Rochester Institute of Technology
RIT Scholar Works
Theses
Thesis/Dissertation Collections
9-30-1997
Sensori-neural deafness and the cochlear implant
Margaret Pence
Follow this and additional works at:
http://scholarworks.rit.edu/theses
This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion
in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact
ritscholarworks@rit.edu.
Recommended Citation
Chief Advisor:
Glen Hintz
Approvals
Date
Associate Advisor:
Robert Wabnitz
Date
:
i - /] -
q'}
Associate Advisor:
Catherine Clark
Date
/~J
'/ f?
I
Associate Advisor:
Dr. Paul Dutcher
Date
/LO/77
Chairperson:
Tom
Lightfoot
Date
/
2-/;I/?
7
~1
II, Margaret Pence, hereby grant permission to the Wallace Memorial Library
of RIT to reproduce my thesis in whole or in part. Any reproduction will not
be for commercial use or profit.
Signature
Rochester
Institute
ofTechnology
A
Thesis
submittedto the
Faculty
oftheCollege
of
Imaging
Arts
andSciences in
candidacy for
thedegree
ofMaster
ofFine
Arts.
Sensori-neural
Deafness
andtheCochlear
Implant
by
Margaret
Pence
Introduction
The inner
earis
part of anintricate
network offluid filled
cavitieslocated
in the
temporalbone
ofthe
skull.Within
this
bony
labyrinth is
thecochlea which
houses
the
organ ofhearing
calledthe
organ ofCorti.
Any
number of
factors,
including
disease,
trauma
and congenital malformationsmay
interrupt
the
processing
of soundthrough the
inner
ear.These
conditions
may lead
to
partial or completehearing
loss.
This
type ofhearing
loss
is
called sensori-neuraldeafness.
Since
the 1950's
medical researchershave
sought to restore some senseof
hearing
in
profoundly
deaf individuals
by
way
ofstimulating
cochlearhair
cells, and or
direct
stimulation ofthe auditory/cochlear nerve.Their
worklead
to
thedevelopment
of adevice
calledthe
cochlearimplant.
Although
not recommended
for
everyone,this
implant
has been
used with atleast
partial success
in
over13,000
peopleworld wide.The
first
portion ofthis
thesis presents generalinformation
concerning
the
anatomy
andphysiology
ofthe
hearing
process, pathologies that causedeafness,
types
ofhearing
aids available, and specificinformation
concerning
the
development
and use of the cochlearimplant.
Surgical
proceduresdescribing
the process ofimplantation
are also covered.The
second portionof
the thesis
reportfocuses
on theillustrations
thatweredeveloped
as part ofmy
investigations
of the structure andfunction
of theinner
ear,particularly
the organ of
Corti
andits
involvement
in
thehearing
process.Their
purposeis
to
visually
communicate complex structures and processes.Over
the
last few decades
concernshave been
voicedboth in
and out ofthe
deaf
community
overthe
use ofimplants,
especially in
children.My
present
information
to aninterested
public.Anatomy
andPhysiology
ofHearing
The
earitself
is
a compound organthat
serves adual
purpose as ahearing
mechanismfor
the
reception of sound and as an organ ofbalance.
Both
functions depend
onthe
movement offluid
through
a speciallabyrinth
within
the
skull and the stimulation of special receptor cells within the complex of theinner
ear.The
earis
composed of threemajor areas: outerear,
middle ear andinner
ear.The
organs ofhearing
andbalance lie
within thebony
labyrinth
ofthe
inner
ear.The
outer earis
composed ofthe
auricle and the externalauditory
canal.
In
humans,
the auricle,the
visible external portion of the ear,is
consideredto
be
vestigial.Ifs
function,
is
to
direct
sound wavesinto
the externalauditory
canalfor better
reception within the ear(transmission
of sound waves).This
function
is
stillimportant
in
many
mammalsbut
has
become
inefficient in
humans.
The
externalauditory
canalis
a short narrowchamber
(2.5
cmlong
by
0.6
cm wide) andextendsfrom
the
auricle to thetympanic
membrane or eardrum.The
canalis
lined
withhairs
and sebaceousand modified apocrine sweat glands that secretecerumen, orear wax.
Both
the
hairs
and wax serve to protectthe earfrom
theinvasion
offoreign
bodies.
The
tympanic
membrane, or eardrum, reverberates with sound wavesthat
travelthrough the
external canal and set the ossicles of the middle earinto
motion.
The
middleear,
ortympanic
cavity,is
a small airfilled
cavity
that
lies
provides a
link
to the nasopharynx andis
usually
closedbut
opensto
allow an equalizationin
pressure withinthe
middle ear cavity.This
is
importantfor
the proper conduction of soundthrough the tympanic
membrane.If
pressure within
the
middle earcavity
is
not equal to external pressure, the tympanic membranemay bulge
in
or out and not allowfor
propermovement against
the
ossicles.This
distortion
of the membranemay
cause conductivehearing
impairment
or other problems.The
middle ear contains thethree
smallestbones in
thehuman
body,
the
ossicles; malleus(hammer),
incus
(anvil)
and stapes(stirrup).
They
arenamed
for
their
shape.These
tiny
bones have
the
important
job
oftransmitting
vibrations ofthe tympanic
membrane to the oval window of theinner
ear, which setthe
fluids
of thebony
labyrinth
in
motion and thus excitethe
receptor cells ofhearing.
The "handle
"of the malleus
is
connected to thetympanic membrane,
or eardrum.The base
ofthe stapesis
set againsttheoval window,
the
incus
serves as the connectionbetween
the other twobones.
The
smallest skeletal muscles are alsofound
withinthe
middle ear.The
tensor tympani
originatesfrom
the
wall of theauditory
tube andinserts
on the
handle
of the malleus.The
stapedius muscle originatesfrom
the
posterior wall ofthe middle ear
cavity
andinserts
onthe
stapes.Both
muscles serve
to
help
controlthe
movement of thebones
against theirrespective membranes thus
protecting
theinner
earfrom very loud
noises.The inner
earis
abony
maze-like structurethat
sitsdeep
withinthe
temporal
bone
andhouses
the
delicate,
sensitive receptor cells ofhearing
andbalance
(orientation).
