JOURNALOFVIROLOGY,Nov. 1983,p.352-360
0022-538X/83/110352-09$02.00/0
Copyright©1983,American SocietyforMicrobiology
Vol.48,No. 2
Isolation of Monoclonal Antibodies That Recognize the
Transforming Proteins of Avian Sarcoma Viruses
LEAH A. LIPSICH, ANITA J. LEWIS, ANDJOAN S. BRUGGE*
Department of Microbiology, StateUniversity of New YorkatStonyBrook, Stony Brook, New York 11794
Received9May 1983/Accepted 1 August 1983
Thirteen clones of hybrid cells which synthesizeantibodies directed against the Rous sarcomavirus (RSV) transforming protein, pp605'', were isolated. Mouse
myeloma cells werefused with spleen cells from mice that had been immunized
with purified pp6Osrc from bacterial recombinants which direct the synthesis of the RSV src gene. The hybridomas which survived the selection medium were
screened by immunoprecipitation of pp605'' from 32P-labeled lysates of RSV-transformed cells. Monoclonal antibodies produced by subclones derived from 13 hybridomas recognized pp6O r' encoded by the Schmidt-Ruppin and Prague strains of RSV and the cellularhomolog of pp6O5''. Antibody from clone 261 hada
high affinity for the viralyesgeneproduct, and antibodies from clones 443 and 463
recognized the transforming proteins encoded by viruses containing the related transforming genesfps andros. Several otherclones hadalowaffinity for the viral yes,fps, and rosgeneproducts which could be detected by in vitro
phosphoryla-tion of the transforming proteins after immunoprecipitaphosphoryla-tion with the monoclonal antibody. All of the monoclonal antibodies allowed phosphorylation of pp60S'C and casein inan immune complex-bound reaction.
Rous sarcoma virus (RSV) has proven to be anidealsystem toinvestigate themechanism of oncogenic transformation by retroviruses.
Ge-netic studies have revealed that a single viral gene product is responsible for the events in-volved inoncogenic transformation (reviewedin
reference 26). The protein product of thisgene,
designated
pp6Osrc,
was first identified byim-munoprecipitation of radiolabeled lysates of RSV-transformed cells,
using
antiserum from rabbits bearingtumors induced by RSV (desig-natedTBR serum) (5).Thepp6OsrC proteinhas been shown to possess a
tyrosine-specific
kinase activity which has been postulated to be essential for the eventsinvolved in oncogenic transformation by RSV (8, 9, 20, 24, 34). Several substrates of
pp60sr(-mediated phosphotransferase have been
identi-fied; however, the functional significance of phosphorylation of these substrates remains to
be elucidated (4, 6, 10-12, 18, 31, 33).
Uninfected cells containageneproductwhich isstructurally andfunctionally analogoustothe viral transforming protein, designated
pp6Oc-src
(7, 29, 32). It is believed that the transforming
gene of RSVwasderived from the cellular gene
encoding
pp6Oc-rc
(36). The cellular src geneproductalsodisplays tyrosine-specific
phospho-transferaseactivity (29, 32).
Thetransforming proteinsencodedbyseveral other oncogenic avian and mammalian
retrovi-ruseshavealsobeen shownto possess
tyrosine-specific protein kinase activity (1, 2, 13, 14, 21, 39). Recently, it has been shown that the RSV
transforming protein shares considerableamino
acidhomology with these other tyrosine-kinase transformingproteins (19, 23, 35, 37).
The antiserum from tumor-bearing animals
which has beenused for the analysis ofpp6Osrcis apolyclonal antiserum. Although this antiserum hasproven tobeinvaluable fortheidentification of this protein and preliminary characterization of itsphosphotransferase activity, thereare sev-eral drawbacks to its use. First, TBR serum
contains antibodiestoviral structuralproteinsas well as topp6Osrc. Second, most of the antisera
raised in rabbits infected with the
Schmidt-Ruppin (SR) strain of RSV do not recognize
pp6Osrcfrom other strains of RSVorthenormal cellular homolog of
pp6Osrc,
and none of the TBR serarecognizenon-gag-encoded regionsof thetransforming proteins fromviruses carryingthe related transforminggenes,fps,yes, orros.
Third,theantigenicdeterminantsrecognized by
TBR are extremely sensitive to denaturation.
Finally, most TBR-derived antibody molecules inactivate the phosphotransferase activity of
pp6Osrc onexogenous substrates.
To circumvent many of theseproblems asso-ciated with TBR serum, we have prepared monoclonal antibodies to the
pp6Osrc
protein. In this study, we report the isolation of13 hybrid352
on November 10, 2019 by guest
http://jvi.asm.org/
353
myeloma cell lines producing monoclonal
anti-bodies against pp60src. We have tested these antibodies for precipitation of pp60src derived from other strains of RSV, the transforming
proteins encoded by thefps, yes, androsgenes,
and thecellularhomolog ofpp60srcinavianand mammalian cells. We have also examined the ability of these antibodies to allow
pp605sr,_medi-atedphosphorylation ofcasein.
MATERIALS AND METHODS
Cellsand viruses. Chickenembryo fibroblasts were prepared from 11-day-old gs-minus embryos (SPAFAS, Inc., Norwich, Conn.). The Prague (PR) (subgroup A) strain of RSV was obtained from T. Parsons; the SR (subgroup A) strain of RSV and Yamaguchi 73 (Y73) sarcoma virus were obtained from H. Hanafusa; PRCIIsarcomaviruswasobtained from K. Beemon; and UR-2 sarcoma virus was ob-tained fromP. Balduzzi.SRD-3T3 cells were obtained by infection of BALB-3T3 cells withSRD-RSV,using polyethyleneglycol.
