OF KERATIN ~ IBRES
Thesis submitted for the degree of
Master of Science
by
Neville L.R. King
January, 1967. Chemistry Dept.,
I am deeply indebted to my supervisors, Dr. J.H. Bradbury and Professor A.N. Hambly, for much valuable inspiration and
guidance.
It is a pleasure to express my gratitude to the staff of the Chemistry Dept. for assistance in many ways, and to the Wool Research Trust Fund for financial support.
SUMMARY
Investigations were carried out on the following keratins
:-Merino 641s wool, Lincoln 361s wool, bibrik, mohair, rabbit fur and
porcupine quill.
Methods for disrupting keratin fibres, particularly by agitation in formic acid, are reported, the separated components being identified by light and electron microscopy. Techniques of amino acid analysis are adapted to meet specific requirements of chromatographic resolution, and to determine amino acids present in small proportions. Analyses of material extracted from wool by formic acid are presented, and a compar-ative study is made of the amino acid composition of the various keratins and their cuticles.
It is concluded that while cell membrane components predominate in material dissolved during short agitations in formic acid, low-sulphur
proteins from the cortex are extracted on prolonged exposure to formic acid. The amino acid, citrulline, not previously detected in non-
medull-ated fibres, was found in cuticle preparations of the keratins investig-ated. The non-crystalline nature of fibre cuticles is discussed in rel-ation to their amino acid composition which is characterised by high contents of cystine, serine and proline. Amino acid analyses of cuticle fractions obtained by treatment of wool in chlorine water and bromine
CON NTS
Acknowledgments.
Summary.
Introduction.
Chapter 1
Materials and Methods
(1) Samples
(2) Cleaning of Keratins
(3) Electron Microscopy (4) Hydrolysis Procedure
(5) Methods of Amino Acid Analysis (6) Defects of Hydrolysis Procedure
Chapter 2
Formic Acid Extracts (1) Experimental
(2) Results and Discussion
Chapter
3
Cuticle and Cortex( 1) I1ethods
(A) Dispersion of Constituents
(B) Separation of Components (2) Results
Yields and Analyses
Page
1
6 7 7 8 8
13
16 18
24
25
(3) Discussion
(A) Assessment of Preparative Procedures (B) Analyses
(i) Comparison of Keratin Compositions (ii) Cysteic Acid
(iii) Citrulline
(iv)
Hydroxy lysine(v)
Kynurenine(vi)
Unidentified Peaks(vii)
Polar Residues(viii)
Conformation(1) Introduction
(A) Allworden Reaction
Chapter 4
Epicuticle
(B) Methods of Preparation (2) Experimental
(3) Results
Yields and Analyses
(4) Discussion
(a) Epicuticle
(b) Cuticle Fraction from Bromine Treatment (c) Chlorine water Extracts
APPENDIX Skin Flakes
Literature Cited Publications
28
4()
4()
41
42
43 43
4J+
45
49
50
51
Nev
epicut icle
endocuticle
~~/miJrofibril
scale
cells
in cuticle
ortical cell
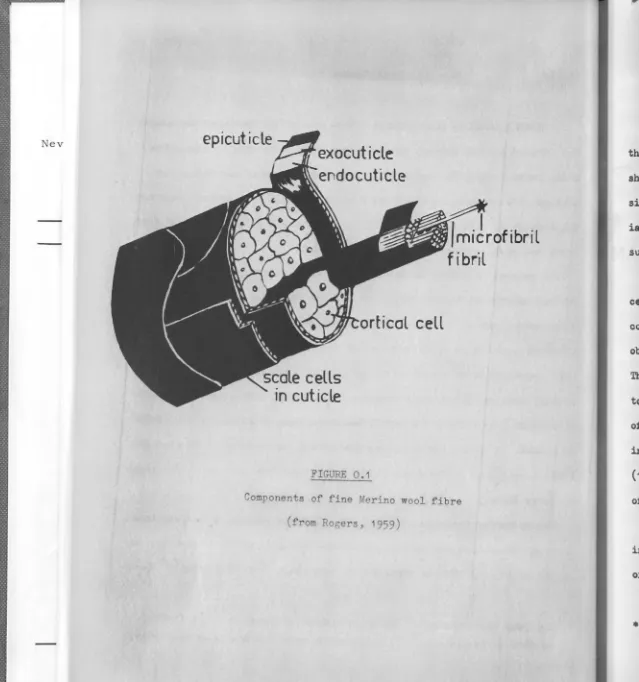
FIGURE 0.1
fibril
Components of fine Merino wool fibre (from Rogers, 1959)
[image:7.663.6.645.8.690.2],.
...
~-1 INTRODUCTION
Histologically, fine keratin fibres consist of two major components-the cuticle and components-the cortex, each of these being structurally complex as shown schematically in Figure O .1. Coarse animal hairs and quills are similar, but contain, in addition, a medulla within the cortex. Substant-ial amounts of skin flakes•, as well as non-protein materSubstant-ial such as grease, suint and dirt, accumulate on fibre surfaces.
The general histology of aniroal fibres was outlined by the nineteenth-century microscopists, and this provided the challenge to separate the components. One of the first attempts was made by McMurtrie
(1886),
who obtained scale cells by dissolution of the cortex in potassiura hydroxide. This procedure draws attention to the greater resistance of the cuticle to many chemical and enzymic reagents. Hock et al (19~1) isolated a tube of cuticle from reduced and methylated wool, upon digestion of the cortex in pepsin. A. method for obtaining the cortex, as reported by Chamberlain (1932), consisted in descaling fibres by drawing them through folded pieces of emery paper.The preceding techniques involve the selective removal of one component, in order to isolate the other. An alternative approach is to disperse most of the cells, and then to separate the components from the mixture. Acid.a,
• The term "skin flakes", used in the manner of Bradbury et al
(1963),
refers to remnants of both epidermal squamae and the inner root sheath •
I
I
---2
alkalis, oxidisizl8 agents, enzymes and bacteria have all been used to
disrupt wool into its constituent cells. After treatment with trypsin
(Burgess, 1934), a partial separation of the smaller scale cells from
el-ongated cortical cells was achieved by fractional sedimentation (Mercer
and Rees, 1946). Mild acid hydrolysis was chosen by Leveau (1956), who
carried out the separation by collecting cortical cells on a sintered glass
filter through which scale cell fragments could pass.
An ideal separation would produce a clean division between cuticle and
cortex, give eax,h in full yield, and eliminate all extraneous contaminants.
However, most separations reported in the past are far from ideal. The
components are often modified by the processes involved. Even mechanically
separated constituents can be chemically modified; Routh (1940) noted th&t
the cystine content of wool decreases on grinding in a ball-mill. A8&in,
many preparations are not free of contaminants. The necessity for careful
examination by optical and electron microscopy cannot be too greatly
emphas-ised - firstly to establish the identity of the component, and secondly, to check for contamination. Furthermore, the isolated fraction might not be
representative of that component in the fibre. The exo- and endo-cuticle
differ in their reactivity toward enzymes, and thus Alexander et al (1963)
claimed that the cuticle prepared enzymatically by Geiger (1944) contained
only the more resistant component. Ag&in, Leveau (1956) found that cortical
cells could be preferentially dispersed from the orthocortex.