Within
the
bony
labyrinth
sits a membranousdistinct
areasbased
on unique structure andfunction;
the
vestibule, cochlea and semicircular canals.The
membranouslabyrinth follows
the contours ofthe
hollow
bony
labyrinth. The
bony
labyrinth
is
lined
with endosteum andfilled
with perilymph whichis
similarin
composition to cerebral spinalfluid.
Thus
the
membraneouslabyrinth
is
"floating"within
ifs
chamber.The
membranouslabyrinth
in turn is
filled
with endolymph whichis
similarin
compositionto
intracellular
fluid.
These
two
fluids
are responsiblefor
conducting
the
sound vibrations thatoccurin
hearing
anddetecting
changesin
body
position.This
paper will concentrate on the movement of soundvibrations
through
the middle earto the cochlea that stimulate thefiring
ofthe
receptor cells ofhearing.
The
vestibule of theinner
earmay
be divided into
two portions, the saccule and utricle.The
utricleis
continuous with the semicircular canals while the sacculeis
continuous withthe
cochlea.Both
these portions contain equilibrium receptorsthatdetect
and report changesin
head
position.Anatomy
oftheCochlea
The
cochleais
a spiral, snaillike
chamber(cochlea
comesfrom
theGreek
for
"snail)
andis
about thesize ofhalf
a splitpea.It
extendsfrom
thevestibule and coils
for
two
and ahalf
turns around abony
pillar called themodiolus.
The
axiallength
of the cochleais
5
mm, the spiral canal extends to31-33
mmlong.
The
membranous cochlearduct
runs through this chamberending
atthe
cochlear apex.The
cochlearduct
andthe
spirallamina
(
abony
extension ofthe
modiolus)divide
the
hollow
cochleainto
three
chambers: scala vestibuli(superior),
scala media(cochlear
duct)
and scalatympani
cochlear
duct
is
filled
with endolymph andhouses
the spiral organ ofCorti,
the receptor organ of
hearing.
The
membraneouslabyrinth
is
supportedby
the perilymphaticfluids
and a network oftiny
blood
vessels.The
construction of the cochlearduct is important in
theunderstanding
ofhow
sound waves are convertedto
nerveimpulses.
There
are a series of membranes that surround and contain theduct.
The
roof ofthe
duct
which separates the scala mediafrom
the scala vestibuliis
called thevestibularmembrane.
The
floor
ofthe
duct is
formed
by
the spirallamina
(from
thebony
modiolus) andthe
basilar
membrane which supports theorgan of
Corti. The
spiralligament
serves as abase
of attachmentfor
thebasilar
membrane andReissner's
membrane.The flexible
nature of thebasilar
membrane
is important
in
the stimulation ofsensory
hair
cells.The
cochlearnerve, a
division
ofthe
8tn cranial or vestibulocochlearnerve, runs
from
theorgan of
Corti
throughthe
modiolus to theauditory
center of thebrain.
The Organ
ofCorti
The
organ ofCorti
sitsatop
thebasilar
membrane andis
madeup
of aseries of receptor
hair
cells and theirsupporting
cells.It is
namedfor Corti
who
first
described its
architecturein 1851.
It
consistsof thefollowing
structure: the pillar cells,Deiter's
cells,Hansens
cells,inner
and outerhair
cells andinternal
and external sulcus cells.All
but
thehair
cells aresupporting
structures.The
hair
cells arethe
receptor cells that causestimulation of
the
afferent nervefibers.
There
areapproximately
3,500 inner
hair
cells and12-20,000
outerhair
cells.The
hairs
or stereocilia ofthe
receptor cells are enmeshedin
agelatinous structure,
the tectorial
membrane that extendsfrom
thelimbus
Fluid
movement within the cochlearduct
causes movementalong
thetectorial
membrane which pull ortweaks
the stereocilia of thehair
cells.(Fig.
1)
At
thebase
ofthe
cells sit afferent nervefibers
that
connect to the cochlear nerve.Stimulation
of thehair
cells causes a release of neurotransmitters whichin
turninitiate
action potential of the nervefibers
and areeventually
transmitted
to the
auditory
center ofthe
brain
wherethey
are translatedinto
"sounds".
The
location
of thehair
cellsalong
the
cochlearduct
correlates to thetype
of soundthat
stimulates them.Cells
nearthe oval window areactivated
by
high-pitched
sounds,
those nearthe cochlear apex are activatedby
the
lowest
frequencies.
Tectorialmembrane
Stereociliaofhaircells
[image:9.570.97.424.337.597.2]directionalmovement of stereocila
Figure 1. Movement
ofBasilar Membrane
An
examination of the closeup
view ofthe
cochlea shows thedifferent
types of cells and orientation
to
each other.The inner hair
cells,located
onthe
modiolar side ofthe
cochlearduct,
areflask
shaped and contain synapticstructures at
their
base.
They
are surroundedby
internal
pillarcells andborder
cells.Outer
hair
cells arelocated
across the tunnel ofCorti
and runin
2-3
rows.These
cells are cylindrical and contain synaptic structures thatconnect
to
both
afferent and efferent nervefibers.
The
outerhair
cellshave
no
internal
support and maintaintheir
shapeby
supporting
cells.They
situpon
Deiter's
cellsthatform
a shallow cup.The
apical surface of the cellis
held in
placeby
a reticular membrane thatalso anchors the stereocilia.(These
cells
may
be influenced
by
the central nervoussystem.)The
stereocilia arelocated
on the apical surface ofboth hair
cells and are arrangedin
rows withthe
fibers
graded atdifferent heights. The
rows oninner
hair
cells arestraight, those on outer
hair
cellsform
aV
orW
shape.The
hairs
areanchored
in
adense
cuticular plate(reticular
membrane) atthe apical surfaceofthe cells.
These
stereocilia are rigid, composed of actinfilaments
bonded
together
in
a'paracrystalline
array'.There
are also sitesalong
the stereociliafibers
thatallow them tobond
to theirneighborproducing
a specificspatialarrangement to the
fibers.