Preparation of pp605rc for immunization. The src gene product waspurified from the particulate fraction ofEscherichia coli cells which carry aplasmid con-tainingthe srcgenefusedto aplasmid containing the UV5 lac operator-promoter and24nucleotides of the
P-galactosidase
gene. Bacteriafrom 500 mlof culturemedium werelysed, treatedwith DNase, and solubi-lizedasdescribedpreviously (15-17). Theparticulate cellular material was pelleted by centrifugation at 16,000 x gfor30min. (This materialwasgenerously provided by Raymond Erikson.) The pellet material wassolubilized byboilingfor1min inelectrophoresis sample buffer (16). pp60src was separated from other bacterialproteins by electrophoresison a7.5% poly-acrylamidegel. pp60sr" wasdetectedby
electrophore-sisof32P-labeled markerpp60srcadjacenttothe bacte-rial matebacte-rial. pp60srcwaseluted from thegel in 0.1% sodiumdodecylsulfate(SDS)-50mMammonium car-bonate.
Preparationofmonoclonal antibodies. A mouse my-eloma cell line of BALB/c origin designated X63-Ag8.653(obtained from the SalkInstitute,SanDiego, Calif.) was used for the fusions. This line does not expressimmunoglobinheavyorlightchains and there-forepermits thegeneration ofhybrids secretingpure monoclonal antibodies. The cells are maintained in culture inRPMI 1640medium(GIBCO,GrandIsland,
N.Y.) supplemented with 10% fetal calfserum(Flow Laboratories, McLean, Va.) and 100 U ofpenicillin and 100 ,ug of streptomycin per ml. Frozen stocks were kept inliquid nitrogen in 10%dimethyl sulfox-ide-90% fetal calfserum, andcells were thawed ap-proximately1 weekbeforeusefor fusion.
Mousespleencells. BALB/c mice (3to 5 weeksold) were injected intraperitoneally three times at weekly intervals with ca. 20 to 50 ,ug of the purified viral pp6Osrc protein cloned in E. coli. The final injection was given intravenously and contained ca. 5 ,ug of
pp6W"rc in phosphate-buffered saline. Fourdays after thefinal injection, the mice were bledand sacrificed by cervical dislocation, and their spleens were re-moved.Spleencells were thenpreparedby teasingthe spleen apart with needles into RPMI 1640 medium.
Erythrocytes were lysed by treatment with 0.83% ammoniumchloride, and spleen cells were then count-edonahemacytometer.
Cell fusionwascarriedoutbyamodification of the method ofLevy and Dilley (25). Briefly, spleen and myeloma cellswerepelleted together inaratio offour spleen cellsto onemyeloma cell. The pelletwasgently suspended in 2 ml of 35% polyethylene glycol (molecu-lar weight; 1,000; Koch-Light, Coinbrook,
Bucking-hamshire, England) in RPMI 1640 medium, and the cells were immediately centrifuged at 230 x g for 6 min.Thepolyethylene glycolwasthen aspirated,and thefused cellsweresuspended in RPMI1640medium supplemented with 20% fetal calf serum and 10% NCTC 109 medium(M.A. Bioproducts, Walkersville,
Md.). The cells were placed in a T-150 flask and incubated overnight at 37°C underan atmosphere of 5% C02-95% air. The following day, hypoxanthine, aminopterin, and thymidine were added to the medium togiveafinal concentration of10-4Mhypoxanthine,4 x 107 M aminopterin, and 1.6 x 10-5M thymidine, and the cells were portioned into 96-well microtiter dishes, allowing ca. 105 spleen cells per well. Two weeks after the fusion, medium samples were taken from the wells containing clones and assayed for antibody. Medium from 500 clones was taken and tested forimmunoprecipitation of radiolabeled pp6Osr' asdescribed below. Of the 500 clones tested, 16 were foundtobepositive for precipitation ofpp60Orc. Posi-tiveclones were transferred to 24-wellmicrotiter dish-esandfed with hypoxanthine-thymidine medium (as above, butomitting the aminopterin). When the cells weresufficiently dense, the media were sampled and again assayed. Clones remaining positive were then grown up, and frozenstocks were made.Cells retained in culturewereroutinely grown in RPMI 1640 medium with 10% fetal calf serum andantibiotics. To ensure monoclonality and long-term stability ofantibody pro-duction, strongly positive clones were subcloned by limiting dilution.
Concentration of monoclonal antibodies from medi-um and preparation of purified IgG. Medium was harvested fromhybridoma cells grownatadensity of ca. 106 cells per ml. This medium was stored under sterile conditionsat4°C until furtherprocessing. Con-centration of the monoclonal antibodies was per-formed by precipitation with60%o ultrapure ammoni-um sulfate (Schwarz/Mann, Orangeburg, N.Y.) and dialysis inone-tenth of the starting volume of phos-phate-buffered saline with 0.02% sodium azide. The antibodieswere storedat 4°C. Freezing and thawing results in a considerable reduction in the titer of the antibody. Immunoglobulin G (IgG)waspreparedfrom the ammonium sulfate-concentrated medium by the followingmethod. Concentrated medium(10ml) was incubated for2h at4°C with 200,i.1 ofswollenprotein A-Sepharose beads (Sigma Chemical Co., St. Louis, Mo.). The beadswere then washed three times with 100mMTris-hydrochloride (pH 8). IgGwaselutedby incubationwith 0.5ml of100mMglycine (pH 3). After sedimentation ofthe beads, the supernatant fraction wasneutralized immediately with 1 M Tris-hydrochlo-ride (pH 8.0). IgG was stored at 4°C and was never subjectedto freezingandthawing. Thefinal concen-tration ofIgGwaslessthan 0.1 p.g/p.l.
Sera. Monoclonal antibody to p19 was obtained from David Boettiger. TBR serum was prepared as
on November 10, 2019 by guest
http://jvi.asm.org/
354 LIPSICH, LEWIS, ANDBRUGGE
describedpreviously (5) fromarabbitbearingatumor induced by RSV. This antiserum containsantibodyto pp6Osrc aswell as tothe gag gene products. Rabbit antiserum directed against the E. coli-produced
pp6Osr, proteinwas agenerousgift fromR. L. Erikson (16).