In the present investigation, components were dispersed by various types
of agitation in formic aoid, and separated by the technique of dif'f'erential
-I I
3
screening (Bradbury and Chapman, 1964).
While satisfactory separation of the major histological components is
difficult, the task of further fractionation is even more formidable.
However, the epicuticle, being the extreme outer layer of the cuticle,
is readily accessible, and has received much attention. The existence of
such a membrane was suggested by McMurtrie (1886), but it remained for
Lindberg et al (1948) to provide definite proof of its presence. These
workers obtained thin membranes from the surface of wool by strong
chlor-ination followed by a supersonics treatment, and also by dissolving most
of the fibre in sodium sulphide, which left epicuticle in the residue. In the subsequent preparations by Golden et al (1955), the fibres were
success-ively exposed to peracetic acid, ammonia and trypsin. Contamination and
chemical degradation are unavoidable in these procedures.
Fibrillar components of cortical cells were revealed by Nathusius (1894),
following treatment with ammonia, and also by Hock et al (1941) who dissected
cortical cells with glass needles. In the present report are presented
analyses of cortical cells disrupted by ultrasonic irradiation (Bradbury
and Chapman, 1964).
Tertiary phosphines and ethanol have been respectively claimed by De
Deurwaerder et al (1964) and Swift and Holmes (1965) to extract cell mem
-brane material. In chapter 2 evidence that several carboxylic acids extract
similar material will be put forward.
Amino acid analyses were, until the advent of automatic column
chrom-atography, tedious and inaccurate operations. However, the older methods
were sufficient to establish reasonable analyses for whole fibres (for
wool, reviewed by Simmonds, 1954), and to show some differences between
the cuticle and the cortex.
Results of Geiger (1944) for wool, and Lustig et al (1945) for human
hair, suggested that the cuticle contains more cystine and serine, but
4
less tyrosine, threonine, pheeylalanine, arginine, tryptophan and histidine
than the parent fibres. The later work of Bradbury (1960) indicated that
LincO'n cuticle is rich in cystine and proline, but is deficient in aspartic
acid and glutamic acid. Present work largely confirms, and also extends
these results.
Epicuticle analyses have been more conflicting. Alexander (1950) and
Elliott(1950) postulated that it w&s composed of lipids, while La.germalm
/
and Graltn (1951) reported finding a carbohydrate component in the
epi-cuticle. However, these claims have been disputed by subsequent workers.
The results of Zahn (1952), Schuring& et al (1952) and Golden et al (1955),
establish that it is predominant]J protein.
Continued interest in the improvement of methods for separating and
analysing histological components, is sustained by the need to attribute
the action of industrial treatments to the appropriate fibre constituent
(Andrews et al, 1966). These authors noted that yellowing and oxidation
of wool are often predominant in the cuticle. ~ain, the task of
charact-erising protein fractions from wool would be simplified, if these were
The aim of this investigation was to perform reliable analyses on
fractions obtained by refined methods of separation.
Asp
CySO.H Thr Ser
FIGURE I.I
Glu
Gly
Alo Val
.'"·:I Pro
-Cit
..
.
• 11 _ I I3 hr 6 hr
8 hr
CA) OrMr of elution of amino acids in Merino wool hydrolysate (t CI-Tyr).
Peoks not drown to scale
x, (al , lb l and (cl ore unidentified peaks
570 mµ
440 mµ
( B) Citrulline-Proline Resolution
Gly
Glu
I
I
c,.
9 hr
z
(1),,.
Met
n-lleu
.
.
ollo-lleu
Leu
II hr
Tyr Phe
...
-·
I12 hr
14hr
(A) continued
(C) Ammonia Cl-Tyrosine
Resolution
Separation of Ornithine from X
CI-Tyr
N~
Orn
A
XLys
_.:·~
.. . .
.
.
: :_,
' ·1
(cl
\, ,...__ J _: : __ _ _ _ _ _
··· ... ··· .... ···
15 hr 16
hr
NH3
Orn
--. ···
Lys
..
.
.
17
hr
Arg
21 hr
(1) SAMPLES
(a) Keratins.
CHAPTER 1
.MATERIALS AND METHODS
6
Merino 64's wool was obtained from a pen-fed sheep kept at
the Division of Animal Physiology, CSIRO, Prospect,
N.s.w.
Lincoln 36's wool had been processed to "top" form a.t the Division of
Textile Industry,
csmo,
Geelong, Victoria. It was the same sample asthat used by Bradbury (1960) for cuticle analyses.
Samples of mohair and rabbit fur were kindly supplied by the Returned
Soldiers and Sailors Woollen Mills, Geelong.
The sample of bibrik, a.Pakistani oarpet wool, was a gift from the
Gordon Institute of' Technology, Geelong.
Quills of the East African crested p11rcupine ( "Hystrix crista.ta") were
a gift from the Taronga Park Zoo, Sydney.
(b) Amino Acids.
Gifts of lanthionine and 3-chlorotyrosine were recieved from
Dr.
D.Shaw (John Curtin School of Medical Research, A.N.U.) and Dr. E.O.P.Thompson (Division of Protein Chemistry, CSIRO, Parkville, Victoria).
All other amino acids were obtained from commercial sources. In particular,
a Beckman mixture of common amino acids was used for general calibration
(2) CLEANING OF KERATINS
Tips of wool fibres were discarded. Grease was removed from all
fibres and quills by Soxhlet extraction with petroleum spirit (B.P. 6o0
-ao0c)
for 8 hr. Some samples of Merino and Lincoln wools were furthercleaned of dirt and skin flakes, by washing in an anionic detergent
sol-ution, 818 described by Bradbury and Chapman
(1964-).
However, it has sincebeen found that ethanolamine is present in formic acid extracts of these
fibres, and that this contaminant arose from detergent remaining in the
fibres after subsequent washing in water. Consequently, other samples
were washed in wa~er only. Fibres were gently agitated for five minutes
in each of six changes of distilled water at
50°c.
However, this treatment is less effective in removing skin flakes, than
is detergent washiil8•
(3) ELECTRON MICROSCOPY
For sectioning, keratin samples were reduced with thioglycollic
acid, stained with osmium tetroxide (Leach et al,
1964-)
and embedded inAraldite (Glauert et al,
1956).
Thin sections were cut with a ServallPorter-Blum microtome, and were collected on grids coated with collodit1-carbon.
Alternatively, a drop of an aq_ueous suspension of the material was
placed on a grid, and the water withdrawn when suspended material had
settled.
In some cases, specimens were gold-shadowed to increase contrast and to
determine dimensions. For this purpose, grids were placed in a bell-jar
The bell-jar was evacuated to <10-4mm Hg and the alloy heated
electric-ally until it had evaporated.
Grids were examined in & J.E.M. electron microscope, model
T-6.
( 4) HYDROLYSIS PROCEDURE
Sample weights were determined after drying at 100°c in va.cuo
8
for 1 hr. Hydrolysis was carried out with distilled 6 ll hydrochloric acid
(1ml per 4 mg protein) in sealed tubes in vacuo at 110°c (eg. Crestfield
et al, 1963). Routinely,hydrolysis was continued for 24 hr., but times
rall8ing from 10 to
140
hr. were used for special purposes. After hydrolysis,concentrated acid was removed under eeduced pressure by means of a
rotary-evaporator, and the hydrolysate made up to the required volume iJl 0.1 ll
hydrochloric acid.