The
different bonds between
thehairs
also affecttheir
movement.The
movement of stereocilia against each other andthe
direction
of movement are thought toplay
a partin
opening
ion
channelsleading
to
changesin
membrane potential and thusfiring
of the cellsalong
the nerve fibers.1
The
generally
accepted model ofhair
cell responseholds
that
deflection
ofthe
basilar
membrane causesdeflection
ofthe
bundle
ofstereocilia of each
hair
cell.The direction
ofdeflection
-towards
oraway
from
the
longest hairs
-determines
the
depolarization
of
the
cell and modulatesthe
activity
of afferentfibers
that
synapse atthebase
of
the
cells.The
majority
of nervefibers
maketheir
synaptic contacts withthe
inner
hair
cells.It is
therefore
assumedthat
it is
the
job
oftheinner
hair
cells to
detect
movement of thebasilar
membrane and to transmit thatinformation
to the central nervous system.2The
analysis of outerhair
cellactivity
has been
moredifficult
thaninner
hair
cells.Outer
hair
cells out numberinner
hair
cellsroughly 3
to1.
It
has
alsobeen
shown thatdamage
to
the outerhair
cells affects the mechanicalvibrations within the cochlea
(efferent
nervefibers
runto
the cochleafrom
the
Central Nervous
System).
One
hypothesis
suggests that the outerhair
cells
may
be
able toincrease
sharpness of mechanicaltuning
of thebasilar
membrane.
Damaged hair
cells maybe unable to control oscillation of themembrane.3
It
is the
inner
hair
cells that seem to respond mostto
sound,95
percentof afferent nerve
fibers
(fibers
that
lead
to thebrain)
innervate
theinner
hair
cells.
These
nervefibers
respondto
sound-specifically
tothe
frequency
ofsound.
The
frequency
is
related to the stimulusthreshold
of thehair
cellsand
thus
the
auditory
nervefibers.
As
sound waves, or mechanical wavespass
along
the
length
ofthe
cochlea,different
cells are stimulateddepending
upon
the
frequency
ofthe
waves.This is known
as'place
coding
offrequency'.
Studies
that
measurethe
response ofdifferent
auditory
nervefibers
to electrical responsehave
been
important
in
the
development
ofthe
cochlear
implant.
Properties
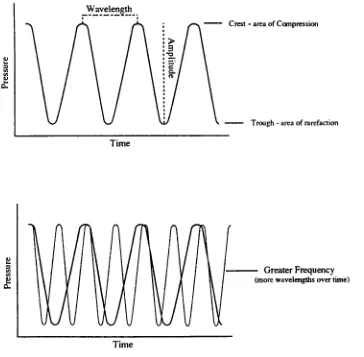
ofSound
Sound
depends
on a medium through whichto travel
-namely
air.It
compresses air molecules which
in
turn
bump
other air molecules and propagate a wave of movement-a new area of compression.
These
series ofcompressions are
collectively
called sound waves.Over
time
anddistance
theoutward
moving
molecules giveup kinetic energy
and the soundeventually
dies. Sound
canbe
depicted graphically
by
use ofS-curves
which can alsodescribe
two
properties of sound-frequency
and amplitude.(Fig.
2)
D.
Wavelength
Time
a.
Crest
-area ofCompression
Trough- areaofrarefaction
Greater
Frequency
(morewavelengthsovertime) [image:12.570.65.429.205.562.2]Time
Figure 2.
Frequency
andAmplitudeThe
crests ofthe
curves represent areas ofcompression, the troughs
representareas ofrarefaction or
low
pressure.The
distance between
two
crestsis
is
defined
as the number of wavesthat
passthrough
a given pointin
a giventime.
The
shorterthe
wavelength, thehigher
the
frequency,
and vice versa.In
humans,
we perceivedifferent
frequencies
asdifferences
in
pitch.The
higher
thefrequency,
the
higher
the
pitch.The
human
ear canhear from 20
to
20,000 Hz
(or
cycles)but is
most sensitiveto those
between
1000-4000 Hz.
Most
sounds are mixtures of severalfrequencies
whichlend
to their richness,a characteristic called quality.
The
intensity
of soundis
dependent
on theenergy
andis
related todifferences
between
areas of compression anddecompression,
or as representedby
the sine wave, to the amplitude orheight
of
the
sound wave.Sound
intensity
orloudness
is
measuredin logarithmic
units calleddecibels. The
decibel
scale as represented on an audiometerbegins
atzero orbarely
audible sound.Every
10
dB
increase
represents a tenfold
(logarithmic)
increase in
soundintensity
or energy.This
correlates toonly
atwofold
increase in
loudness.
The
normal range ofhearing
for
ahealthy
adult earis
from
0 to
over120
dB.
Severe
and profoundhearing
loss
may
occur with overexposureto
soundintensities
over90
dB.
Sound Transmission
andThe
Hearing Pathway
Hearing
occurs when sound waves transmitted tothe
inner
ear, via air,bone
andfluid,
reach and stimulate the receptor cells within the organ ofCorti.
This
occurs through a process of transference within the middle earthat
amplifiesthe
wave energy.Sound
waves arefunneled
into
the earthrough the
pinna(outer
ear)and travel
to the
middle ear where vibrations againstthe tympanic
membrane set
it in
motion,
vibrating
at the samefrequency.
The
motion ofthe tympanic
membraneis
transferred,
to the
oval window oftheinner
ear,
pressure on
the
oval window whichthen
sets thefluids
of theinner
earin
motion.The
tensor tympani muscle can alterthe tension
of the tympanicmembrane and control
the
level
of vibrations.The
stapedius muscle candampen
vibrations,if
needed,
by
pulling
the
stapesaway
from
the ovalwindow.
The
ossicles provide avery
efficient means oftranslating
sound wavesfrom
the airto
fluid
vibrations within theinner
ear.The
areaof the tympanic membraneis 17-20
times larger
than that ofthe oval window and thus results
in
pressure onthe
smaller window22
times
that of pressure onthe
tympanic membrane.Because
the cochlearfluid
is
highly
resistant to motion,this
increase
in
pressureis
necessary
toovercome
any
impedance to
movement.Normally
only
0.1
percent of soundenergy
is
transferred tofluid,
the
restis
reflectedback
at the air-fluidinterface.
It is the
job
of the middle ear componentsto
reduce that reflection andincrease the transference
ofenergy
tothe
fluids
of theinner
ear.4Once
setin motion,
the cochlearfluid
or perilymph within theinner
ear swings the
basilar
membraneup
anddown
causing
the cochlearduct
to oscillate.Sound
waves ofdifferent frequencies
travelalong
the cochleafrom
the
oval windowto the apex orhelicotrema back
to the round window.Low
frequencies
travel
along
thelength
ofthe
cochlearduct
from
the
oval tothe
round window,
high
frequencies
(and
therefore shorterwavelengths)fail
to travelthe
entiredistance.
