelltypes.Thegreatest
percentage of phosphotyrosine-containing
pro-teinswasfound inafractionwith sizesof83,000
to 100,000 daltons, which includes the virus-coded presumptive transforming protein P85
that has several phosphotyrosine residues in
vivo (Beemonand McNelly,unpublished data).
In addition, relatively large levels of
phospho-tyrosine-containing proteins were observed in
the
37,000-
to68,000-dalton
size classes (datanot
shown).
To quantitatively analyze the fraction of the total phosphotyrosine recovered in proteins of varioussizes present in cells transformedby SR-A
RSV,
the recovery ofradioactivity from the gel was monitored. When electroelution wasperformedfor 20h,itwasfoundthat radioactiv-itywasobtained from all sizeclasseswith rela-tively equal efficiency. The proportion of the totalamountofphosphoprotein inacell
present
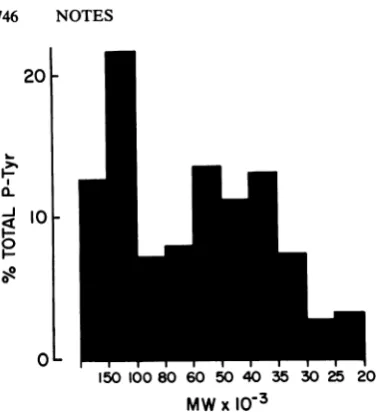
in each size class was determined by summing recoveriesofphosphoaminoacids from uniform samples ofeluted proteins. The percentage of totalphosphotyrosinerecoveredwasthen calcu-lated for each size class. Figure 2 shows the resultof suchananalysisofphosphoproteinsin RSV-transformedcells. These dataarefrom the
same experiment as in Fig. 1 and in Table 1, experiment 1. Although most of the total phos-photyrosine recovered was present in proteins of35,000to60,000 daltons,there was a surpris-ingly large amountof the totalphosphotyrosine presentinproteinsof100,000to150,000daltons and even larger. If there were a bias in the efficiency ofelution,it should favor the smaller proteins, making this result even more striking. These data suggest that a relatively large number ofdifferent cellular proteins are
phos-phorylated at tyrosine residues as a direct result oftransformation by RSVorPRC II and estab-lish a minimum number of 10 potential
sub-strates of the various tyrosine-specific protein kinases in a cell. The actual number of such proteins isprobably muchlarger,since the sepa-ration method used here provided no resolution between proteins of similar size.Thus, the sub-strate specificity oftyrosine-specific protein ki-nases appears to bebroader than was suggested by experiments in which potential substrates werefractionated by two-dimensional gel elec-trophoresis. Such studies detected only a few phosphotyrosine-containing proteins in trans-formed cells having sizes of 36,000, 28,000, 43,000and46,000 daltons(8, 9, 25,26).
Themostsurprisingfinding of this study was thelarge number ofphosphotyrosine-containing protein molecules having sizes greater than 100,000 daltons in RSV-transformed cells. The identity or absolutenumberofthese proteins is notknown. Suchproteins accountedfor approx-imately 30% of the phosphotyrosine in RSV-transformed cells, as determined in this
assay,
but had notbeen detected by two-dimensional gel analyses. Thisdiscrepancy could be due to the presence of a large number of different
phosphotyrosine-containing
proteins (each oflow abundance), to chargeheterogeneity, or to the inability of these large proteins to be re-solved by isoelectric focusing. Vinculin, a
on November 10, 2019 by guest
http://jvi.asm.org/
0.8r
0.71-0.6
0.5
0.41-
0.31-
0.2-
0.1-L
100 80 60 5040 35 302520
MWx
io-3
PRCII
I
150 100 80 60 50 40 35 30 25 20
MW x 10-3
FIG. 1. Relative abundance of phosphotyrosineinindividual size classes of cellularproteins.Uninfected, SR-ARSV-transformed, and PRC II-transformedchicken embryofibroblastswere grown on35-mmculture dishes
andlabeled with 2to6mCi32P1 (ICN) for4h (3). Cellswerelysedat4°Cin radioimmune precipitationbuffer, clarifiedbycentrifugation (2), and the supernatants werebroughtto afinalconcentration of 2% SDS-10%
