JOURNAL OF VIROLOGY, Dec. 1974, P. 1337-1342 Copyright i 1974 AmericanSociety for Microbiology
Vol. 14, No. 6 Printed inUJ.S.A.
Bacillus subtilis DNA Polymerase III Is Required for
Replication
of
the Virulent Bacteriophage
/e
URI LAVI, ALONA NATFIENBERG, AMIRAM RONEN, AND MENASHE MARCUS
Department of Genetics, The Hebrew University of Jerusalem, Jerusalem, Israel
Received forpublication 29 July 1974
The virulent phage (eofBacillussubtiliswv Achcontainshydroxymethyluracil
in its DNA requires host DNA polymerase III for its DNA replication. DNA
polymerase IHts mutant cellsinfectedwith (eat restrictive temperatures do not
supportphage DNA synthesis. However, (egrowsnormally bothatlowand high
temperatures inthe mutant'sparent strainandinspontaneous DNA polymerase
III+ revertantsisolated from themutant strain. Temperature-shift-down
experi-ments with (e-infected cells having thermosensitive DNA polymerase III (pol
IHI>) indicate that at 48C the thernmolabile DNA polymerase III is irreversibly inactivated and has to be synthesized de novo after the shift to 37 C, before phage DNA synthesis can begin. Temperature-shift-up experiments with (e-infected mutant cells show that,phage replication isarrested immediatelyafter
the temperature shift and indicate that (e requires DNA polymerase III
throughout its replicationstage.
Bacteriophage (e contains
hydroxymethylu-racil in its DNA inplace of thymine. This phage
belongs to a group of large, double-stranded
DNA phages of Bacillus subtilis (1, 11, 14, 21,
26). The lytic cycle of-behas been characterized
(13, 24-26). Roscoe (25) found that host DNA synthesis is arrested in (e-infected cells several
minutes after infection andphage DNA
synthe-sisbegins.Inboth (eandphage SPOl, which is
relatedto (e, several observationsindicate that
phage DNA synthesis dependsonthesynthesis
of a phage-specific DNA polymerase. Thus it
wasf6und, thatanewDNApolymeraseappears
in SPOl-infected cells (32, 33). Both (e and
SPOlhave been found togrownormallyinDNA
polymerase I- mutant cells, indicating that
DNA replication ofthese two phages does not
dependonthe host DNApolymeraseI (16, 32).
Moreover, DNA synthesis of (e and SPOl is
resistant to the drug
6-(p-hydroxyphenylazo)-uracil (HPUra) (18, 27), which inhibits DNA
polymerase III (2, 10, 20). This suggests that
DNA synthesis of these two'phages does not
require DNA polymerase III. Similar results
wereobtained withother B.subtilisphageslike
SPO2(27) and PBS2 (23). t
Weshow in thispaperthat host DNA
polym-erase III isrequired for (e replication
through-out thestageofphage replication.
We will discuss the apparent contradiction
between ourresults andotherstudies basedon
HPUra inhibition of DNA polymerase III (16,
18, 27), which indicate that this enzyme isnot
involved in (e DNAsynthesis.
MATERIALS AND METHODS
Materials andmethods, unless otherwise indicated, were aspreviously described (18).
Bacterial strains. The following strains of B. subtilis 168wereused: 2344(polA-, thy-)designated 2344pol III+ (2) and 2355 (polA-, polC", met-) designated 2355pol IIP (3) were describedby Bazill
and Gross. Strains U2355 (polCts, met-, ile-) desig-nated U2355polIIP and R2355 (polA-, met-, ile-) designated R2355pol III+ were derived from strain
2355.
Phage strains. Phage 0e wild-type strain and phage SPOl wild-type strainwereused.
Media. The standard growth medium used throughout the experimentswasC+ (22).
One-step growth experiments. These experi-ments were performed as described by Sonenshein andRoscoe (29).