When
pressure waveshit
thebasilar
membrane,they
setthe
entiremembrane
into
motion.Differences
in
thefibers
within the membranedetermine how
greatthe
effect willbe. Fibers
nearthe
oval window orbase
ofresonate
in
responseto
low
frequencies. Thus
before
everreaching
the
receptor
hair
cells, sounds aremechanically
processedbased
on theirfrequency
and wavelength.The
organ ofCorti
sitsatop
the
basilar
membrane withinthe
cochlearduct. It is
madeup
of rows of cochlearhair
cells and theirsupporting
cells.Afferent
nervefibers
of the cochlear nerve sit at thebase
of thesehair
cells.Up
anddown
vibrations ofthe
basilar
membrane cause a"tweaking"
of the
stereocilia of
the
hair
cellsthat
are enmeshedin
theoverlying
tectorialmembrane.
This
"tweaking" stimulates theopening
ofion
channelsin
thehair
cell membranes whichleads
to
the generation of receptor potentials.Neurotransmitters
releasedby
the
hair
cells stimulatethe
cochlear nervefibers
attheir
base
initiating
action potentials that are sentalong
the afferentauditory
nerves to the brain.5The
afferent nervefibers originating
from
thecochlea come
together
to makeup
theauditory
or vestibulocochlear nerve.The
cellbodies
ofthese
neurons arefound
in
the spiral ganglion at the centerofthe cochlea.
The
auditory
nervetravels to the
cochlear nucleilocated
in
the pons.Secondary
nervefibers
travelto the
opposite side ofthe
pons andmay
projectto the superior
olivary
nucleus orto
theinferior
colliculus ofthe
midbrain.At
the
level
of theinferior
colliculus,impulses
from both
sides provideinformation
as to thelocation
of sound origination.From
the
inferior
colliculus
the
nervefibers
travelto
the medial geniculate nucleus ofthe
thalamus where all
fibers
synapse.At
thislevel
there
is
crudediscrimination
of tone and
direction
of sound.From the
thalamus,
impulses
travel to the
auditory
cortexin
the
superior temporal gyrus andinsula
whereinformation
where
loudness
and a precisediscrimination
of pitch occurs.There
are aprogressively
larger
number of neuronsinvolved
from
the cochlea to theauditory
cortex.Efferent
stimulitravel
along
the
same route.Pathologies
that
causedeafness/Types
ofDeafness
Any
number offactors,
including
disease,
trauma,
ototoxicdrugs
andgenetic
malformation,
may
interrupt
theprocessing
of sound through theinner
ear.These
conditionsmay
lead
to partial or completeloss
ofhearing
ordeafness.
Hearing
loss may
rangefrom
the
inability
tohear
a certain pitch tothe complete
inability
todetect any
sound.There
is
no agelimit
onhearing
loss
either,it
may
occurin
young
or olddepending
on the cause.The
type ofdeafness is defined
by
the cause ofthe
hearing
loss
-either conductive or
sensorineural.
Conduction
deafness
is
causedby
the
interruption
of the transmissionof sound vibrations to the
fluids
oftheinner
ear.Any
number offactors may
cause
this
including
impacted
ear wax, theinsertion
offoreign
objects, andruptured ear
drums. This
type ofhearing
loss
generally
involves
structures ofthe
outer and middle ear.The
most common causes of conductiondeafness
are otitis media
(or
inflammation
of the middle ear)in
children, andotosclerosis
("hardening
of theear"
or
fused
ossicles) often a problem relatedto
aging.Otitis Media
canlead
to abuild-up
offluids
withinthe
middle earwhich
may
interfere
with the vibrations of the tympanicmembrane,
oftencausing
it
tobulge.
Normally
the
auditory
orEustachian
tube
allowsfor
the
drainage
of normal anddiseased fluids
from
the middle earinto
the
nasopharynx.
This
can alsoact as apathway
for
infection
to enterthe
middlemore
horizontally
thanin
adults.Inflammation
ofthe
mucous membranelining
the
Eustachian
tube
may
also causethe tube
to closepreventing
drainage
andin
chronic cases resultin
a negative pressure within the middleear.
This
conditionis
generally
causedby
allergies,
colds,inflamed
tonsils,
etc. and can
usually be
treated with medication orsurgery.6Otosclerosis,
orfusion
of the ossicles to each other or to the ovalwindow,
inhibits
vibrations andtherefore
interferes
with the transmission ofsound waves.
There
may
be
some vibrationthrough the
skull,but
it is
poorly
transmitted
compared to the usual passagethrough
the ear.Otosclerosis,
andother
factors
inhibiting
movement ofthe
ossicles,may
be
treated surgically.Hearing
losses
causedby
otisis media and otosclerosis areusually
treatableand
therefore temporary.
Sensorineural
deafness
occurs when thereis damage
to neuralstructures and
may
occur anywherealong
the
auditory
pathway,from
thecochlear
hair
cells tothe
auditory
cortex.Again
this type ofhearing
loss
may
be
partial or complete andhas
many
causes,both
congenital and acquired.Partial
hearing
loss is
oftenthe
resultof aging, the gradualloss
ofhearing
receptor cells
(hair
cells) throughoutlife.
This
type ofhearing
loss
starts withhair
cells atthebasal
turn andgradually
progressestoward the apex.It
is
characterized
by
high
frequency hearing
loss.
Extremely
loud
noises, orprolonged exposure
to
noise can also cause celldamage. Tumors
anddisease
(such
asmeningitis)
can causeirreversible
damage
to receptor cells andneurons.
Ototoxic
drugs may actually
destroy
hair
cells,depending
onthe
patient,
dosage
andlength
oftreatment.Antibiotics
ofthecategory
aminoglycosides,
especially
in
conjunction withdiuretics,
may be
ototoxic.may
causetemporary
damage.
Other
factors may
include head
injurieswhich
lead
to reducedblood
supply
tothe
inner
ear, andillnesses
that resultin
high
fever for
prolonged periods.Hair
cells are more susceptible toinjury
thansupporting
cells orauditory
neurons.And
alarge
percentage ofhair
cellsmay
be lost
withlittle
or no
degradation
of cochlear neurons.The loss
of neurons seems to parallelinjury
to the
supporting
cells,especially
Deiter's
and pillar cells.Total
loss
of thesesupporting
cells almostalwaysleads
to
severeloss
of cochlear neurons.The
nerve endingsactually
attach to thebase
ofhair
cellsby
desmosomes,
thenerve
fibers lie
in invaginations
of the cell membranes of theDeiter
and pillar cells.In
some casesthe
auditory pathway
tothe
brain
, or thebrain
itself
may
be
affected.While the
outer, middle andinner
earmay
be
intact,
those withcentral
hearing
disorders may
notbe
able tointerpret
the
soundsthey
"hear".