2-mercaptoethanol-50 mM Tris-hydrochloride(pH 6.8)-10%glycerol. Afterbeing boiled for 2min,sampleswere
electrophoresedondiscontinuous SDS-polyacrylamidegels,usingthebuffers of Laemmli (19). The resolvinggel
wasapproximately 13cmhigh, 14cmwide, and 2mmthick and contained 0.1% SDS, 15%acrylamide, and 0.09%obisacrylamide. After electrophoresis, together with molecular weight markers (Bio-RadLaboratories), wetgelswereautoradiographed for 5to20min. Subsequently,the gelswereslicedhorizontally into10fractions, each approximately 1 cm high. Both the autoradiograms and the molecular weight markers were used to standardize fraction boundaries from geltogel. Proteinswereelectroeluted from each gel slice in 5to10ml of buffercontaining 0.1% SDS, 0.05 MNH4HCO3, and 5% ,B-mercaptoethanol for 6to20 h at100to 200 mA. Recovery was monitored by themeasurement of Cerenkov radiation. Proteins wereprecipitated with 20%o trichloroacetic acid, and the proteinpelletwaswashed withchloroform-methanol(2:1). Proteinswerepartially hydrolyzed with 200 ,ld of 6 N HCI for 1 hat100°C.Phosphoamino acidswereresolvedby two-dimensional electrophoresisoncellulosethin-layer plates (E. Merck Labs), firstatpH 1.9andsubsequentlyatpH3.5bythe procedure of Hunter and Sefton (15); 0.5 Fg of each phosphoamino acid marker (phosphoserine and phosphothreoninewere obtained fromSigma ChemicalCo.; phosphotyrosine was agiftfrom T.Hunter) was coelectrophoresedwith the radioactivesamplesand visualizedby stainingwithninhydrin.Afterautoradiography of thethin-layer plates, phosphoaminoacidscomigratingwiththemarkerswereeluted withpH1.9buffer,and theirradioactivitywasquantitated byscintillationcounting.The relativeabundance ofphosphotyrosineineach gel fraction is plottedas afunctionof the molecularweight (MW) of theproteinsin that sizeclass.
130,000-dalton cytoskeletal protein, has also
beenidentifiedasapotentialsubstrate ofpp60(rc
(27). However, the number of molecules of
vinculinphosphorylated ontyrosine residues is
muchtoolow (B. Sefton, personal
communica-tion) to account for any significant fraction of
the phosphotyrosine-containing proteins larger than 100,000 daltons detected in this study. In
PRC II-transformed cells, the large amount of
phosphorylation of the viral protein P105 at
tyrosine residues (Fig. 1; 2, 9, 22) obscures
detectionbythis method ofphosphorylated
cel-lular proteins in this sizerange, making it diffi-culttocomparetoRSV-transformed cells.
The 36,000-dalton protein, which appearsby
two-dimensional gel analysis to be the major substrate of RSV transformation-induced phos-phorylation (also of transformation by several
otherretrovirusesorof activation ofthe epider-mal growth factor receptor-associated kinase
activity [8, 9, 11, 12, 14, 25, 26]),accounted for
no morethan15%of the totalphosphotyrosine
on proteins in this analysis (Fig. 2). It is not
knownwhether there areadditional
phosphoty-c
0
._
C) 0
I.-
L-8
7
6
C
I-S
-4
0-R 3
2
NOTES 745
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.505.73.468.54.331.2]746 NOTES
20
I-.
-J
F--J
al
1O0
0'
L
150 10080 60 50 40 35 30 25 20 MWx103
FIG. 2. The relative abundance of phosphotyro-sine-containing proteins of different sizes in SR-ARSV-transformed cells. The data from RSV-trans-formed cellsshown in thelegendtoFig.1 and Table1, experiment 1,has beenreplottedhere todemonstrate theproportionof the totalphosphotyrosine-containing proteinsrepresentedin eachsize class.
rosine-containing proteins presentin the
35,000-to40,000-dalton size classwhich would diminish
further the fraction of totalcellular phosphotyro-sine present in the 36,000-dalton protein. Both
RSV- and PRC II-transformed cells contained
several substrates of tyrosine-specific protein kinases in the30,000-to60,000-dalton size class.