RESULTS
Kinetics ofphage DNA synthesisinaDNA
polymerase III"' mutant strain. Preliminary
experimentshave shown that (edoes not direct
its DNA synthesis in either (e-infected U2355 pol IIP's (DNA polymerase
Illts)
cells or in (e-infected 2355polIIP- (DNA polymeraseIIlt", DNApolymeraseI-) cells at 48 C. On the otherhand, phageDNAsynthesisis normalat48 C in
(e-infected 2344polIII+ (DNA polymerase I-)
cells. We therefore performed our experiments
with the double mutant strain 2355pol IIt11
(DNA polymerase IIIts, DNA polymerase I-)
which is more suitable for studies of DNA
synthesis in vitro thanthesingle mutantstrain
U2355pol IIPs (DNA polymeraseIII").
1337
the
on November 10, 2019 by guest
http://jvi.asm.org/
LAVIET AL.
The results presented in Fig. 1 describe the synthesisof DNA inuninfected and
oe-infected
cells, in the presence and absence of HPUra.
2
E.b
L
4-
3-
2-
0-0 10 20 30 40 50 60
MINUTES AF TE R NFECTICN
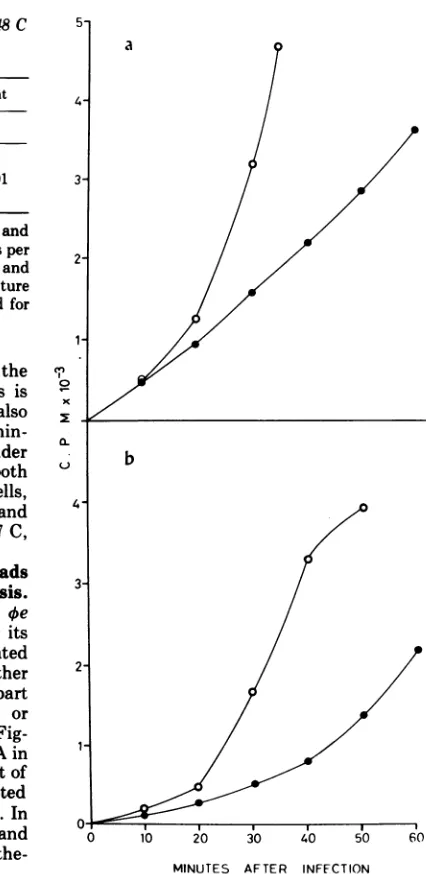
FIG. 1. DNA synthesis in oe-infected cells at 37 and 48C in presence and absence of HPUra. B. subtilis strains 2344pol III and 2355pol III'S were
grownat37Cto2 x1011cellsperml. Oneminute
be-fore infection,eachculturewasdividedintoeight
por-tions, four of which are transferred to 48C. HPUra
(finalconcentration 0.15mM) wasthenadded where
indicated. [2-14C]uracil (final concentration 5 ,ugl
ml; 0.1 ,uCilml) and 0ewhere indicated (MOI= 10)
wereaddedatzerotime.Sampleswereremovedanld
treatedwith alkali to hydrolyze labelled RNA. DNA
wasprecipitatedwith trichloroacetic acid and
radio-activitywasdetermined. (a)37C; (b)48C.Symbols:
b~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
These results show that in
oe-infected
2355polIII' cells, phage DNA is synthesized at 37C
but not at 48 C. On the other hand, in
4e-in-fected 2344pol III+ cells-from which 2355pol III8 was derived-phage DNA is synthesized both at 37 and 48C. Even at 37 C the rate of phage DNA synthesis in (e-infected 2355pol
IIPt cells is slower than in le-infected 2344pol
III+ cells. As expected, bacterial DNA
synthe-sis is inhibited by HPUra and in 2355pol IIIt
cells DNA synthesis isarrested at 48C.
In
oe-infected
cells, DNA synthesis isresist-ant toHPUrafromabout10min afterinfection,
at which time host DNA synthesis is arrested
(18, 25). We conclude that the DNA
synthe-sizedunder theseconditions,fromabout10min
after infection, is phage DNA. We therefore
omitted in the following comparable
experi-ments the HPUra treatment. In accordance
with these findings, Table 1 shows thatat 48C virtually no mature phage is produced in (e-infected 2355pol IIIt" cells. At thesame
temper-ature, theaverageburst size of(egrown inthe
parental strain 2344pol III+ is 25. At 37C the
averageburst size of( egrownin bothstrainsis
about 100.