Complex
hearing
rehabilitationmay
bring
about someimprovement.
Depending
uponthe cause ofhearing
loss,
cochlearimplants
may
be
anoption
for
persons with severe or profound sensorineuraldeafness.
These
auditory
prosthetics act astiny
transmittersconverting
sound waves to electricalimpulses
that stimulate the cochlear neuronsdirectly.
Implant
surgeries aimto
preserve as much of theremaining
organ ofCorti
as possible.This includes
trying
not todisrupt
thebasilar
membrane orReissner's
membrane and to prevent contamination of the perilymph which can
lead
tosecondary
nerve degeneration.7Types
ofHearing
Aids
20
to90
dB.
Individuals
with profoundhearing
loss may
not evendetect
sound above90
dB.
Although
someloud
noisesmay
be
discerned,
theseindividuals
cannotrely solely
onhearing
for
communication.Tests
conductedby
an audiologist will measurethe
degree
ofhearing,
as well asspeech
descrimination.
The basic
function
of ahearing
aidis
to make soundslouder
and thuseasier to understand,
especially
speech.There
are several types available andare
usually
usedin
conjunction withlip
reading
toimprove
communicationwith
the
hearing
community.In
generalhearing
aids all work the same way.A
microphone picksup
sound waves and converts them to electrical signals.
These
signals are sent toan amplifier that
increases
their strength,
abattery
provides theenergy
tooperate.
The
receiverthen
converts the amplified signalsback
into
soundwaves and
directs
theminto
the ear via aspecially
fitted
ear coupler.This
amplification and
reshaping
of sound wavesmay
help
compensatefor
weaknesses
along
the
neural pathway.Microphone
*=&
Amplifier
t=5>Receiver
=|^ Ear
Coupler
The
types ofhearing
aidscurrently
available are;In
the
Ear
(ITE),
Canal
Hearing
Aids
(ITC),
Behind
the
Ear,
Eyeglass
aids,Body
worn aids, and specialfunction
aidsthat include
Cochlear
Implants.
The
latter
weredeveloped
for
those with severe
hearing
impairment
that cannotbenefit
from
traditional
amplification
devices.
The
development
of theCochlear Implant
The idea
ofelectrically
stimulating
theauditory
systemis
not new.Interest
began in
the
field
with experimentsby
Volta in 1800. He
inserted
developed
electrolytic cells.Volta
experienced a sensation whichhe
described
later
as"a
blow
to thehead,
followed
by
a soundlike
the
boiling
ofa viscousfluid.
"^Despite
his
unpleasant
results,
interest
continued andby
thelate
19th
century
thefield
was referred to as"electro-otiatrics".
Many
of thediscoveries
madehave
influenced
thedevelopment
of the cochlearimplant
overthe
last
30
years.During
the1960's Drs William
andHoward
House
began
studies ofelectrical stimulation on patients
undergoing
middle-ear surgery.In 1961
onedeaf
patient received a multi electrodedevice
which wassubsequently
removed
due
toswelling
and redness.Premature
publicity
andinappropriate
insulation
materialslead
to problems and research was stoppedfor
severalyears.
In
thelate 60's
engineerJack Urban
joined
the surgical team and threepatients were
implanted in
1969-70.
By
thenimproved
materialshad been
developed
in
partdue
to pacemakerresearch.Several
othersin California
were also
conducting
researchin
thefield
of electrical stimulation withboth
animal and
human
subjects.(Robin
Michelson
atU
ofC,
San
Francisco
andBlair Simmons
at Stanford)9Within
ten yearsinterest in
theimplants
ranged
from
enthusiasmto
outrage.The
1970's
saw muchdebate
overthe
use and
implications
of theimplants
andin
general pitted the clinicalcommunity
against the scientific community.House
andUrban
reported thefirst
long
termimplantations
and concludedthat
thedevice
was nowready
for
widespreadtesting
anddevelopment.
They
cautioned againstuncontrolled
implantation
and use and suggested"careful investigation
by
teams
ofsurgeons,
electronics experts and rehabilitation personnel."10In
many
concluded that theimplant
should notbe
pursued sinceit
would neverbe
able torestore "normal"hearing. Dr. House
and others countered thatsome sensation of sound was
better
than
none and that studies shouldcontinue.
"We
areentering
a new era of otology.For
the pastthirty
years wehave been in
the
conductivehearing
loss
era...but
we are nowentering
theera of
the
sensory
hearing
loss..."11From
that
time
through the present,researchers
have
been
looking
tofully
understand thebasic
principles ofhearing
thatunderliethe
success of theimplants.
Today
cochlearimplants
have
become
acceptedauditory
prostheticdevices for both
children and adults.It
has
been
established through avariety
of studies that
the
implants
are effective optionsin
the rehabilitation of theprofoundly deaf.
Although
resultsvary
depending
onthe
medical, social andpsychological situation of the
individual
patients, almost all receive somebenefit
from
the
implants.12The
best
results
have
occurred withpost-lingually
deaf
adultsandaccording
to several studies,pre-lingually
deaf
children
(Miyamoto,
etal.1993, 1997,
Clark
&
Cowen,
1995).
Benefits
are alsoshown
to
improve
with continued use.Post
-operative rehabilitation andrealistic expectations are vital
in
the success of theimplant.
Of
coursecontinued evaluation of the
device
is
essential.Advancing
technology
brings
about new modifications
in
their use and therefore new questionsin
regardto
evaluation.Surgical
risks andlong
term evaluations should continue.13Benefits
Just
what are thebenefits
providedby
the
implants
and when shouldthey
be
consideredfor
use?First
it
shouldbe
understoodthat
implants
areimplants
may
help
theseindividuals
hear
speech at normal conversationallevels,
be
more aware ofsafety
andwarning
sounds,
lipread
(speechread)
better,
have
clearer speech, and monitorthe
rhythm andloudness
of theirown speech
better.