Theseproteinsarelikelytoincludethose previ-ously identified by other methods (8, 9, 15).An
exception to the apparent similarity between
RSV- andPRCII-transformed cellsin phospho-tyrosine-containing proteins has been observed invinculin, which isnotphosphorylated in PRC II-transformed cells (27).
Since uninfected cells are likely to contain several tyrosine-specific protein kinases, in ad-ditiontopp60c-src (the cellular homolog of viral pp60V-src),it isnotpossibletocomparesubstrates
ofkinases encoded byv-srcandc-srcsimply by
comparison of phosphotyrosine-containing
pro-teins in transformed and nontransformed cells. Itisnotknown, furthermore,whether kinases in
additiontopp60-src itselfareresponsiblefor the
elevated level of tyrosine phosphorylation of
proteins in RSV-transformed cells. However,
the profiles of phosphotyrosine-containing
pro-teins, although not identical, are not grossly
dissimilar between uninfected and
RSV-trans-formed cells. These results are consistent with
thehypothesis that the various viraland cellular
tyrosine-specific kinases sharemanyof thesame
protein substrates with perhaps only
quantita-tive differences in preference for different sub-strates.
Inconclusion,itappearsthat there are several potential substrates of ASV-codedor-activated protein kinases which needtobe further charac-terized,particularly those in the high-molecular-weight region. The apparently large number of substrates ofprotein kinases makes it easier to explainthe
pleiotropic
natureof the transformed phenotype. However, it makes it much more difficult to ascertain which of these substrates are essential for transformation.This workwas supportedbyPublic Health Service grant CA-23896 from the National Cancer Institute.
LITERATURE CITED
1. Barbacid, M.,K.Beemon,andS. G. Devare. 1980.Origin andfunctionalpropertiesof themajorgeneproductof the Snyder-Theilenstrain of felinesarcomavirus. Proc.Nati. Acad. Sci.U.S.A. 77:5158-5162.
2. Beemon, K. 1981. Transforming proteinsofsomefeline andavian sarcoma viruses arerelated structurally and functionally. Cell 24:145-153.
3. Beemon, K., and T. Hunter. 1978. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J.Virol. 28:551-556.
4. Brugge, J. S.,and R. L.Erlkson.1977.Identification ofa
transformation-specific antigeninducedbyanavian sar-comavirus. Nature(London)269:346-348.
5. Burr, J. G., G.Dreyfuss,S.Penman,andJ.M. Buchanan.
1980.Association of thesrcgeneproductof Rous sarco-mavirus withcytoskeletalstructuresofchickenembryo fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 77:3484-3488.
6. Coliett, M.S.,and R. L. Erikson. 1978. Protein kinase activity associated with the avian sarcoma virus gene product. Proc. Natl. Acad.Sci.U.S.A. 75:2021-2024.
7. Collett, M.S.,A. F. Purchio,and R. L.Erikson. 1980. Avian sarcoma virustransformingprotein,pp6tYrc,shows protein kinaseactivityspecificfortyrosine.Nature (Lon-don) 285:167-169.
8. Cooper, J. A.,and T. Hunter. 1981.Changesinprotein phosphorylation in Rous sarcoma virus transformed chicken embryo cells. Mol. Cell. Biol. 1:165-178. 9. Cooper, J. A.,and T. Hunter.1981. Fourdifferent classes
of retroviruses inducephosphorylation of tyrosines
pres-entin similar cellularproteins.Mol.Cell. Biol. 1:394-407. 10. Erikson, R. L., M. S. Coliett, E. Erlkson, and A. F.
Purchio. 1979. Evidence that the avian sarcoma virus transformninggene product is a cyclic AMP-independent protein kinase. Proc. NatI. Acad. Sci. U.S.A.
76:6260-6264.
11. Erikson, E., R. Cook, G. J.Miller, and R. L.Erikson. 1981. The same normal cell protein is phosphorylated aftertransformation by avian sarcoma viruses with unre-latedtransforming genes. Mol. Cell. Biol. 1:43-50.