Immunoprecipitation. Forthepreparation of animal cell extracts, cultures were labeled for4 h with32Pi
(0.5 mCi/ml, carrier-free; ICN Pharmaceuticals Inc., Irvine, Calif.) in phosphate-free medium. Cellswere lysed and clarified asdescribedpreviously (5). Either ammonium sulfate-concentrated mediaorpurified IgG
from the hybridoma cell lines were utilized for im-munoprecipitation of radiolabeled lysates. Lysates
were incubated 45 min with each monoclonal anti-body, 20 min with goatanti-mouseIgG (Meloy Labo-ratories, Springfield, Va.), and precipitated with the proteinA-containing bacteria,Staphylococcusaureus (22). The bacterial-bound immune complex was washed three times with radioimmunoprecipitation
(RIPA) buffer (5), and the immunoprecipitated pro-teins were eluted and analyzed on 7.5%
SDS-poly-acrylamide gels.
Detection ofphosphotransferaseactivity.i. Phosphor-ylationof casein. Protein kinase activitywas assayed
by phosphorylation of casein. pp6O,r' bound to S. aureus which had been immunoprecipitated with monoclonal antibodies was suspended in 5 mM
MgCl2-20 mM Tris-chloride (pH 7.2) and incubated with 5 pg of casein(Sigma). After theaddition of10 p.Ciof[y-32P]ATP (ICN), thereactionwas incubated at4°C for 20 min. The reactionwasterminatedbythe addition ofSDS-sample buffer and subjectedto elec-trophoresison10%SDS-polyacrylamidegelsfollowed byautoradiography todeterminethephosphorylation
ofcasein.
ii. In vitro phosphorylation of pp6osrc, pp90Yes,
ppllofPs,
andpp68srS.
In vitro phosphorylation ofpp6O-src wasassayed byutilizingpp6o0-sr, boundtoS. aureus which had been precipitated from a lysate prepared from a12-day embryonic chicken brain as described above. The immunecomplex was suspend-ed in 5 mM
MgCl2-20
mMTris-chloride (pH 7.2)-10,uCi of[-y-32P]ATPandallowed toincubateat4°Cfor 20min. TheprecipitateswerewashedoncewithRIPA medium and thensuspendedinSDS-samplebuffer and subjected toelectrophoresis on 7.5%
SDS-polyacryl-amidegels followed byautoradiography todetermine
phosphorylation ofpp6Osrc. Asimilarprocedure was utilizedfor detection ofphosphorylation of the trans-forming proteins from Y73-, PRCII-, or UR-2-trans-formed chicken cells, except that 5 mM MnCI2 re-placed MgCl2.
RESULTS
Weisolated13hybridomacelllinesproducing antibodies which recognize
pp6Osrc.
These celllines were prepared byfusion ofmyelomacells with spleen cells of BALB/c mice immunized with thesrcproteinextracted fromE. coli cells
carrying
aclonedsrcgene.Five hundred clonessurvived the selection procedure, and medium from each clone was screened for antibody directedagainstpp6Osr, byimmunoprecipitation of
lysates
from32P-labeled
PR-RSV-transformed chicken cells. Sixteen wells contained clonesJ. VIROL.
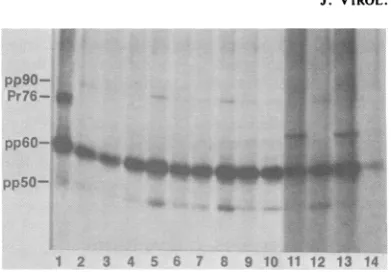
pp9O- ..S...
Pr76-
pp5O-1 2 3 4 5 6 7 8 9 10 11 12 13 14 FIG. 1. Proteins immunoprecipitated from 32P-la-beled Prague RSV-transformed chicken cells. Alysate from a 100-mm culture of 32P-labeled Prague RSV-transformed chicken cells was immunoprecipitated with 5 ,ul of TBR serum (lane 1) or 100,u1 of concen-trated medium from hybridoma cells as described in the text. Lane2,monoclonal antibody 69; lane 3, 78; lane 4,127; lane 5, 191;lane 6, 199; lane 7, 200; lane 8, 261; lane 9, 273; lane 10, 327; lane 11, 443; lane12, 450; lane 13; 463;lane14, 492.
positive for pp6Osr( precipitation. All 16clones
weresubcloned by limiting dilution to assure the
monoclonality of each hybridoma. Of the origi-nal 16clones, only 3 clones have ceased
produc-ingantibodymolecules which recognize
pp60src.
Figure 1 displays an autoradiogram of a gel
containingthe
32P,-labeled
proteinsimmunopre-cipitatedfrom Prague RSV-transformed chicken
cells, usingTBR serum(lane 1) or concentrated
medium from the antibody-producing hybri-doma cells (lanes 2 through 14). TBR serum
immunoprecipitated pp60src as well as Pr76, the
initial translation product of the gag gene, and twocellular proteins of Mr 90,000 (90K) and 50K which are associated in a complex with a small percentage of pp6Osr( molecules (3, 28). The
monoclonal antibodies immunoprecipitated
pp60src aswell asthe two cellular proteins pp9O andpp5O. Antibodies443 and 463 (lanes 11 and 13)consistently precipitated lesspp9O andpp5O relative to pp6O
rc,
suggesting that pp9O andpp5Omayinterferewith recognition of the deter-minants recognized by these antibodies. Anti-bodies 443 and 463 also precipitated another
32p-labeled protein ofMr 68K which was found to contain phosphoserine and phosphotyrosine
(datanot shown). The identity of this protein is underinvestigation. Allof the monoclonal anti-bodies which recognized pp6Osr, from cells in-fected with PR-RSV also recognized SR virus-encoded pp6osrc (including mutant viruses derived from the PR and SR strains of RSV which carry temperature-sensitive defects in thesrc gene; data not shown). The identity of
pp601S1C was confirmed by partial proteolytic
cleavagewith V8 protease (data not shown). Cross-reactionwith cellularpp6OS'. Figure 2A
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.491.255.451.64.201.2]displays theimmunoprecipitation of32P-labeled proteins fromuninfected chicken cells.IgGfrom all of the hybridomas precipitated a protein which comigrated with the 60K protein precip-itated with serum directed against the pp60src protein expressed in E. coli (16). Theidentity of
these proteins aspp6Osr( wasconfirmed by par-tial proteolytic peptide analysis with V8 prote-ase (data not shown). In addition, monoclonal
antibodies 273 and 327 (lanes 7 and 8) were
found to specifically immunoprecipitate the hu-man and mouse cellularsrc proteins (data not
shown; other antibodies not tested).