(5) METHODS OF AMINO ACID ANALYSIS
(A) General
A Technioon Amino Acid Analyser, running 21 hr. chromatograms,
was used. Amino acids were separated on an ion-exchange column, 127 cm x
0.63
cm by gradient elution (Piez and Morris, 1960). The eluent was reactedwith a ninhydrin reagent, and colorimetric estimations made at 570 mµ and
440 aµ (Moore and Stein, 1954). Te.urine, norleucine, and
OC-amino-f3-guanidino propionic acid, which do not occur in animal fibres, were chosen
as internal standards, and run with ea.oh hydrolysate, to correct for any
variations of the analyser. They appear near the beginning, middle and
end of the chromatogram, respectively.
but in some cees larger a.mounts were run, in order to estimate amino
acids present in low proportions.
9
Routinely, values from duplicate runs were averaged, these being within
±
3%
of the mean, except in rare instances.(B) Identification and Chromatographic Resolution of Amino Acids.
Those amino acids which comprise the bulk of wool protein are
well known (eg. Simmonds, 1954), and their positions on Technicon
chromat-ograms have been firmly established by running authentic samples. When
investigating an hydrolysate, peaks coinciding with these positions on the
chromatogram were attributed to the correspondill8 amino acids, in the
ab-sence of any evidence to the contrary. However, in a few cases, two amino
acids simultaneoualy left the column during elution (Figure 1.1).
( i) Ci trulline and Clrni thine.
On some ohromatogeama, the ratio of the optical densities at 570 mµ
and 440 mµ, for a peak in the position of proline, differed from the value
for this amino acid (0.14). It therefore seemed that a second amino acid,
P, contributed to this peak, and evidence that it was citrulline follows:
(a) Under normal elution conditions, peaks corresponding to authentic
samples of citrulline and proline, coincided on the chromatogram.
(b) Under the conditions used for resolution of citrulline from proline
(described later), authentic samples of citrulline continued to appear in
the same position as P (Chapman, 1965, private communication).
(c) The ratio of the optical densities at 570 aµ and 440 mµ was the
same for both P and citrulline (5.2).
(d)
In
most hydrolysates containing P, a peak O, appearing in the--10
ition of ornithine was also observed. Citrulline is known to be partially
converted to ornithine on acid hydrolysis (eg. Rogers, 1958 a), and thus
if an bydrolysate contained citrulline, the presence of ornithine would
also be expected.
Hence there is strong evidence for P and 0 being citrulline and ornithine, respeoti vely.
Although the contribution made by each of citrulline and praline to the combined peak may be estimated from the optical densities at 570 mµ and 440
mµ, it is more accurate to resolve the two amino acids. This may be achieved by appropriate adjustment of the elution conditions (eg. a.reslfes etal., 1965).,
but the method used in the present investigation was one developed mainly by
Chapman (1965, private communication)., and reported by Bradbury et al (1966).
The temperature of the column is decreased from 6o0c to 15°c when aspartic
acid begins to emerge. Lowering the temperature reouces the rate o£ elution of all amino acids, but glutamic acid and citrulline are delayed to a greater
degree than proline, which then appears before glutamic acid on the
chromat-ogram (Figure 1.1). If the temperature is returned to the normal 6o0c when
glutamio acid has been eluted from the column, resolution of the remaining
amino acids is not impaired.
G.rnithine almost coincides with a peak, X, whose identity has not been
established, on a. typical chromatogram. This anomalous peak appears even
when the normal buffer gradient alone is run on the column, and thus it is
not a constituent of hydrolysates. In addition to the ammonia peak, an
ammonia in the eluting buffers. The emergence of this plateau, together with X, can be delayed by incorporating a short column (28cm x 1cm) in
the buffer line between the autograd* and the main column. This auxiliary column was packed with Aminex, a sulphonated polystyrene resin similar to that used in the main column. By this means ornithine could be separated from X. Concomitant with its delayed elution, the ammonia plateau is con-verted to an extremely broad peak, and Xis no longer distinguishable
(Figure 1 .1 ) •
(ii) 3-Chlorotyrosine.
An authentic sample of 3-chlorotyrosine coincided with ammonia on a normal chromatogram. However, the position of 3-ohlorotyrosine is very sensitive to column temperature. Resolution was achieved by decreasing the tempersture from
6o
0c
to 30°c, 20 min. after the exit of phenylalanine from the column. The temperature was returned to6o
0c
immediately after the elution of ammonia. 3-Uhlorotyresine then appeared on the chart mid-wayh
between ammonia and ornitine (Figure 1.1). /\
Following a run at
6o
0c,
some hydrolysates were eluted under the aboveconditions. If' on the second chromatogram, an additional peak appeared in the position of 3-chlorotyrosine, this was regarded as sufficient for id-entification as that amino acid. Such a peak arose only from hydrolysa.tes
of samples treated with cllorine water.
u
12
(iii) allo-Isoleucine.
On many chromatograms, a small peak, MI, appeared between methionine
and isoleucine. Withihe similar conditions used by Hamilton
(1963),
ethionine, cystathionine, djenkolic acid, norvaline and allo-isoleucine
are eluted in this region. Of these, however, only allo-isoleucine ran
in the same position as MI, under our conditions. A sample of MI in the
eluting buffer solution was collected from the column, the collection
ceasing ten minutes before the emergence of n-isoleucine. This fraction
of the eluent was saturated with barium hydroxide and heated at
130°c
for24 hr. in a sealed tube (conditions similar to those used by Ehrlich,
1904,
to racemise isoleucine). After precipitation of barium as the sulphate,
the supernatant was freeze-dried and redissolved in
0.1
M hydrochloricacid. When reloaded on the analyser column, the sample gave peaks in the
positions of MI and n-isoleucine. In spite of the anomaly that a peak also
appeared in the position of leucine, it was concluded that MI was allo- iso
-leucine.
(iv) Ethanolamine.
An extra peak, apuearing between phenylalanine and ammonia, was
regarded as ethanolamine, since it ran in the same position as an authentic
sample, and it arose only from extracts of wool treated with a detergent
containing ethanolamide.
(v) Methionine Oxidation Products.
Authentic samples of methionine sulphoxide and sulphone were eluted
just ahead of aspartic acid (Figure 1.1). A small peak appeared in this
position on numerous chromatograms and was attributed to methionine sulphone,
-ox
since methionine sulphide largely reverts to methionine during acid
"
hydrolysis (Ray and Koshland, 1960).
(vi) DNP-Lysine and DNP-Ornithine.
Reference samples of €-2,4-dinitrophenyl-lysine and
S-2,4-dinitro-phenylornithine required buffers of higher pH for elution from the column, With a citrate beffer of pH 6.0 (0.8 w.r.t. Na+)
a-amino-{3-guanidino-propionic acid emerged after 350 min, S-DNP-ornithine 480 min later, and E-DNP-lysine after a further 320 min. Peaks in these positions were looked
for, af'ter treating Merino cuticle with 1-fluoro-2,4-dinitrobenzene.