12. Erikson, E., andR. L.Erikson. 1980. Identification of a cellularprotein substrate phosphorylated by the avian
sarcomavirus transforming gene product. Cell 21:829-836.
13. Feldman, R. A., T. Hanafusa, and H. Hanafusa. 1980. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell 22:757-766.
14. Hunter, T., and J. A. Cooper. 1981. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 humantumor cells. Cell 24:741-752.
15. Hunter, T., andB. M. Sefton. 1980. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl. Acad. Sci. U.S.A. 77:1311-1315.
16. Hunter, T., B. M. Sefton, and K.Beemon.1980. Studies
onthe structureand function of the avian sarcoma virus transforming-gene product. Cold Spring Harbor Symp. Quant. Biol. 44:931-941.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.505.51.243.58.265.2]17. Hunter, T., B. M. Sefton, and K. Beemon. 1980. Phosphor-ylation of tyrosine: A mechanism of transformation shared by a numberof otherwise unrelated RNA tumor viruses, p. 499-514. In B. Fields, R.Jaenisch, and C. F. Fox (ed.), Animal virus genetics. Academic Press, Inc., NewYork.
18. Kawai, S., M. Yoshida, K. Segawa, H. Sugiyama, R. Ishizaki, and K. Toyoshima. 1980. Characterization of Y73, an avian sarcoma virus: a uniquetransforming gene and its product, aphosphopolyprotein with protein kinase activity. Proc. Natl. Acad. Sci. U.S.A. 77:6199-6203. 19. Laemmli, U. K. 1970. Cleavage of structural proteins
during the assembly of the head of bacteriophage T4. Nature (London)227:680-685.
20. Levinson, A. D., H. Oppermann, L. Levintow, H. E. Varmus, and J. M. Bishop. 1978. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell 15:561-572.
21. Levinson, A. D., H. Oppermann, H. E. Varmus,andJ.M.
Bishop. 1980. Thepurified product of the transforming geneof avian sarcoma virusphosphorylatestyrosine.J. Biol. Chem.255:11973-11980.
22. Neil,J. C., J. Ghysdael, and P. K. Vogt. 1981. Tyrosine-specific protein kinase activity associated with p105 of avian sarcoma virus PRC II.Virology109:223-228. 23. Pawson, T., J. Guyden, T.-H.Kung,K.Radke, T.
Gil-more, and G. S. Martin. 1980. A strain of Fujinami sarcoma viruswhich is temperature-sensitiveinprotein phosphorylationand cellulartransformation. Cell 22:767-776.
24. Plimmer, R. H. A. 1941. Esters of phosphoric acid. IV. Phosphoryl hydroxy amino acids. Biochem. J. 35:461-469.
25. Radke, K., T.Gilmore, and G. S. Martin. 1980. Transfor-mation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell 21:821-828.
26. Radke, K., and G. S.Martin. 1979. Transformation by Rous sarcomavirus: effects of src geneexpression on the synthesis and phosphorylation of cellular polypeptides. Proc. Natl. Acad. Sci. U.S.A. 76:5212-5216.
27. Sefton, B. M., T. Hunter, E. H. Ball, and S. J. Singer. 1981. Vinculin: acytoskeletal target of the transforming protein of Rous sarcoma virus. Cell 24:165-174. 28. Sefton, B. M., T. Hunter, K. Beemon, and W. Eckhart.
1980. Evidence that the phosphorylation oftyrosine is essential for cellular transformation by Rous sarcoma virus. Cell 20:807-816.
29. Sefton, B. M., T. Hunter, and W. C. Raschke. 1981. Evidence that the Abelsonvirus protein functions in vivo as aprotein kinase that phosphorylates tyrosine. Proc. Natl. Acad. Sci. U.S.A. 78:1552-1556.
30. Van de Ven, W. J. M., F. H. Reynolds, and J. R.
Stephenson. 1980. Thenonstructural components of poly-proteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinaseactivity. Virology 101:185-197. 31. Witte, 0. N., A. Dasgupta, and D. Baltimore. 1979. Abelson murine leukemia virusproteinisphosphorylated in vitro to form phosphotyrosine. Nature (London) 283:826-831.
VOL.42,