Figure 2B displays the phosphorylation of pp6O'-src'afterimmunoprecipitationwithIgG
puri-fied from the hybridoma medium. This assay wasperformed withahighlyconcentratedlysate from fresh chicken brain tissue and appears to be a more sensitive assay of pp60'-sr, than immunoprecipitation of32P-labeled chicken cell
lysates.Allofthemonoclonalantibodies
precip-itated 60K protein which was phosphorylated aftertheadditionof[-y-32P]ATP tothe immuno-precipitated proteins (antibody 199 notshown). The identity of this 60K protein which was
phosphorylated in this assay as
pp6O'-sr(
wasconfirmedbypartial
proteolytic peptide
analysiswith V8 protease. These results suggest that all
ofthemonoclonal antibodiesrecognize determi-nants which are shared with pp6O(sr. In both
assays shown in Fig. 2, antibodies 273 and 327
precipitatedhigherlevels ofpp6Osr' than didthe
othermonoclonal antibodies.
Cross-reaction with other viral transforming proteins. Recentinvestigations havefound that the
protein
products ofthetransforming
genesof other avian sarcoma viruses bear considerableamino acid sequencehomology with
pp6Osr(
(19, 23, 35, 37). To determine whether any of themonoclonal antibodies to
pp6Osrc recognize
these related
proteins,
we examinedlysates
fromchicken cells infected with avian sarcoma
viruses containing the yes,fps, and ros genes. These viruses included Yamaguchi 73 (Y73)
(21), UR-2 (14), and PRCII (27), which encode
transforming proteins of 90K (pp9Oyes), 68K
(pp68ros)and 110K
(pp110JfPs)
molecularweight,
respectively. These
transforming
proteins
aregag gene fusion
products,
andantibody
whichrecognizes thegag portion was used as a
posi-tive control in these experiments.
Figure 3 shows the immunoprecipitation of
32P-labeled
proteins from Y73-transformed chicken cells. Itcan be seen thatantibody
261(lane 8) precipitated greater levels of
ppgoYes
thandid monoclonal
antibody
top19
(lane 1),thegag-encoded protein. Three other monoclonal
antibodies (443, 450, and492;lanes 11, 12, and 13, respectively) precipitated lesseramounts of
pp9Oyes
MONOCLONAL ANTIBODIES TO pp60r'( 355
A
-pp6
1 2 3 4 5 6 7 8 9 10 11 12 13 14 B
o -ppGO
1 2 3 4 5 6 7 8 9 10 11 12 13
FIG. 2. Proteins immunoprecipitated from
32P1-la-belednormal chicken cells. (A) Autoradiogram of a gel containing the proteins immunoprecipitated from a
lysate prepared from three 100-mm cultures of32p;
labeled normal chicken cells with 20 ,ul of IgG from hybridoma cell line 69 (lane 1), 78 (lane 2), 127 (lane 3), 191(lane 4), 200 (lane 5), 261 (lane 6), 273 (lane 7), 327 (lane 8), 443 (lane 9), 450 (lane 10), 463 (lane 11), 492 (lane 12), anti-bacterial
pp6O"s
(lane 13), orantimouse IgG alone (lane 14). (B) Proteins phosphorylated in vitroafter immunoprecipitation of alysateofchicken brain with purified IgG as described in the text. Lane 1,monoclonal antibody 69; lane 2, 78; lane 3, 127; lane 4, 191;lane 5, 200; lane 6, 261; lane 7, 273; lane 8, 327; lane 9, 443; lane 10, 450; lane 11, 463; lane 12, 492; lane 13,antimouse IgG alone.To determine whether the monoclonal
anti-bodies which were negative for
pp9O
I'precipi-tation in the above experiment could indeed
recognize
pp9oYe"
in a more sensitive assay, weperformed an invitrophosphorylation reaction.
Figure 3B demonstrates the
autophosphoryla-tion of
pp90'ez
bound to the monoclonal anti-bodies. Unlabeled lysates of Y73-transformedchicken cellswere immunoprecipitatedwith the monoclonal antibodies followed by incubation with
[_y-32PIATP
and MnCl,. In this in vitroreaction,
pp9oYes
wasphosphorylated.Precipita-tion with monoclonal antibody to p19 and 261 (lanes 1 and 8) allowed the greatest level of
pp90We3
phosphorylation; however, lesser amountsofphosphorylationweredetectedafter precipitation of monoclonal antibodies443,450,A
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.491.254.441.66.339.2]356 LIPSICH, LEWIS, AND BRUGGE
A
pp90- so
1 2 3 4 ' 8 9 lO1I 2 13
1 2 3 4 5 67 810 V12 1419M
FIG. 3. Proteins immunoprecipitated from Y73-transformed chicken cells. (A)Autoradiogramofagel
containing the proteins immunoprecipitated from a
100-mmculture of
3'Pi-labeled
Y73-transformedchick-en cells as described in the text. Lane 1, anti-p19 antibody; lane 2, concentrated medium from
hybri-doma 69; lane 3, 78; lane4, 111; lane 5, 127; lane 6, 191;lane7, 200;lane8,261;lane9, 273;lane10,327;
lane 11, 443;lane12,450;lane13,492.(B) Autoradio-gramofa gel containingthe proteins phosphorylated
afterimmunoprecipitationfrom alysateof Y73-trans-formed chicken cellsasdescribed in thetext. Lane1,
anti-p19 antibody; lane 2, concentrated medium from monoclonalantibody69;lane3, 78;lane4, 127;lane5, 191; lane6, 199;lane 7, 200; lane 8, 261; lane9, 273;
lane 10, 327;lane 11, 443;lane 12, 450; lane 13, 463;
lane14, 492; lane 15, antimouseantibodyalone.