(6) DE ~CTS OF HYDROLYTIC PROCEDURE:
During the acid hydrolysis conditions employed, some amino
acids are modified in the following ways:
(i) Tryptophan in the presence of other amino acids is destroyed, but
if tryptophan alone is subjected to the hydrolytic procedure, it largely
survives (Olcott and Fraenkel-Conrat, 1947). No tryptophan determinations
were made in this investigation.
(ii) Citrulline is partly converted to ornithine (eg. Rogers, 1958a).
(iii) A small fraction of 1-n-isoleucine is racemised, giving rise to
D-allo-isoleucine, ( oore et al, 1958). These authors also mentioned that
oxidation of methionine occurs to a slight extent. In the present
invest-igation, some chromatograms showed small peaks in the positions of methionine sulphone and allo-isoleucine, but these were not so large as to appreciably
a.ff'ect the methionine or isoleucine contents of samples analysed. As
14
"allo II form increased with time of hydrolysis, being about 170 after 24 hr.
and 51{, after 140 hr.
(iv)Glutamine and asparagine are quantitatively hydrolysed
to glutamic acid and aspartic acid respectively (eg. Greenstein and Winitz, 1961). Thus in the tables of amino acid analyses, the values quoted for
these dicarboxylic amino acids, include the contents of the corresponding
amides.
(v) Chloro- and bromo-tyrosines may be formed owing to
im-purities in the hydrochloric acid used for hydrolysis (Sanger and Thompson,
1963), These were, however, not evident when a lerge amount (4 mg) of
erino wool hydrolysate was run under the conditions described for
resol-ution of 3-chlorotyrosine.
(vi) Cystine is partially destroyed, a small fraction being
detected as cysteic acid (eg. Wilcox et al, 1957), Decomposition of
various other amino acids also occurs (eg. Smith and Stockell, 1954),
Values for cystine, serine, threonine and tyrosine, obtained from extended -time hydrolyses of Merino wool were extrapolated to zero time of hydrolysis,
This showed that the 24 hr. values should be increased by the following
small amounts
serine
9%,
cystine3
%
,
threonine 6%, and tyrosine tP/4.Other amino acids which are present
in need of correction. Although similar decreases were observed for the
above amino acids in Merino cuticle, this may not be so for all other
samples (eg.
Hill, 1965).
Prolonged hydrolyses, were, however, notper-formed in other cases, and uncorrected values are given in the tables,
these being regarded as sufficient for comparison purposes.
There was no evidence thai.t hydrolysis was not complete in 24 hr.
1·5
0 w 1·0
....
u
<I
a:
....
X w 0-5~ 0
0
15
010 w
....
u<I
a:
....
Xw
~
0
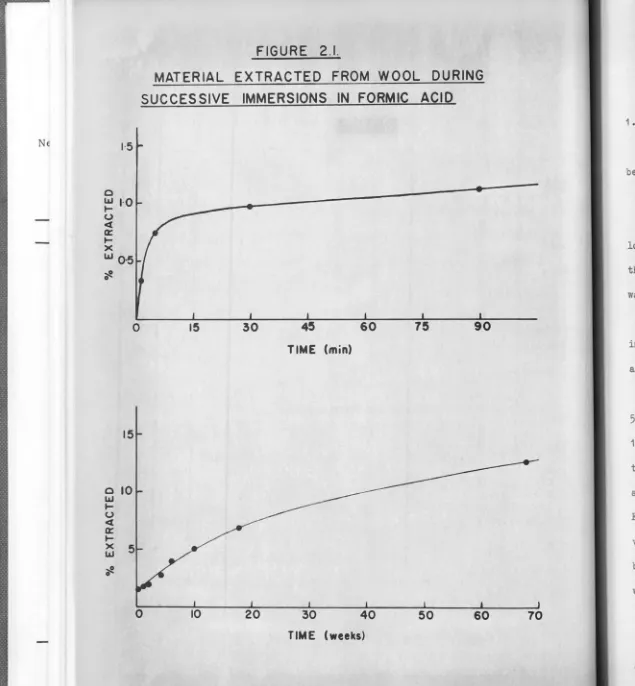
FIGURE 2.1.
MATERIAL EXTRACTED FROM WOOL DURING
SUCCESSIVE
IMMERSIONS IN FORMIC ACID
15 30 45 60 75 90
TIME (min)
0 10 20 30 40 50 60
TIME (weeks)
be f
lots the was
E
imme as
1
50ml
19
time agit For vol bye were
[image:27.670.10.645.10.696.2]1. Experimental
CHAPTER 2
Formic Acid Extracts
All solvents were A.R. grade. Each was distilled and checked to
be free of residue before use.
(a.) Extraction.
16
6 g water-washed Merino wool was immersed in successive 100ml
lots of
98%
formic acid. At various stages during the68
week treatment,the wool was filtered off, immersed in fresh formic acid, and the filtrate
was freeze-dried to obtain the extract.
Extracted material was similarly obtained from 50 mg Merino cuticle
immersed in successive 10ml lots of formic acid. The cuticle was prepared
as described in Chapter
3.
1 g lots of Merino wool were subjected to ultrasonic irradiation in
50ml
of either formic acid or dichloroacetic acid (Bradbury and Chapman,1964). In addition, 2 g lots of Merino wool were agitated for various
times in 50ml formic acid, by means of a laboratory shaker. After each
agitation, dispersed material in suspension was collected by centrifugation.
For this purpose, the dichloroacetic acid solution was diluted with an equal
volume of formic acid. The dichloroacetic acid extract was then obtained
by evaporation under reduced pressure at 100°c, while formic acid extracts
were recovered by freeze-drying.
(b) Dialysis
2 g water-washed Merino wool was agitated in 50 ml formic acid
50ml fresh formic acid for a further
3
hr. Following centrifugation,solutions from both treatments were diluted with equal volumes of water,
and dialysed in Visking tubing against six changes of distilled water over
five days. Some precipitation occurred on dilution of the formic acid
solutions with water, and the amount precipitated increased throughout
the period of dialysis. Finally, the material in suspension and in
sol-ution within the bags was recovered by freeze-drying.
(c) Analyses of Extracts.
(i) Carbohydrates.
The Molisch test was applied to extracts, both before and after refluxing with 2 M hydrochloric acid for 1 hr 45 min.
The anthrone reagent of Dreywood (1946) was also employed, (ii) Lipids.
Extractions with chloroform-methanol (2:1) and ethanol-ether
(3:1) were carried out at room temperature and under reflux, for periods
of 1 hr. in each case. Dissolved material was recovered by means of a
rotary-evaporator. The extracts were reconstituted in chloroform-methanol
(2:1) and mixed with a 'Zfo aqueous calcium chloride solution. After
cent-rifuging, the uJper methanol-water phase containing non-lipid material was
drawn off, and the chloroform phase evaporated to dryness. (iii) Amino acids,
Amino acid analyses were performed as described in Chapter 1.
(iv) Elementary analyses.
These were carried out by the Australian Microanalytical
18
2. Results and Discussion.
Graphs showing the amount of material extracted during successive
immersions of Merino wool in formic acid are displayed in fig. 2.1.