They
offer greater awareness of environmental noise andtherefore,
improved
safety.Those
whobecame
deaf
as post-lingual adultsmay
be
able tounderstand speech without speech
reading
and even use the telephone.The
overall objective of
the
implant is
toimprove
the
ability
of thedeaf
tocommunicate with
the
hearing
world.This
generally leads
toimproved
psychological and social
benefits
as well. 14Possible Side Effects
It
mustbe
understoodby
the
recipientthat
thereis
no guarantee theimplant
will work.Any
number offactors may
interfere
with properfunctioning.
Careful
evaluation,both
psychological and physical,before
thesurgery helps
to
ensure success.15If
thereis
a mechanical problem with the
coil or
electrodes,
additionalsurgery may
be
neededto
repairor replace thedevice. External
partsmay
alsobe damaged
or malfunctionbut
canbe
corrected without surgery.
Another
possible side-effectthat
may
occuris
infection
atthe
surgical site or around theinternal
coil.Again,
treatment andsurgery may
be
necessary
to
treat the problem.Muscles
in
theface may
become
weak orpartially
paralyzeddue
to
surgery.The
proceduresinvolved
are
in
closeproximity
to several nervesincluding
the
facial
nerve whichmay
be
damaged
or affectedin
someway.16Usually
this
problemis
temporary
andwill resolve
itself in
time.
Lastly,
the
auditory
nervefibers
in
the
cochleamay
not respond
to
the
electrical signalimpulse,
or theremay be
some otherform
possible that calcificationand new
bony
growthmay
occur around theelectrode
in
the
cochlea andblock
the
signals.Once
again,
secondary surgery
may
be
necessary.17Follow
up
treatment
with patientsmay be
very
important in
thesuccessful use of
the
implant.
Negative
feelings
ordisappointing
resultscause some patients to
stop using
the
implant.
They
may
simply
stop
wearing
the external coil and speech processor.
How
theImplant Works
The
cochlearimplant
is
an electronicdevice
that is
composed of twoparts: one that
is
surgically
implanted
within the cochlea(inner
ear) and onethat
is
worn externally.The
external microphone picksup
sound andchanges
it
to
electricalimpulses
which arefed
into
a speech processor, alsoworn externally.
The
processortranslates
the electrical signalinto
anelectrical code which
is then
sent to an external coilheld
in
placejust
behind
the ear
by
a magnet orheadset. The
codeis
transmitted through the skin tothe
internal
coil whichin
turnis
sentalong
the electrodeslocated
within thecochlea.
The
electrodes stimulatethe
auditory
nervefibers
directly
and themessage
is
sentalong
the
auditory
nerve to thehearing
processing
centers ofthe
brain. As
coding
systemsimprove,
sodo
the signals sent tothe
brain.
The
number of electrodesnecessary for
stimulationhas been
studied and willcontinue
to
be developed
andimproved.
Some
ofthe
majordifferences
among
commercial cochlearimplants
include;
the
location
of the external microphone, method of soundprocessing, the number,
type and placement of electrodes, stimulationmode,
back
telemetry
and theprogramming
systems utilized.18work with a specific
internal
device. The
coding
strategy
is
carried outby
thespeechprocessor and refers
to
theway
soundsare processed."Coding
strategies are
designed
primarily
to
optimize speechunderstanding
ratherthan the enjoyment of sounds such as music and environmental
although
these
other sounds should notbe
unpleasant.19A
channelis
a singleline
ofinformation
about a signal thatis
transmitted
to theinner
ear.These
lines
of communication run parallel toeach other.
Electrodes
referonly
to the number of electrical contacts that areinserted
into
the cochlea(the
electrode array).The
number of channels andelectrodes
may
ormay
notbe
the same.Modern implant devices have
4
ormore channels.
Studies
have
determined
that
four
channels are the amountnecessary
to understand speech, provided optimal conditions.20Single
andtwo
channel usershave
been
able to resolve ambiguitiesin
speechin
combination with
lip
reading.Observations have
evenbeen
made with2-channel users who
felt
they
werereally
"hearing".
The illusion
was theresult of
very
familiar
sound patternseasily
recognizedin
combination withthe temporal
cuesfrom
thelip
reading.It
was observedthat
some patternswere so
"well learned
androbustly
stored"
they
couldbe
understood withonly
"a minimal amount of spectral detail."21The
number of electrodes neededis
still underdebate.
There
may be
apoint of
"diminishing
returns"
from
toomany
electrodes.Until
there
is
better understanding
of speechprocessing
and researchersdetermine
how
best
tolocate
the
electrodes,it
is
a matter orquantity
over quality.The
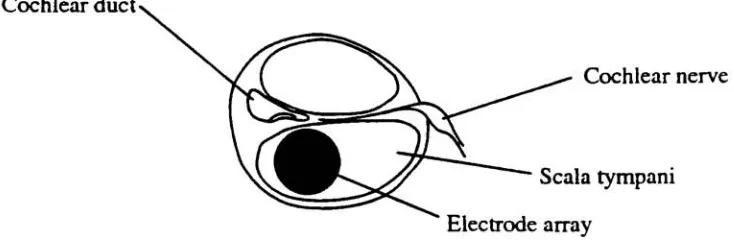
placement of
the
array
withinthe
scala tympaniis
alsoimportant.
Positions
close to the medial wall offer closer contact to
the
ganglion cells withinthe
along
the
length
ofthe cochlea.The
distance between
electrodesis
alsoimportant
so that thereis
minimaloverlap between
neural stimulation.22Cochlear
duct^Cochlear
nerveScala
tympani [image:25.570.87.454.130.254.2]Electrode array
Figure 3. Positionofelectrodearraywithin scalatympani
Another
difference among
implants
are theprocessing
strategies used,either
analog
or pulsatile.With
aCA
(compressed
analog) processor,electrical stimuli are carried
simultaneously
on adjacent channels.There is
some
danger
that
signals willinterfere
with eachother,
however
thereis
continuous
information.
With
the pulsatileCIS
(Continuous
Interleaved
Sampler)
there
is
a staggered arrangement of pulsesalong
parallel channelsthat
helps
alleviatethis interference.
However,
this
causeslarge
"following
distances"
between
successive pulsesthat
may
lead
to gapsin
information.23Brief
changesin in
acoustic signalsmay
be
missed.Different
soundinformation
has
its
own pattern orformant
(predictable digital
signal).Recognition
and simplification ofthose
patterns occursin
the
speechprocessor.