J. VIROL.
13) and which was identical to
pp110PS
by partial proteolytic peptide analysis with V8 pro-tease (data not shown). The phosphorylated protein of Mr 60K did not show any partial peptides identical to those of pp60(-5r. Longerexposureof thisgel revealed faint protein bands
in other lanes which comigrated with
ppllOfPS.
Thisexperiment was repeated with more
mono-clonal antibody anda more highly concentrated lysate of PRCII-infected chicken cells to
in-creasethesensitivity of thisassay. Under these conditions, several monoclonal antibodies
pre-cipitated a 110K protein which wasidentical to
ppllOfPs
by peptide analysis. Thelevel of phos-phorylation ofppllOWPS
wasconsistently at leastA
B
.
4
1 2 3 45
-pp110
6 7 8 910 11 12 13 14 15
9I .e
.S
vo. * pp68
and 492(lanes 11, 12,and 14,respectively). The
identity of these proteins as pp90We' was
con-firmed by peptide analysis with V8 protease (datanotshown). Theprotein migrating slightly faster than pp9OYes in lanes 4, 5, and 7 did not share any peptides with pp9OX'e, whereas pro-teins precipitated by antibodies 261, 443, 450,
and 492 were identicaltopp9OYe.
Since the in vitro reactiondescribed above for
Y73-transformed chicken cells proved to be moresensitive than the direct immunoprecipita-tionof32P-labeledlysates,the
autophosphoryla-tion reacautophosphoryla-tion wasutilized fordetermining
cross-reactivitywith other viraltransforming proteins. Figure4Ashows theautophosphorylationofthe
PRCIItransforming protein, pp11OfPs, boundto the monoclonal antibodies. Antibodies 443 and
463(lanes10and12)precipitateda110Kprotein
whichcomigratedwith the
pp1l0fPS
proteinpre-cipitated by monoclonal antibody to p19 (lane
1 2 3 4 5 6 78 9 10 11 12 1314 15
FIG. 4. Proteins immunoprecipitated from PRCII-or UR-2-transformed chicken cells. (A) Autoradio-gramof a gel containing the proteins phosphorylatedin vitro after immunoprecipitation from a lysate of PRCII-transformed chicken cells as described in the text. Lane 1,purified IgG from hybridoma69; lane 2, 78; lane 3, 127; lane 4, 191; lane 5, 199; lane 6, 200; lane7, 261; lane 8, 273; lane 9, 327; lane 10, 443; lane 11, 450; lane 12, 463; lane 13, 492; lane 14, anti-p19 antisera;lane15, antimouseIgGalone. (B) Autoradio-gram of agel containing theproteinsphosphorylated after immunoprecipitation from a lysate of UR-2-transformed chicken cells as described in the text. Lane 1, purified IgG fromhybridoma 69; lane 2, 78; lane3, 127; lane 4, 191; lane 5, 199; lane 6, 200; lane 7, 261;lane 8, 273; lane 9, 327; lane 10, 443; lane 11, 450; lane 12, 463; lane 13, 492; lane 14, TBR antiserum; lane 15, antimouse IgGalone.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.491.54.242.57.302.2] [image:5.491.260.448.241.511.2]MONOCLONAL ANTIBODIES TO pp603'' 357
10-fold less than thatfound with antibodies 443 and 463, and the relative levels of phosphoryla-tion varied for each monoclonal antibody in multiple experiments. The 110K protein band detected in the control reaction of Fig. 4A was
notrelated toppllOPs and was not reproducibly detected in other experiments. These results suggest that some of themonoclonal antibodies have a low affinity for
ppllfP
and that thesereactions are carried out at the lower limit of detection. When PRCII cells are labeled with 32p;, ppllOP can only be detected with antibod-ies 443 and 463.
Figure 4B shows asimilar in vitro phosphor-ylation of the UR-2 transforming protein, pp68r"s, bound to the monoclonal antibodies. Antibodies 443, 463, and 492 (lanes 10, 12, and 13) precipitateda68Kprotein which comigrated with pp68r"s precipitated with TBR serum. TBR
serum contains antibodies togag-specific
deter-minants ofpp68r0s and was used to precipitate
pp68r0S because the monoclonal antibodytop19 didnotprecipitate pp68r"S efficiently.The identi-tyofpp68r"swascorroborated by peptide
analy-sis with V8 protease. We alsofound low levels
ofprecipitationofpp68r"swithmonoclonal anti-bodies other than 443 and 463 when this assay was performed under the moresensitive
condi-tions described above for ppllOfPS. pp68ros
could also be detected by immunoprecipitation
of [35S]methionine-labeled lysates of UR-2-in-fectedchickencells(data notshown).
Protein kinaseactivity. Todetermine whether anyof the monoclonal antibodies interfere with the phosphotransferase activity ofpp6Osrc,
pro-tein kinaseactivitywasassayed by phosphoryla-tionof exogenous casein inanimmune
complex-bound reaction (Fig. 5A). An identical plate of 32P-labeled cells was immunoprecipitated with the same amount of IgG to demonstrate the
relativeamountofpp60fsr precipitated with each monoclonalantibody (Fig. 5B). Figure5Ashows phosphorylation of casein after immunoprecipi-tation ofunlabeled lysates of SRD-RSV-trans-formed 3T3 cells. It can be seen that
pp6OsrC
bound to all ofthe monoclonal antibodies was
abletophosphorylatecasein. The relative levels
of casein phosphorylation generally reflect the level of 32P-labeled
pp6O"r
shown in Fig. 5B.Theprotein of Mr60Kwhichwas phosphorylat-ed in the in vitro reaction (Fig. SA) is
pp6Osrc.