Weisolated severalspontaneous DNA
polym-erase III+ revertants from strain 2355pol IIPI".
These revertants grow well at 48C. They have lost the mutagenic character of their
parent strain (3) and have an active DNA
polymerase III bothat 37 C andat 48 C. How-evertheserevertantsstillcarrythe DNA
polym-erase I- mutation. The growth of be in the
various revertant clones isolated is normalboth
at37and48 C. The datapresentedinFig.2and in Table 1 were obtained with the revertant R2355pol III+. They clearly show that in
pe-infected R2355
pol
III+ cells phage DNA syn-thesis andprogeny production are normalbothat 37and48 C.
Requirement of protein synthesis for phage replication in a temperature shift down experiment. Figure 3 described the kineticsof
DNAsynthesis in cells infected with(eat48 C
and transferred to 37 C 21 min after infection,
in presence or absence of chloramphenicol
(CAP), an inhibitor ofproteinsynthesis. These
results show that in (e-infected 2355pol IIP8
*, 2344pol III+, uninfected;0, 2344pol III+,
unin-fected +HPUra;A, 2344pol III+,infectedwith4e;*,
2344polIII+, infected with(e +HPUra; 0, 2355pol IP', uninfected; U, 2355pol III"', uninfected +
HPUra; 0, 2355pol III", infected with 0e; *, 2355polIII"', infected with Oe +HPUra. The vertical bars indicate thatall thepoints (<y; 0; *; 0; *) of theappropriatetreatmentsfall within the ends of the bar.
1338 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.499.67.248.129.537.2]DNAPOLYMERASEHI IS REQUIREDBYOE
TABLE 1. Averageburstsizeof(egrown at 37 or 48C instrains 2344polIII+, 2355 poliP8,and
R2355pol III+
Burst size ofjegrown at Hoststraina
37C 48C
2344pol III+ 100 25
2355 polIfu 95 <0.001
R2355potIII+ 110 32
aB.subtilis strains:2344 pol III+, 2355polIIPand
R2355pot III+ were grown at 37 C to 3 x 108 cells per ml. The cells were infected at an MOI of0.1 and shaken at 37 C for 10 min. The adsorption mixture was treated with anti-serum, diluted and plated for assayofphage (see Materials andMethods).
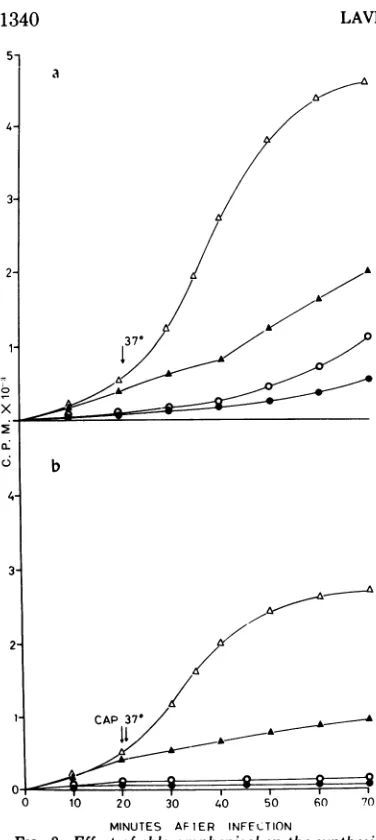
cells, phage DNA synthesis begins after the temperature shift only if protein synthesis is
allowed to continue. Protein synthesis is also required for bacterial DNA synthesis in unin-fected cells of strain 2355polIIP? grown under similar conditions. Onthe other hand, in both uninfected and (e-infected 2344pol III+ cells,
DNA synthesis is not blocked at 48 C, and continues after the temperature shift to 37 C, eveninpresence ofCAP.