There
does
not appearto
be
any
clear advantage of onecoding
strategy
overthe
other atthis time.Patient
Evaluation
The implant is
not a"cure"
for
deafness.
Recipients
mustunderstandthat
they
do
not return normalhearing.
Wearers
describe
the
produced as
being
electronic and staticy.Careful
evaluation ofpossiblerecipients
is
conductedbefore
surgery
to
ensure that the patient understandsthis.
CT
scans are performed prior tosurgery
todetermine
the possibleextent of
damage
and status ofthe
cochlea andsurrounding
area.The
surgeon should
know
the
particularanatomy
as well as possiblebefore
surgery,
for
instance,
if it
willbe
possible to enterthe cochlea through theround window or
if it
willbe
necessary
to
perform a cochleostomy.Co-axial
and coronal plane views
help
to
determine
the presence offractures,
otosclerosis or malformations.24
It is
especially
important
to visualize thebasal
turnofthe
cochlea andany
intracochlear
calcification.Mastoid
air cellsof the
surrounding
area are prone toinfection
from
otitis media andmay
lead
to mastoiditis which needs
to
be
treated with antibiotics.Fibrous
bony
growthmay
develop
in
this
area and causeblockages.
Meningitis
oftenleads
toossification of the round window, new
bone
growthup
to6
mmalong
thescala tympani
is
possible.Other
tests
may
determine
the extent of nervedamage.
Promontory
stimulationmay
determine if
the
cochlear nervefibers
will
be
ableto
be
stimulated.25Several
factors
tobe
consideredin
patient selectioninclude;
is
ahearing
aidadequate,
has
the
patienthad
any
earinfections,
histology
ofthe
cochlea, population and
viability
of ganglion cells, general medical andpsychosocial situation of
the
person.There is
somedebate
aboutthe
use ofimplants in
patients thatusehearing
aids successfully.Are
theimplants
at allnecessary
ordo
they
improve hearing?
Studies
suggest thatthe
implants
canoffer significant
improvements
for
hearing
aid wearers.26population of cochlear neurons
remaining, the
level
oflanguage
and speechdevelopment
present, theability
ofauditory
associated areasto
ascribemeaning
to the
8tn cranial(vestibulocochlear)
nerve stimulation.As
with allhearing
aidsthe
level
of expectation of the patientis
alsocritical.
It
takes time
and practicetobecome
accustomed to new sounds.Depending
onthe
length
ofdeafness,
the
absence of soundmay
have
become
very familiar
-a return
to
"noise"may be
aconfusing
experience.Success
requires
motivation,
perserverence and patience.New implant
users willneed to workwith
their
audiology
team
to makeany necessary
adjustments.They
will needto
participatein
an aural rehabilitation program.A
history
ofthe
patientis important
todetermine
the successfulcandidate.
The
best
resultshave
been
seenin
post-lingually
deaf
adults.It
is
easier
for
patientsto
learn
to "recode"information if
they
have heard
soundsbefore. The
soundsheard
by
theimplant
wearers are "robotic"in
nature andif
associated withmemory may
have
greater meaning.The
shorter the periodof
deafness,
the
better,
especially
under threeyears.The
outcome of success withthe
device
will alsobe determined
by
thepatient's environment.
Those in
a positive, well-educatedspeaking
environment will
tend to
have better
use of thedevice
and will continue touse
it.
The
best
candidates,according
to
Simmons
(1985),
arethose
living
in
ahearing
world with astrong
motivationto
continueto
do
so.The
decision
toimplant
and the success afterdepends
very
much onthe
patients supportsystem of
friends
and family.27Age
also seems tobe
animportant
factor.
Those implanted
asteenagers
constitutethe
biggest
group
of"non-users".
Due
to the currenttrendtoward
earlierimplantation,
studiesthat
focus
onimportant.28
The
implant is
based
on electrical stimulation via a transmitter placedin
the scalatympani
ofthe
cochleathrough the
round window.Other
possible areas
that
may
be
affected or willdetermine
the
success of theimplant;
viable neural elements ofthe
acoustic nerve,higher auditory
pathway,
receptor areas ofthe
auditory
cortex.Implant Research
-The Past
30
Years
The
originalimplants
used priorto
1978,
whether single or multichannel
devices
usedbroad-band analog
electrical waveforms that excitedbroad
regions ofauditory
neurons synchronously.Unlike
the normal cochleain
whichdifferent
resonancesspecifically
effect thefiring
ofhair
cells, thisbroad
range stimulation still alloweddescrimination
of consonants and"temporal
Cues"provided
by
the
implant.
The
prostheticdevice
washelpful
as a supplement
to
lip
reading
andthe
identification
of"classes"
of sounds.
Studies
conductedduring
the1970's
and80's
provided valuableinformation
that
enabled vastimprovements in
the
design
ofthe
Nucleus
cochlear
implant
speech processorin
wideuse today.Studies
by Tong
andcolleagues
in
Australia,
providedimportant
information
concerning
thedescrimination
offrequency,
intensity
and patterns of stimulation.Hartman,
et al. studied
the
response of theauditory
nerve to electrical stimulation anddetermined
that the spread of neuralactivity
wasdetermined
by
thelocation
and orientation of electrodes
in
the cochlea.29Important
studiesby
Shannon
at
the
University
ofCalifornia,
San Francisco
(1983-93)
measuredhearing
thresholds,
uncomfortableloudness
and the temporalprocessing
ofimplant
patients.
It
wasdetermined
that implant
usershad
ahigh
sensitivity
to
stimulation was much smaller
than that
of normalhearing.
Users
coulddescriminate
small changesin
intensity
(less
than
1
dB)
but
with smallerdynamic
ranges,they
couldonly discern
20-30
stepsin
intensity
compared to200+
stepsin
normalhearing.
Frequency
descrimination
was similar tonormal
hearing
for low frequencies
(<300Hz)
but
absentfor
high
frequencies.
The implant has
no mechanism to separatedifferent
frequencies
of electricalstimulation.