Initially there is rapid dissolution of 0.8% material in 6 min., followed
by a slower rate of dissolution. Blackburn and Lowther (1951) attributed
this two stage behaviour to a configurational change from a- to~- keratin when wool is immersed in formic acid. However, Bendit (1966) has shown
that the a X-ray diffraction pattern is retained for immersion times of a
few hours, but weakens on prolonged exposure to formic acid.
Dissolution is faster if wool is shaken, or subjected to ultrasonic
irradiation, in formic acid. Again, dissolution is more rapid if
dichloro-acetic acid is substituted for formic acid. During 15 min. ultrasonic
irradiations,~
2%
of the wool dissolved in formic acid, but.£!.7%
indi-chloroacetic acid (Bradbury et al, 1965a).
Results of the dialysis experiment are shown in table 2.1.
TABLE 2.1
Dialysis of Formic Acid Extracts from Wool.
Before Dialysis
After Dialysis
Weight of Extract (mg)
5
min. extract. Subsequent3
hr. extract.10.0
2.4
13.6
8.2
Thus 24'>/4 of the initial 5 min. extract and 60'/4 of the subsequent 3 hr.
[image:30.622.53.611.17.695.2]19
This suggests that the material in the 3 hr. extract is of higher
average molecular weight than that in the 5 min. extract. Hence, the initial rapid rate (fig. 2.1 ) is associated with dissolution of
comparat-ively low molecular weight material.
Analysis of the formic acid extracts indicated that the recovery of
amino acids, and thus the protein content, increased with the time of
treat-ment. Table 2.2 shows that the extract from a 5 min. agitation on a
lab-oratory shaker had 10% protein, while that from a 96 hr. treatment had 62}6
protein. Concomitantly, the ash content decreased from 13% for the 1 hr.
extract to
7.5%
for the 12 hr. extract, and the lipid content from 6ofo to46fe respectively.
The composition of the extracted protein changed markedly with time of
treatment. However, in their high contents of glyeine and tyrosine
(com-pared to wool) as well as their low cystine content, these extracts bear
certain resemblances to material claimed to originate from cell membranes
by De Deurwaerder et al, 1964 (analysis reported by Andrews et al, 1966).
The present extracts, though, have much higher contents of the acidic and
basic amino acids than the material obtained by these workers.
The above extracts were derived from detergent-washed wool, and were
shown to contain residual detergent, by the presence of ethanolamine in
each extract. The detergent used, Gardinol BW, contained "coconut
ethan-olamide" as a foam stabilizer (Hendy, 1965, private communication).
As a consequence of drying each sample at 100°c in vacuo, prior to weighing
[image:31.621.119.611.15.697.2]considerably reduced. However, a content of 2fc, was found in the 1 hr.
extract when it was analysed without drying.
20
When water-washed wool was shaken in formic acid, felting was more
severe than it w~s for detergent-washed wool. This resulted in less
diss-olution and less dispersion of material during the agitation. Amino acid
analyses on the 1 hr. formic acid extract from this treatment indicated a
protein content of 2~. Values 6or individual amino acids were close to
those for the same extract from detergent-washed wool. The ash content
was 13% and 61% lipid could be extracted by both chloroform-methanol and
ethanol-ether.
No positive test for carbohydrates was obtained on any of the extracts;
nor were amino sugars detected on the amino acid analyser.
Fraser et al, (1963) pointed out that remnants of mitochondrial membranes
as well as sebum and cell membranes, contribute to the lipid content of
wool. A detailed analysis of the lipids extractable from the interior of
wool fibres has been given by Anderson and Leeder (1965).
From the above and other analyses, as well as the electron microscopy
studies of Chapman, Bradbury et al (1965b) concluded that cell membranes
were dissolved by such solvents as formic acid, dichloroacetic acid and
trifluoroacetic acid. The electron micrographs showed cell membrane
structures to be considerably modified or completely removed, while the
fibril-matrix assembly in the cortical cells was unaltered.
Since the lipid content of the extract decreases as the agitation in
are not dissolved as whole units but that the portions rich in lipids
are extracted first, and the bulk of the protein dissolved later.
However, after 50 weeks' immersion, 11.5% of the fibres had dissolved
(fig. 2.1) suggesting that in this time, some material must originate
from regions other than cell membranes. The cell membrane content was
estimated to be 5 -
7%
by Alexander and Earland (1950), while Lundgrenand Ward (1963) quoted
8%.
When separated cuticle was subjected tosuc-cessive immersions in formic acid, 5% dissolved in 50 weeks. Since the
cuticle constitutes.£.!_ 1o% of the fibre, cuticle dissolved in this
treat-ment would have represented only 0.5% of the original wool. Although the
two series of immersions were not strictly comparable, the preceding
fig-ures indicate that extracted cuticle would comprise not more than 6% of
the material dissolved from wool during an immersion of 50 weeks
follow-ing the 1 hr. Vibromix agitation required to produce the cuticle (chapter
3).
Accordingly, dissolved cuticle would make only a small contribution to
the material extracted between 18 weeks and 68 weeks (E68)• Since
7%
ofthe wool had dissolved in 18 weeks and a further
5.8%
dissolved in thenext 50 weeks (fig. 2.1), the bulk of E68 would not consist of cell
mem-branes, as these are preferentially dissolved and account for only
5-8%
of the fibre. Thus E68 must arise mainly from the cortex.
In table 2.2 are included amino acid analyses of material extracted
during immersion between 6 weeks and 10 weeks (E10) as well as analyses
of E68· The latter extract, compared to wool, is low in cystine, proline,
22
leucine and lysine. In these respects, it resembles the low-sulphur
proteins of wool (eg. Harrap and Gillespie,
1963).
Available evidencefavours association of the low-sulphur protein fraction with the
micro-fibrils in wool (eg. Crewther et al,
1965).
Thus it appears that aport-ion of the microfibrillar component may be extracted on prolonged
immers-ion of wool in formic acid. This accords with the observation of Bendit
(1966)
that the a X-ray diffraction pattern is weakened on extendedexpos-ure of keratins to formic acid. It also supports the conclusion of Leach
et al
(1964)
that reagents which do not attack disulphide bonds might beexpected to attack the microfibrils more rapidly than the matrix. These
workers studied the extraction of wool with boiling 0.01 M hydrochloric
acid and observed that a similar low-sulphur fraction was preferentially
extracted.
Further, table 2.2 shows little difference i~ composition between E10
and E68, and hence proteins from the cortex also represent a substantial
proportion of the earlier extract, E10 • However, since only
4
%
of thewool had dissolved in 6 weeks (fig. 2.1), some cell membrane material
would still be present at this stage, and contribute to the extract.
Thus, while it is concluded that cell membranes comprise the bulk of
the extract during immersion times of several days, it is apparent that
low-sulphur proteins from the cortex dissolve on prolonged immersion, and
TABLE 2.2
23
AMINO ACID AlALYSES OF HYDROLYSATES OF FORMIC ACID EXTRACTS (mole%)
Amino Acid Ala Arg Asp Cit CyS03H 1 - Cys 2 Glu Gly His Isol Leu Lys Met Orn Phe
5 min.
5.96
5.32
8.220.36
0.55 1.01 12.20 16.952.07
3.39
7.20
4.12 1.22 0.304.57
Pro
2.97
Ser
9.23
Thr
4.62
Tyr 5
.42
Val
4. 78
Recovery**10.