Differences in the level of phosphorylation of
pp60Jrc
also appearto reflect the ability ofthe monoclonal antibody to precipitate32P-labeled
pp605rc. Although antibodies 273 and 327 (lanes 7and8) allowedphosphorylation of casein to a
lesserextentthanthe other monoclonal
antibod-ies, this was not reproducible in other
experi-ments. Monoclonalantibodies 127, 199, and 200 showedasimilarphosphorylationof
pp60s1'
andA
*I IgG
4_.__ -VW pp60
46 -I _ -casein
B
1 2 3 4 5 6 7 8 9 1011 12 13
6-.
to.- gm
,
-4-F
6~~~~~~~~~~~~~~~~~~~~~~.
-pp6O
1 2 3 4 5 6 7 8 9 10 11 12 13
FIG. 5. Phosphorylation of casein by pp6Osrc
bound to monoclonal antibodies. (A) Autoradiogram
of a gel representing the phosphorylation of casein afterimmunoprecipitationofpp6o0r5 from SR-D-trans-formed 3T3 cells as described in the text. Lane 1, antimouse IgGalone;lane2,purifiedIgGfrom
hybri-doma69; lane 3, 78; lane 4, 191;lane 5,200; lane 6, 261;lane 7,273; lane 8, 327; lane9, 443; lane 10,450; lane11, 463; lane12, 492; lane 13,TBR-Bantiserum. (B) Autoradiogram of a gel containing the proteins
immunoprecipitated from an equivalent amountofa
32Pi-labeled
SRD-3T3 cell lysate as that used in (A) Lane1,antimouseIgGalone;lane2,purified IgGfrom hybridoma69; lane 3, 78; lane 4,191; lane5, 200;lane 6, 261; lane7, 273; lane 8, 327; lane 9, 443; lane 10, 450; lane 11, 463; lane 12, 492; lane 13, TBR-B antiserum.casein(datanotshown). The TBR serum used in this assay to precipitate pp6Osr, also allowed caseinphosphorylation. IgGwasnot phosphory-lated after immunoprecipitation of pp6Osrc by any of the monoclonal antibodies. In a similar experiment with lysates from Y73-transformed chicken cells, it was found that
pp90Ye`
which was immunoprecipitated with antibody 261 also phosphorylated casein.VOL.48, 1983
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.491.250.445.77.420.2]358 LIPSICH, LEWIS, ANDBRUGGE
TABLE 1. Recognition of proteins by monoclonal antibodies
Protein Recognized by
monoclonal antibodies'
pp60.src. All
pp60 ....273, 327> 69, 78, 443,
463 >other
pp90yes 261 >443, 450 >492
ppllOfvs
... 443,463pp68r0s...43,463
U> Indicatesmoreefficientimmunoprecipitation.
DISCUSSION
In this study, we report the isolation and preliminary characterization of monoclonal anti-bodies to pp605rc from 13 hybridoma cell lines. To determine the extent of cross-reactivity of thesemonoclonal antibodies andtoestimate the number ofdifferent antigenic determinants that
are recognized by these reagents, we screened
normal cells and cells infected with viruses carrying transforming genes other than the src gene(Table 1). All of the monoclonal antibodies
recognized pp6f051 from Prague and SR RSV-infected chicken cells and the cellular homolog
ofpp601rt. Antibodies 327 and 273 appeared to precipitate pp6O'-1'' more efficiently than the
other antibodies. One monoclonal antibody, 443, recognized all of the viral transforming proteins: pp60src,
pp110fPS,
pp68'05, and, to a lesserextent, pp90)'e. Antibody 463 hada simi-lar pattern of cross-reactivity to 443 in that it precipitatedpp110fPS,
pp68r0S, and pp6051s, butwe have not yet detected pp90OeS precipitation
by 463. This suggests that these antibody
mole-cules do not recognize the same epitope on pp6O5"". It is noteworthy that both antibodies precipitated the same 32P-labeled 68K protein
fromRSV-transformed cell lysates (Fig. 1,lanes
11 and 13).
Several of the monoclonal antibodies
ap-peared to have weak cross-reactivity with pp9OYes,
pp110fP',
and pp68r,, that wasdetect-able inaninvitrophosphorylationassaybutwas
not detectable by immunoprecipitation of
pro-teinsradiolabeledinvivo. It isnotclearwhether
this weak recognition is due to amino acid
sequence differences or to different
conforma-tional arrangements of each of the epitopes in
thevarious transforming proteins. We are pres-ently attempting to vary the conditions of
im-munoprecipitationtooptimize forrecognition of
these proteinsby the different monoclonal
anti-bodies. Preliminary experiments comparingthe
efficiency ofprecipitation of the pp60" protein
expressed in E. coli with that of pp60"'S from
RSV-transformed chicken cells indicates that several of the monoclonal antibodies recognize
the E. coli-produced protein much more
effi-ciently than the protein from transformed chick-en cells. That is not an unexpected result since the antigen used for immunization was a dena-tured form of the bacterialprotein. This protein is distinct from the RSV src protein in that it containseight amino acids from ,-galactosidase at its amino end. Since all of the monoclonal antibodies recognizeda52Kcleavage product of pp60src which has lost the amino terminal se-quences ofpp60*"' (datanot shown), the mono-clonal antibodies wouldnotappeartorecognize the 3-galactosidase-derived sequences. There-fore, differences in the precipitation of the two
forms ofpp605'' could reflect differences in the conformation ofpp605'' molecules derived from either the bacterial or the animal cell environ-ment.
This battery of monoclonal antibodies should be valuable for investigations of pp6O`r", its normal cellular homolog, and the related
trans-forming proteins from other avian sarcoma vi-ruses. Monoclonal antibodies provide the tech-nology to perform immunodetection assays without contaminating reactivities which are
present in polyclonal serum. Parsons and
co-workers have previously reported the isolation of a monoclonal antibody which recognizes
pp60"-"'' (30).Monoclonal antibodies 273 and 327
willconsiderably improvethemeansfor analysis
of the normal cell src protein since most sera
from animals bearing RSV-induced tumors ei-ther do not recognize pp6O('-5' or have a low titer of antibodies to this protein. None of the
RSV-specific TBR sera which have been
de-scribed previously recognize
transformation-specific regions oftheviral
fps,
ros,oryes geneproducts. With the exception of
pp1301Ws
(13),theseproteins havebeen characterizedby using antibodies directed against the gag-specific
re-gion of these proteins.