Inactivation of DNA polymerase III leads toimmediatearrestof phage DNA synthesis.
The data presented above indicate that (e
requires an active DNA polymerase III for its DNAreplication. Wehave further investigated
this requirement in order to find out whether DNApolymeraseHI isrequired only during part of the stage of phage DNA synthesis, or throughout the stage of phage replication.
Fig-ure 4shows that thesynthesis of phage DNA in
(e-infected 2355pol IIP cells,as well asthat of
bacterial DNA in uninfected cells, is arrested
immediately upon transfer from 37 to 48 C. In the control experiment with uninfected and
phage-infected 2344ppo III+ cells, DNA synthe-siscontinues throughout the experiment.
DISCUSSION
B. subtilis has three distinct DNA
polymer-ases(2, 8-10,20). Severalobservations indicate that theproductofthepolCgene, DNA
polym-erase IH, participates in the replicationofthe bacterial chromosome. Thus-, mutants
produc-ing temperature-sensitive DNA polymerase III
cannotsynthesize their DNAatrestrictive
tem-peratures. Someofthese mutants werefoundto bemutagenic (3, 9). AmutagenicDNA
polym-erase was found also in another system (30).
Moreover, the drug HPUra which
specifically
blocks bacterial DNA synthesis in vivo (4) has been foundtoact by inhibitingDNA
polymer-ase II(10, 17). The roleof DNApolymeraseIIis
0
x
a-U.
MINUTES AFTER INFECTION
FIG. 2. DNA synthesis in (be-infected R2355 pol III+ cells at37 and 48 C. Strain R2355 pol III+ was grown at 37C to 2 x 10' cellsper ml. One minute
before infection, the culture was divided into four
portions, two of which were transferred to 48C.
[2-14Ckuracil(final concentration 5ug/ml;0.1;XCi/ml)
and(ewhere indicated(MOI= 10)wereaddedatzero
time. Samples were removed and radioactivity in DNAwasdeterminedasdescribed inFig.1.(a)37C; (b) 48 C. Symbols: *, R2355pol III+, uninfected; 0,
R2355polIII+,infectedwith(e.
not clear. DNA polymerase I participates in
repairDNA synthesis (15).
Rutberg et al. (27) have found that DNA synthesis of the temperate B. subtilis phage
VOL.14,1974 1339
on November 10, 2019 by guest
http://jvi.asm.org/
LAVI ET AL.
a
b
0 10 io 30 40 50 60 70
[image:4.499.66.254.52.472.2]MINUTES AFIER INFELTION
FIG. 3. Effect ofchloramphenicol onthesynthesis of bacterial andphageDNA ina
temperature-shift-down experiment. Strains 2344pol III+ and2355pol Il'" weregrownat37C to 2x 108cellsperml. One minutebefore infection,each culturewasdivided into
four portions, all ofwhich were transferred to48C. [2-'4C Juracil (final concentration5Ag/ml;0.1 ACi/mI)
and 4e where indicated (MOI = 10) were added at
zero time. Chloramphenicol (CAP, final
concentra-tion 100ug/ml)wasadded20minafter infection.One minute later, theshift to37Cwasperformed.
Sam-ples were removed and radioactivity in DNA was
determinedasdescribed inFig. 1.(a) Shiftto37Cin absenceofCAP; (b)shiftto37Cinpresenceof CAP. Symbols: A,2344pol III+ uninfected; A,2344pol III+
infected with 4e; *, 2355pol III'S uninfected; Q,
2355polIII'sinfectedwith ke.