2%
o/'oWool in
Extract
o.8
Agitation Time* 1 hr. 12 hr.
6.23
6.16
7.28
0.19 0.122.07
10.5014.39
1.74 3.91 8.21 4.661.20
0.18 4.334.03
8.33 4.876.20
5-47
26.9%
1.0
6.715.96
7.25
0.280.32
3.20
10.18 11.881.70
4.18 8.874.96
1 .170.13
3.904.58
8.62 5 .14-4-.88 6.15 4-1.9% 1.696
hr.6.70
5.87 7.820.23
0.263.60
10.37 9.4-2 1.674.20
11.4-0 4-.59 1.28 0.203.68
5.18 8.07 5.28 4.026.31
62.1% 7 .4-97.31
14.08<: 0.03 0.12 0.52
13.23
4.911.56
4.69 11.235.04
1.61 <0.03
2.78 2.32
6.57
4.973.94
7.641.2
7.68 7.52 14.02<: 0.03
0.15
0.67
16.184.15
1.354.02
11 .4-04.65
1.360.38
2.26 2.106.65
4.42
3.82 7.3584.8'/4
5.8
*
2 gm detergent-washed Merino wool agitated in 50ml formic acid on a laboratory shaker.1 Material dissolved from water-washed Merino wool during a 4- week
immers-ion in fresh formic acid following successive immersions for 6 weeks (E10),
and during a 50 week immersion following immersions for 18 weeks (E68)
[image:35.632.52.616.15.704.2]FIG URE 3.1
MATERIAL DISPERSED FROM WOOL BY SHAKING TREATMENTS.
0 uJ
6
5
4
~ 3
uJ
~ (/)
0
~ 2
0
0 3 6
Dichloroacetic Acid.
•
~ - - - E t h a n o l . (50° C}
9 12 15 18 21
SHAKING TIME (hr)
z
20%• ...
96 hr
[image:37.720.6.710.13.641.2]1. Methods.
CHAPTER
3
Cuticle and Cortex.
(A) Dispersion of Constituent Cells.
24
Initially, Merino and Lincoln wools were disrupted by
u~ltra-sonic irradiation in formic acid, dichloroacetic acid, or dimethyl
sulph-oxide, according to the method of Bradbury and Chapman (1964).
Subsequently, it was found that cells could be dispersed on merely
agitating fibres in various liquids by means of a laboratory shaker (Brad
-bury et al, 1965b). Fig. 3.1 shows the rate at which material was dispersed
when 2 gm. detergent-washed wool was shaken in 50 ml. formic acid and
di-chloroacetic acid at room temperature, as well as in ethanol at 50°c.
Skin flakes were first to be liberated in each treatment, but after shaking
for 3 hr. in formic acid, scale cells predominated. After 48 hr. in formic
acid there were just as many cortical cells as scale cells in the dispersed
material. On shaking detergent-washed wool in trifluoroacetic acid for 3
hr., 1% was liberated, but only very small yields were obtained, in this
time, when water, acetic acid, dimethyl sulphox ide or e thanol (at room
temperature) was used as the dispersing medium.
Of the liquids which gave satisfactory yields of dispersed material in
reasonable times, formic acid was probably the least degradative, and it
was chosen for routine use.
Finally, the folloVling technique due mainly to Chapman (1965, private
communication) was adopted, since it gives greater yields than the lab
25
irradiation. 1.5 g samples of fibres were cut into short lengths (0.5 -1.0 cm) and agitated in 50 ml. formic acid (98%) by means of a Vibromix agitator, oscillating at 100 c/s and operating at maximum amplitude. After an initial treatment of 5 min. to disperse skin flakes, the fibres were teased apart and subjected to a further agitation for
55
min. in fresh formic acid at room temperature. The material dispersed by this second treatment was centrifuged, and the formic acid solution withdrawn.The extract was obtained from this solution by freeze-drying.
The above method is similar to that used by Hock et al (1941) but is
milder, in that the fibres were exposed to formic acid for only 1 hr.,
w ereas these workers continued the immersion for several weeks.
Examination of residual fibres by optical microscopy showed that the treatment was very uneven, because of partial felting. Completely descaled fibres could not be obtained. In treatments of longer duration, cortical cells are liberated in abundance, but some fibres still retain their cuticle.
(B) Separation of Dispersed Components.
Dispersed material, collected by centrifugation in the above
treatment was washed thoroughly with water and ethanol. Cuticle, cortical
PLATE 3, 1
tlect on micro rnph of ~ection of cuticle prep·r~tion obtained
·Fit t · ng Uerino wool in formic ncj d.
,es are a tifa s 0 s'rain· 1 roce u e
cu
2.
*
55
M
B
M
R
ana
electron microscopy. An electron micrograph of a typical section of a cuticle preparation is shown in Plate 3.1.
2. Results.
(A) Yields of Separated and Dissolved Components.
26
For the various fibres subjected to the Vibromix treatment,
yields are listed in Table 3.1. In every case the liberation of cortical
cells was slow. In order to obtain large quantities of the latter, it is
preferable to use ultrasonic irradiation, by means of which~ 20}{> of the cortex can be dispersed in 1 hr. (Bradbury and Chapman, 1964).
*
TABLE
3.1
Yields from Vibromix Treatments* in Formic Acid.
Fibre Cuticle(%) Cortical Cells(%) Dissolved Material(%)
Merino 64's
Bibrik
Mohair
Rabbit fur
1.0
2.2
0.9
0
.7
0.1
0.1
0.1
0.1
2.5
3.7
1.5
3.2
In each treatment, 1.5 g fibres agitated in 50 ml. 98}& formic acid for
55 min. following preliminary agitation for 5 min. (B) Amino Acid Analyses
For each of the keratins investigated, cuticle and whole fibre
analyses are compared in Tables 3.5 to 3.10, while a summary of the data
is presented in fig. 3.2.
[image:42.619.33.611.16.695.2]reas-27
onable agreement with corresponding results of previous workers. This is especially so for wool, but less so for the other fibres. Analyses for
Merino and Lincoln wools as well as mohair have been reviewed by Crewther et al (1965), while Rogers (1962) reported analyses of rabbit fur. However there have been no previous analyses of cuticle derived from fibres other than wool.
The analysis of erino cuticle obtained by ultrasonic irradiation (table
3
.
4),
reported by Bradbury et al (1965a), has subsequently been largelyconfirmed by Inglis et al (1965) and Andrews et al (1966).
At the commencement of this investigation, citrulline was not known to be present in non-medullated animal fibres, and since modified conditions are required for accurate determinations of this amino acid, its content is not included in all tables.
Values for ornithine obtained with the auxiliary column in the buffer line are indicated where they occur in the tables; in other cases, orni-thine estimations are considerably less reliable, owing to peak X (chapter 1).
Values of less than 1%, have much larger percentage errors, than higher values for amino acids. To improve accuracy, ten or twenty times the
normal 0.4 mg load of amino acids was run on the analyser column. Although resolution of adjacent large peaks was impaired, enhancement of well-sep-arated small peaks enabled more reliable estimations of the corresponding amino acids. Values obtained from such high concentration runs are marked in the tables.