Temporal
processing
appeared normalfor
implant
wearers.They
candetect
gapsin
stimulation andhave
normalrecovery
time
from
adaptations
from
previous stimulus.Overall
implant
patientshad relatively
normal
ability
tofollow
temporal
fluctuations
but
noability
todescriminate
frequencies
above300 Hz
(on
single channel electrodes).During
the70's
and80's
there
were continuedimprovements
ofcommercial
devices
thanks
toinformation
gatheredfrom
research.Actual
performance
in
patients often exceeded expectationsby
researchers.Most
recently,
developments
in
cochlearimplant
signalprocessing
have
resultedin
dramatic
improvements
in
performance.New
processing
strategies,
suchas
CIS
-continuous
interleaved
sampler, showimproved
performance withmultichannel speech processors,
better
than with single channel processes.(Some
multichannel users still receive no spectral place cues andfunction
only
atthe
level
of single channel users.It is
notJcnown if
this
is
due
to poornerve survival or
failure
to
adjust thedevice
properly.)30Implant
researchhas
alsoled
to abetter
understanding
of thehearing
process
in individuals
withoutimpairment
as well.Studies
have
shown thatloudness
is
actually
perceivedby
two
distinct
mechanismsin
the
brain,
onefor
low
frequencies,
another processfor high
frequencies.
number of
safety
concerns that couldonly be
answered over time.Would
there
be
long
term negative effects onthe
auditory
nervous systemfrom
continuous exposure to electrical
fields?
Would
electrical stimulationprecipitate
bone
growthin
the
cochlea and the areasurrounding
theauditory
nerve, would
it
causethe
death
ofany remaining
neural elementsrendering
itself ineffective?
Would
the
devices
have
to
be
replaced and would thislead
to
further
trauma?
Looking
back
attwenty
years of carefulmonitoring
andresearch,
it
would seem thatthe
implants
canbe
usedsuccessfully
andsafely
with
little
or nodetrimental
effects.Implant devices have
proven stable anddependable.
Even
somedevices
thathave been
removed and re-implantedstill
function
well.Hermetic
sealing,
aprocessdeveloped
for
pacemakers waskey
in
early
technological
developments.
It
preventsleakage
ofany
kind
into
the
implant,
from
body
fluids,
that
might cause corrosion orshorting
ofelectrical circuits.
Once
adevice
has been
implanted
for
a year, thefailure
rateis
less
than
1%
(von
Wallenburg
Brinch
1995).
Another
safety
concern, and adebate
stillin
progressis
theimplantation
ofpre-lingually
deaf
children.If
a childis
congenitally
deaf,
canthey
stillbenefit
from
use oftheimplant,
oris
someauditory
experiencerequired?
Also
will therebe
detrimental
effects to the stilldeveloping
auditory
system?Studies
wouldindicate
that thereis
benefit. The
patterns ofstimulation provided
by
the speech processors arerich
enoughfor
thedeveloping
nervous system toform
connections that will allowthem
toprocess
information
in
much the sameway
as a normalhearing
child.Some
results question
the
appropriateness of the stimulus-"wrong
information"such as
that
presentedin
situations with alot ofbackground
noise,
coulddevelopment in later
years.31Surgical
Procedures
Since
the
1970's therehave been
a number ofimplant devices
on themarket.
The
majortypes
include
the
single-channelintracochlear implant
and
the
multichanneldevice,
most often used.The
type ofdevice
usedmay
depend
upon economic and clinical considerations.The
single channelimplant
mostwidely
usedhas been
a modelintroduced
by
House
andUrban
in 1972.
A
number offactors,
including
safe surgical technique and easein
manufacturing,
enabled thisimplant
tobe developed
early.Most
single-channel
devices
workin
the same manner and are similarin
design.
The
surgical procedure
involved
in
placement of thedevice is virtually
thesame.32
One
possible advantage ofthe single channelis
thatit
may
be
placedoutside
the
cochlea, againstthe
round window, although studieshave
shownthat
better
results occurwhenthe
electrodeis
passed a shortdistance
(6
mm)into
the scala tympani.In
children and patients that arebeing
implanted
for
tinnitus
suppression,it is
still preferable toleave
the electrode outside thecochlea.
The
objective ofthe
multichannelimplant
is
to take advantage of the"spatial
representation offrequency
in
the cochlea"so
that
speechmay
be
interpreted in
theprofoundly
andtotally
deaf
toimprove
communication.The best
way
to
take advantage of this spatial stimulationis
to
place anelectrode
array along
the
basilar
membrane withinthe
scalatympani,
beginning
atthebasal
turn ofthe cochlea.(Fig.
4)
The
array
is
alsoflexible
and tapered
to
follow
the
turn of the cochlea and allowfor easy
placement,
and
if
necessary, replacement.33anatomical variations exist.
Fibrosis
orbony
growth presentin
the
basal
turncan
be
removedup
to
6
mmto
allowfor
insertion
of the electrode.Or
if
completely
filled,
the electrodemay
need tobe
placed around the turn.Alternatively,
an extracochlear multichanneldevice may
be
necessary.Electrode
Array
insertedintoscalatympani toa
depthof24mm
Base- High
Frequency
Apex- LowFrequency
Fig. 4 Electrodeinserted intocochlea
The
surgical proceduresdiscussed
here
are well established and generalfor
avariety
ofimplants,
based
onthe
techniquesfor
implanting
theMelbourne
/Cochlear
multichannel receiver.34The
surgeon's
understanding
ofthe
relevantanatomy
is
vitalin
the
success ofthe
implantation.
It is
necessary
to
be completely
familiar
withthe
anatomy
ofthe
round window andbasal
turn
ofthe cochlea, sinceit is
possiblethat
abony
overhang may
obscurethe
surgeon's view ofthe
window.More
extensive
drilling
may be
requiredto
exposethe
round window.Experience
[image:32.570.77.484.171.406.2]There
are severalincisions that
may
be
used to exposethe implant
area.
They
include
the postauricularinverted-U,
extended endaural,C-shaped and
inverted-L.
A
generous margin around the site of the proposedimplant
providesflexibility
for
the surgeonin
case of unforeseen problems.A
dummy
packageis
placedin
the
approximate position of theimplant
siteand marked.
Depending
on the experience and preference ofthe surgeon, theskin and subcutaneous
fascia
may
be
raisedseparately from
thedeep
fascia
and periosteum.36
Tension
sutures
may
be
used tohold
theflap/s
out oftheway.
Extra
precautions, such asbi-polar
coag, aretaken
to preventdamage
tosoft
tissue.
Once
the skullis
exposed, alimited
mastoidectomy
is
performed toexpose the mastoid