Monospecific
antibodydirected against the
transformation-specific
re-gions of these proteins willmake it possible to
focus on the transforming protein without the
additionalrecognition ofthe gag geneproducts. Large-scalepurification of theseantigensis pos-sible by using the monoclonal antibodies for
immunoaffinity chromatography.
All of the monoclonalantibodies described in this report allowed
phosphorylation
of casein inanimmunecomplex-boundassay.This
provides
arapidand simplemeansofassaying phosphor-ylation of exogenous substrates. This also pro-videsanassay which canbe usedtoinvestigatethe normal cellular src protein from unlabeled material. We have not yet determined whether
the src protein from lower eucaryotes can be
recognizedbyanyof the monoclonal antibodies.
From the preliminary analysis of the cross-reactivities ofthese monoclonal
antibodies,
we canpredictthatthisbatteryof antibodies recog-J. VIROL.on November 10, 2019 by guest
http://jvi.asm.org/
nizes at least six different antigenic determi-nants. None of thesedeterminantsappearstobe
within the amino-terminal 8,000 daltons of
pp60"',
sinceall oftheantibodies recognized the 52K cleavage product ofpp6O0"' generated dur-ing lysis of RSV-transformed cells (data not shown). Since all of the monoclonal antibodies recognized thecellular homolog of pp60"'(, they apparently do notrecognize thecarboxyl-termi-nal 12amino acidswhich are unique to pp6o'-"( (38). Finer-detailed mapping and competition assays are required todistinguish other
specific-ities.
ACKNOWLEDGMENTS
We thankJoe Cirrone and Diane Darrow forassistance in
thescreeningandcharacterizationof the monoclonal antibod-ies.WeareindebtedtoTonaGilmerand Raymond Eriksonfor providing pp60"' expressed in E. coli. We thank Piero Bal-duzziandKaren Beemon and Hidesaburo Hanafusafor
sup-plyingus with UR-2, PRCII. and Y73 sarcoma viruses. We
alsothankSandraBurns fortypingthis manuscript.
This work was supported by grant CA 27951 from the NationalCancer Institute. L.L. is supported byafellowship
fromtheMuscularDystrophy Association. LITERATURECITED
1. Barbacid, M., A. V. Lauver, and S. G. Devare. 1980. Biochemicaland immunologicalcharacterization of poly-proteinscodedforbytheMcDonough. Gardner-Arnstein. and Snyder-Theilen strains of feline sarcoma virus. J. Virol. 33:196-207.
2. Blomberg,J.,W.J. M.VandeVen,F. H.Reynolds, Jr., R. P. Nailwaik, and J. R. Stephenson. 1981. Synder-Theilenfeline sarcomavirusP85 contains asingle phos-photyrosine acceptor site recognized by its aissociated proteinkinase.J.Virol. 38:886-894.
3. Brugge, J., E. Erikson, and R. L. Erikson. 1981. The specificinteractionofthe Roussarcomavirus transform-ing protein. pp6tprc. with twocellular proteins. Cell 25: 363-372.
4. Brugge,J. S.,and D.Darrow.1982.Roussarcoma virus-induced phosphorylation ofa 50.000 molecular weight cellularprotein.Nature(London)295:250-253. 5. Brugge, J. S.,and R.L.Erikson.1977.Identificationofa
transformation-specific antigen induced byanavian
sar-coma virus.Nature(London)269:346-348.
6. Burr, J.G.,G.Dreyfuss,S.Penman, and J. M. Buchanan. 1980.Associationof thesrc geneproduct of Rous sarco-ma viruswithcytoskeletal structuresofchicken embryo fibroblasts. Proc.NatI. Acad. Sci. U.S.A.77:3484-3488.
7. Collett,M.S.,E.Erikson,A. F.Purchio, J.S.Brugge, and R. L. Erikson. 1979. A normal cell protein similar in
structureandfunction tothe avian sarcomavirus trans-forming gene product. Proc. Natl. Acad. Sci. U.S.A. 76:3159-3163.
8. Collett, M. S.,and R. L. Erikson. 1978. Protein kinase activityassociatedwiththeaviansarcoma virussrcgene
product. Proc.Natl.Acad. Sci. U.S.A. 75:2021-2024.
9. Collett, M. S.,A. F. Purchio,and R. L. Erikson. 1980. Aviansarcomavirus-transformingprotein.pp6O0. shows proteinkinaseactivity specific fortyrosine.Nature (Lon-don)285:167-169.
10. Cooper, J. A., and T. Hunter. 1981. Changes in
pro-teinphosphorylationin Roussarcomavirus-transformed chickenembryocells. Mol.Cell. Biol. 1:165-178.. 11. Cooper, J. A., and T. Hunter. 1983. Identification and
characterization of cellular targets for tyrosine protein kinases. J. Biol. Chem.258:1108-1115.
12. Erikson, E., and R. L. Erikson. 1980. Identification ofa
cellular protein substrate phosphorylated by the avian
sarcoma virus-transforming gene product. Cell 21:829-836.
13. Feldman,R., and H. Hanafusa. 1980.Characterizationof
protein kinase activity associated with the transforming
gene product ofFujinami sarcomavirus.Cell 22:757-765.
14. Feldman, R. A., L.-H. Wang, H. Hanafusa, and P. C. Balduzzi. 1982. Avian sarcoma virus UR2 encodes a
transforming protein which is associated with a uinique
proteinkinase activity. J. Virol. 42:228-236.
15. Gilmer, T.,and R.L. Erikson. 1981. Rous sarcomia viruIs
transforming protein, pp6O0''. expressed in E. co/i.
func-tionsasaprotein kinase. Nature(London)294:771-773.
16. Gilmer, T. M., and R. L. Erikson.1983. Development of
anti-pp6OG' serum with antigen produced in Esc/erichia
(0/i.J. Virol. 45:462-465.