-sonic irradiation were kindly supplied by Mr. G.V. Chapman.
3.
Discussion.(A) Assessment of Preparative Procedures
(i) Disruption
28
In the disruption process, the organic acid swells the fibres, and
dissolves a small portion of the protein, while mechanical agitation
lib-erates the constituent cells. Such a process would be defective if there
was (a) chemical modification, (b) dissolution of cellular material, or
(c) selective dispersion of a particular fraction of any component.
(a) Chemical modification:
Previous workers have found no appreciable hydrolysis of peptide
bonds when proteins are immersed in formic acid for several days at room
temperature, but formylation of the hydroxyl groups of serine and threonine
may occur (eg. Smillie and Neurath, 1959; Narita, 1959). However, any such
esters formed during the preparations of this investigation would have
re-verted to serine and threonine during subsequent hydrochloric acid hydro
l-ysis.
Dimethyl sulphoxide is generally regarded as non-degradative (Koenig
and O'Connell, 1960), but the viscosity of certain polypeptides decreases
slowly in dichloroacetic acid (Doty et al, 1956) presumably due to peptide
bond fission.
~xposure of aqueous solutions of proteins to ultrasonics for several
hours causes pronounced chemical changes, reflected by alterations in
vis-cosity and spectral properties (eg. Prudhomme and Grabar, 1947; Khenokh
I I
:
I . ' ITABLE
3.2
29
Al,INO ACID ANALYSES OF POWD.Eru:D ME.IHNO lOOL BE ORE AND AETlill ULTRASO IC
IRRADIATION (mole%)
Amino Acid
Alanine Arginine
Aspartic Acid Cysteic Acid
½
CystineGlutami.o Acid
Glycine Histidine Isoleucine Leucine Lysine ethionine Phenylalanine Proline Serine Threonine Tyrosine
Valine
Powdered
erino wool
5.46
6
.63
6.45
0.09
9
.5
2
11. 31
8.91
0.89
3.12 7.71 2.990.50
2.85
6.14
10.24 6.074.16
5
.
59
97.5
After Ultrasonic Irradiation in
Formic Acid
5.27
6.92
6.41
0.10
10.14
11 .1+7
9.09
0.93
3.177.86
2.89
0.502.97
6.309.95
5.90
4.065
.52
96.0Dichloroacetic Acid
5.50
6.63
6
.
37
0
.1
2
11.62
0.90
3.01+
7.5
8
2.
8
7
o.43
2 .84
6.33
10.90
9
8.5
lo Recovery*
---* In all tables, this refers to the recovery of anhydroamino acids, as a
[image:45.618.46.607.24.698.2]I I
I I
I
TABLE
3.3
.Afr1INO ACID ANALYSES 01'' HYDtlOLYSATES 01'' M}.:RI1'10 WOOL, CORTICAL CELLS AND
DISRUPTED CORTICAL CBLLS (mole ~)
30
Amino Acid erino Wool Cortical Cells+ Disrupted
Formic Acid Dichloroacetic Cortical Cells+
Alanine
Arginine
Aspartic Acid Citrulline
Cysteic Acid
½
CystineGlutamic Acid Glycine
Histidine
Isoleucine
Leucine
Lysine
l,1ethionine Ornithine Phenylalanine Proline Serine Threonine Tyrosine Valine
%
Recovery+
5-31
6
.
80
6
.
34
0
.
045*
0
.
08
10.44
11.
86
8.57
0
.
93
3.11
0
.
50
0.028*
10.216
.47
3.95
5.50
95.5
5.58
6.72
6.71
0
.
004*
0.19
9.01
11
.
62
0
.
8
1
3.31
7
.
71
2.81
0
.
47
0
.
016*
3
.
08
5
.
83
10.57
5.32
4
.
35
5
.
81
96.2
acid Formic Acid
5
.
54
6.92
6
.
81
11.64
0
.72
3.22
7.93
2
.
90
o
.40
3
.15
6
.
02
10.03
5
.
82
4
.
0
1
95
.2
5.54
6
.
57
6
.69
0
.1
8
9
.
66
11.56
9
.62
0
.
83
3
.
27
7.55
2
.
82
0
.
45
3
.
02
5
.
91
1
0
.32
5
.
54
4
.21
96.2
Prepared by ultrasonic disintegration in the medium indicated.
* high analyser column load (4 mg), auxiliary column in buffer line.
I
TABLE
3.4
AMINO ACID ANALYSES O HYD OLYSATES OF .IBHI O CUTICLE (mole %)
Amino Acid Ala Cuticle* from Agitation
Arg 4.61
Asp 3.14
Cit 0.31
CyS03H 0.23
1
- Cys 15.81
2
Glu 8.21
Gly 9. 79
His 0.99
Isol 2.48
Leu 5.51
Lys 2 .80
Met 0.36
Orn 0.171+
Phe 1 .63
Pro 10.2~
Ser 13.28
Thr 4.57
Tyr 2.89
Val 6.91
"/o Recovery
99o2
*
Cuticle from Untrasonic Irradiation in Formic acid Dichloroacetic Dimethyl
0.69 15 .19 8.54 9.08 0.85 2.37 5.77 2.82 0.33 1.75 9.45 13. 74
5 .10 2.86
6.71
96.8
Acid Sulphoxide
5.83 4.91 4.25 0.23 13.50 8.93 9.26 0.92 2.66 6.30 3.05 0.37 2.03
9 .16
13.40
4.93
3.04
6.50
98.4 5.38 5.06 4.56 0.27 13.00 9.97 10.20 1.07 2.57 6.25 2.81
o
.46
1.98 8.1513.59
4.96 2.86 6.15 ean Cuticle Analysis 5.64 4.77 3.91 0.31 0.36 14.38 8.91 9.58 0.96 2.52
5.96
2.87
0.38
0.171
1 .85
9.26
13.50
4.89
2.91
6.57
Cuticle produced by agitating 2 gm wool in 50 ml formic acid for 3 hr.
by means of a laboratory shaker.
+ High analyser column load (2.4 mg), auxiliary column in buffer line. 31
[image:47.617.59.606.14.701.2]-TABLE
3.5
32
AMINO ACID ANALYSES OF HYDROLYSATES OF RINO WOOL AND CUTICLE (mole%) Amino Acid
Alanine Arginine Aspartic acid
Citrulline
Cysteic acid
½
Cystine Glutamic acidGlycine Histidine Isoleucine Leucine Lysine Methionine Ornithine Phenylalanine Proline Serine Threonine Tyrosine Tryptophan Valine
%
RecoveryMerino Wool
5.31
6.80
6.34
o.045**
0
.08
10.44
11.86
8.57
0.93
3.11
7.65
3.05
0.50
0.028**
2.90
5.90
10.216.47
3.95
o.45*
5.50
95.5
Cuticle5
.64
4.77
3.91
0.31
0.36
14.38
8.91
9.58
0.96
2.52
5.96
2.87
0.38
0
.171
++1 .85
9.26
13.50
4.89
2.91
0.34*
6.57
9
7.2
+ Mean cuticle analysis, Table
3.4.
%
Deviation of Cuticle from Parent Fibres.+ 6
-
30
- 38
+600
+350
+
38
-
27
+
12
+
3
- 19
- 22
6- 24
+500
- 36
+
57
+
32
-
24-- 26
- 24
+
19
* Derived from values given by Inglis et al
(1965).
[image:48.623.41.603.14.684.2].---
~
~
...
---""'·
TABLE
3.6
AMINO ACID ANALYSES OF HYDROLYSATES OF LINCOLN WOOL AND CUTICLE (mole%)
Amino Acid
Alanine
Arginine
Aspartio Acid
Citrulline
Cysteic Acid
1
Cystine2
Glutamic Acid
Glycine Histidine Isoleucine Leucine Lysine Methionine <Arni.thine Phenylalanine Proline Serine Thraonine Tyrosine Valine
% Recovery
Wool
5.65
7 .4-76.75
<0.03 0.13 9.8812.66
6.91
o.
74-3.63
7.95
2.82o.4-9
<0.032.56
6.89 10.306.31
2.626.18
Cuticle+5.93
4.80 0.16o.87
16.50 9.127.98
0.552.34-5.50
2.28 0.26<
0.031.38
11.10
94-. 7
%
Deviation of Cuticle from Parent Fibre.+5
-36
-49
+570
+
67
- 28
+ 15
- 26
- 36
- 31
- 19 - 4-7 - 46+
61
+ 30
- 25
- 20 + 21
+ Prepared by ultrasonic irradiation in formic acid.
33
--TABLE
3.7
AMINO ACID ANALYSES OF HYDROLYSAT.ES OF BIBRIK AND CUTICLE (mole%)
Amino Acid
Alanine
Arginine
Aspartic acid
Citrulline
Cysteic Acid
1
Cystine 2Glutamic Acid
Glycine Histidine Isoleucine Leucine Lysine Methionine <lrnithine Phenylalanine Proline Serine Threonine Tyrosine Valine
%
RecoveryBibrik Fibres 5.44
6.79
6.25
0.27
0.36
10.30
13.81
7 .01+
0.79
3.167.03
2.78
o.46
11.272.84-Cuticle+ % Deviation of Cuticle
from Parent Fibres
6.03
+ 114.67
- 31
3.20
-
49
0.26
-
40.67
+86
15.09
+ 468.92
- 35
8.18
+ 16o.66
- 16
2.35
- 26
5.67
-
19
2.35
-
15
0.30
-35
0.17
+ •1.37
-
4211.65
+59
13.92
+23
4.79 - 24
2.28 - 20
7.45
+36
96.6
+ Prepared by Vibromix treatmen~ in formic ~cid.
I
[image:50.615.53.606.14.701.2]-
~TABLE J.8
35
AM.INO ACID ANALYSES OF HYDROLYSATES OF MOHAIR AND CUTICLE
(mole%)
Amino acid Mohair Cuticle+
%
Deviation of CuticleFibres from Parent Fibres
Alanine
5.78
6.05
+5
Arginine
7
.01+-4.51
- 36
Aspartic acid
6.50
3.26
- 50
Citrulline
<
0.03
0.34
+ 00Cysteic acid
0.18
0.58
+2201 Cystine
9o65
15.20
+58
2
Glutamic acid
13.17
8.30
- 37
Glycine
7.08
8.68
+23
Histidine
0.76
0.76
0
Isoleucine
3.70
2.26
- 39
Leucine
7.77
5.77
- 26
Lysine
2.84
2.44
- 14
,, .Methionine
o.47
0.26
- 45
,;
<lrnithine
.:::::0.03
0.15
+•Pheeylalanine
2.67
1-35
- 50
Proline
6.50
10.91
+68
Serine
10.31
15.30
+ 48Threonine
6.71
4.66
- 31
Tyrosine
2.86
2.19
- 23
Valine
6.00
6.91
+15
%
Recovery99.5
91.4
+ Prepared by Vibromix treatment in formic acid.
[image:51.616.41.606.14.681.2]-TABLE
3.9
AMINO ACID ANALYSES OF HYDROLYSATES OF RABBIT FOR AND CUTICLE (mole%)
Amino acid
Alanine
Arginine
Aspartic acid
Citrulline
Cysteio acid
1
Cystine2
Glutamic acid
Glycine Histidine Isoleucine Leu.cine Lysine Methionine Clrnithine Phenylalanine Proline Serine Threonine Tyrosine Valine
% Recovery
Rabbit
Fur
5.00
6.12
5.50
o.
71
0.35
12.38 12.698.54
1.742.70
6.67
2.880.71
0.10
2.65
7.30
10.30
5.60
3.03
4.92
+Cuticle
%
Deviation of Cuticle from Parent Fibres5.08
+ 23.76
- 39
4.26
- 23
1.80 +154
o.48
+37
14.12 + 14
11.94
-
610.68
+ 251.13
-
351.96
-
276.24
-
6
4.13 +
43
0.59
-
17
0.82
+720
1.83
- 31
8.30 +
14
11.87
+ 15
3.50
- 37
1.88
- 38
5.74 +
17
98.0
+ Prepared by Vibromix treatment in formic acid.
[image:52.620.52.606.13.702.2]37
AMINO ACID ANALYSES OF HYDROLYSATES OF PORCUPINE QUILL AND CUTICLE (molefo)
Amino acid
Alanine
Arginine
Aspartic acid
Citrulline
Cysteic acid
½
Cystine Glutamic acid(.;.lycine Histidine Isoleucine Leucine Lysine twethionine Ornithine Phenylalanine Proline Serine Threonine Tyrosine Valine
'lo
RecoveryQuill+
6.22
6.12
7 .41
0.85 0.14 5.28 13.33 11.62 0.85 8.27 2.87 0.31 0.28 3.61 6.11 7.82 4. 71
6.44
101.5 Cuticle* 6.23 6.60 7 .12 0.17 0.15 7.30 12.0010. 78
0.79
3.00
8 .18
2.85
0.27
<
0.032.94 7.00 8.32 5.32 5.57 5.39 93.7
%
Deviation of Cuticle from Parent quill0
+ 8
- 4
- 80
+
7
+ 38
- 10
-
7
- 7
+ 3
- 1
- 13
-100
- 19
+ 15
+
6
+ 13
- 14
+ 10
1
* Obtained by dissection.
+
[image:53.615.36.605.13.683.2]1
!
I
1
:
I
I
I
I
L
I
I
1
I
I
[image:54.614.20.601.17.677.2]I
FIGURE
3
.
2
l
-AMINO ACID COMPOSITION
17
16
•
b
IS
V
14
Q
13
R R
12 ,q
Q
II q
10
~
m
,
I
9 "' b
8
~ Q Cl
q
7
l&.I q
..J 0
6 RQ
~
R
Q
5 ml b
~I
4 m
3
I
2
Q
R Mm
m ab'
i.,tE.
0 lo\! E q
[image:55.660.7.632.15.705.2]~
ANO CUTICLES
Whole
Cuticle Keratin
17
Merino M m
Lincoln L 1 16
Bibrik B b e
Mohair E e
Rabbit R r
15
I I
Quill Q q
b 14
m
13
12
~
II10
9
q
Q 8
R
('
b
q
7
_!,
Q ~
Q L
6
R
q q
RQ 5
4
3
2
--+--- ---+---1---4---t-O