17. Gilmer, T. NI., J. T. Parsons, and R. L. Erikson. 1982.
Constructionofplasmidsforexpressionof Roussarcoma
virus transforming protein, p6O'r, in Escherichia (oli.
Proc. NatI. Acad. Sci. U.S.A. 79:2152-2156.
18. Gilmore, T. D., K. Radke, and G. S. Martin. 1982.
Tyrosine phosphorylation of a 50K cellular polypeptide
associated with the Rous sarcoma virus transforming
protein, pp6O Mol. Cell. Biol. 2:199-206.
19. Hampe,A.,I. Laprevotte, F. Galibert, L. A. Fedele, and
C.J.Scherr. 1982. Nucleotide sequencesof feline retro-viral oncogenes (v-fes) provide evidence fora family of
tyrosine-specific protein kinasegenes. Cell 30:775-785.
20. Hunter, T., and B. Sefton. 1980. Transforminggene
prod-uctof Rous sarcoma virusphosphorylatestyrosine. Proc. NatI. Acad. Sci. U.S.A. 77:1311-1315.
21. Kawai, S., M. Yoshida, K. Segawa, R. Sugiyama, R. Ishizaki, and K. Toyoshima. 1980. Characterization of Y73,an avian sarcomavirus: a uniquetransforminggene
and its product, a phosphoprotein kinase activity. Proc. Nat]. Acad. Sci. U.S.A. 77:6199-6203.
22. Kessler, S. W.1975. Rapidisolation ofantigensfromcells
witha Staphylococcalprotein A-antibody absorbent:
pa-rametersofthe interactionof antibody-antigen complexes withproteinA. J. Immunol. 115:1617-1624.
23. Kitamura, N.,A.Kitamura,K.Tovoshima,Y.Hirayama, andM. V'oshida. 1982. AviansarcomavirusY73genome sequenceand structural similarity ofitstransforminggene
producttothat of Roussarcomavirus.Nature (London) 297:205-208.
24. Levinson, A. D., H. Oppermann, L. Levintow, H. E.
Varmus, and J. B. Bishop. 1978. Evidence that the trans-forming geneofavian siarcomia virusencodes a protein
kinaseassociated withaphosphoprotein. Cell15:561-572.
25. Levy, R.,andJ. Dilley. 1978. Rescueofimmunoglobulin secretion from humanneoplasticlymphoid cells by
somat-iccell hybridization. Proc. Natl. Acad. Sci. U.S.A. 75: 2411-2415.
26. Linial, M., and D. Blair.1982. Geneticsof retroviruses.p.
649-784. In J. Weiss (ed.). RNA tumor viruses. Cold SpringHarborPress,Cold Spring Harbor. New York. 27. Neil, J.,NI. L.Breitman, and P. K.Vogt.1981.
Chairacter-ization ofa 105.000 molecular weight gag-related phos-phoprotein from cells transformedby thedefectiveavian sarcomavirus PRCII. Virology 108:98-110.
28. Oppermann, H., A. D. Levinson, L. Levintow, H. E. Varmus, J. M.Bishop, and S. Kawai. 1981.TwocellIlar
proteins that immunoprecipitate with the trzansforming protein of Roussarcomavirus. Virology 113:736-751. 29. Oppermann, H.,A.D. Levinson, H. E. V'armus, I,.
Levin-tow,andJ.Ml. Bishop. 1979. Uninfectedvertebratecells contain itproteinthatis closely relatedtothe productof the avian sarcoma virus transforminggene (src). Proc.
NatI. Acad. Sci. U.S.A.76:1804-1808.
30. Parsons,S. J., D. J. McCarley, C. M. Ely,D. C.Benjamin, and J. T. Parsons.1983. Isolation and partial
characteriza-tionofamonoclonalantibodytothe Rous sarcoma virus
transformingproteinpp6O"'(. J.Virol. 45:1190-1194. 31. Radke,K., T. Gilmore,andG. S.Martin. 1980.
Transftr-mation by Rous sarcoma virus: a cellular substrate for
on November 10, 2019 by guest
http://jvi.asm.org/
360 LIPSICH,LEWIS, AND BRUGGE transformation-specific protein phosphorylation contains phosphotyrosine.Cell 21:821-828.
32. Rohrschneider, L. R., R. N. Eisenman, and C. R. Leitch. 1979.Identification ofaRoussarcomavirus transforma-tion-related protein in normal avian andmammaliancells.
Proc.NatI. Acad. Sci.U.S.A. 76:4479-4483.
33. Sefton, B. M., T.Hunter, E. H. Ball, and S. J. Singer.
1981.Vinculin: acytoskeletal targetof the transforming protein ofRoussarcomavirus.Cell24:165-174.
34. Sefton, B.M.,T. Hunter, K. Beemon,and W. Eckhart.
1980. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma
virus. Cell 20:807-816.
35. Shibuya, M., and H. Hanafusa. 1982.Nucleotidesequence
ofFujinamisarcomavirus:evolutionaryrelationship ofits
transforming genewith thetransforming genes of other sarcomaviruses.Cell30:787-795.
36. Stehelin, D., H. E. Varmus, J. M. Bishop, and P. K. Vogt.
1976. DNArelatedtothetransforminggene(s)ofavian
sarcoma virusis present innormalavian DNA. Nature (London)260:170-173.
37. Takeya, T., R. A. Feldman, and H. Hanafusa.1982.DNA
sequenceof theviraland cellularsrcgeneofchickens.I.
Complete nucleotide sequenceofan EcoRI fragment of recovered aviansarcomavirus which codes for gp37and
pp605".J.Virol.44:1-11.
38. Takeya, T., and H. Hanafusa. 1983. Structure and se-quence of the cellulargenehomologoustothe RSVsrc
geneand the mechanism forgenerating the transforming
virus.Cell 32:881-890.
39. Witte, 0. N., A. Dasgupta, and D. Baltimore. 1980.
Abelsonmurineleukemia virus proteinisphosphorylated
in vitrotoform phosphotyrosine. Nature(London) 283:
826-831.
J.VIROL.