0105 issensitive to HPUra in HPUra-sensitive
host strains, but is resistant to the drug in
HPUra-resistant mutant strains. Neville and
Brown (20) and Cozzarelli and Low (5), have found that HPUra-resistant B. subtilismutants produce a mutated DNA polymerase IIIwhich isinsensitivetothedrug. Therefore, the results obtained by Rutberg et al. indicate that host DNA polymerase III is required for 0105DNA synthesis. DNA synthesis in the coliphages
OX174
and M13 was also found todepend on host DNApolymerase III (7, 19).The synthesis of DNA of the B. subtilis phagesPBS2,SPO2,andSPOlaswellasthat of
oe
is resistant to inhibitionby HPUra. In cells infectedwith these phages, newDNApolymer-aseactivities weredetected (23, 27, and
unpub-lished data, respectively). However, no new DNA polymerase activity has been found in
extracts of ¢105-infected cells (27). Rutberg et
al. (27) suggested that all the B. subtilisphages
which arecapable ofdirecting the synthesis of their DNAinthepresenceofHPUra inducethe synthesis of a phage-specific DNApolymerase.
Indeed, a new DNA polymerase was purified
fromphageSPOl-infectedcells (33).Therole of thenewDNApolymerasewhichappears incells
infectedwith thephages SPO2, PBS2, SPOl,or
4e
isnotyet known. The coliphages T4, T5, and T7 were also found to induce the synthesis of their own DNA polymerases. Genetic studies have shownthat theseenzymes areessentialfor thesynthesisofphage DNA (6, 12,31).We were interested in understanding the mechanism whereby qe arrests hostDNA syn-thesis several minutesafterinfection (16, 18,24, 25). Thisphage inducesoninfection the
synthe-sis ofbothaninhibitor ofthe hostthymidylate
synthetase and a deoxythymidinetriphosphate
nucleotidohydrolase (dTTPase) (13, 24, 25).
Previous studies have shown that depletion of
the dTTP pool in
4e-infected
cells is not theonly cause for host-DNA arrest by this phage.
Upon infectionwith adTTPase mutantphage,
the arrest of host DNA synthesis takes place
even in thymine requiring cells provided with
exogenous thymine (18). Another
oe-directed
mechanism is known to be responsible for
arresting thesynthesisofhost DNA.Mutants in
which this mechanism does not operate (18),
and consequently do not shut off host DNA synthesis, aredesignated as bda- (forbacterial
DNA arrest-). We have shown earlier (16) that
in toluenized preparations of
ke-infected
cellshost DNA synthesis is blocked in a manner
which closely resembles DNA arrest in vivo. Similar (unpublished) results were obtained withlysatesof
oe-infected
cellsspread on cello-phane disks (28).It is conceivable that host DNA arrest is
effectedbyaninteractionbetween thebda-gene
J. VIROL. 1340
on November 10, 2019 by guest
http://jvi.asm.org/
DNA POLYMERASE III IS REQUIRED BYOE
O~~~~~~
.
G
0
~~~~
o
b 8
4/
3-
2-#04
0 10 20 30 40 50 60
[image:5.499.48.232.56.512.2]MINUTES AFTER INFECTION
FIG. 4. Kineticsof bacterial and phage DNA syn-thesis inatemperature-shift-up experiment. Strains 2344pol III+ and2355pol III'sweregrownat37Cto 2
x 108cells per ml. One minutebefore infection,each culturewas divided intotwoportions.At zerotime,
[2-14CJuracil(finalconcentration 5ug/ml;0.1,uCi/ml) and be where indicated (MOI = 10) were added. Portionsweretransferred from37to48Catdifferent times after infection. Samples were removed and
radioactivity in DNAwasdeterminedasdescribed in
Fig. 1. (a) Uninfected cells, symbols: 0,2344pol III+
incubatedat37C; +,2344polIII+ transferredto48C at 30 min; <, 2355pol IIPs incubated at 37C; Q, 2355pol III's transferredto 48 Cat0min; x, 2355pol
lIII transferred to 48C at30 min. (b) e-Infected
cells, symbols: A,
2344pol
III+incubated at 37C;
*,2344pol
LIIk
transferredto 48Cat 25min;*,
2355polIII"
incubated at 37C;
A, 2355pol III" transferred
toproduct and the host DNA polymeraseIII. We investigatedthispossibility by measuring the growth of 4e in a thermosensitive DNA
polymerase III host. It is evident that no
oe
DNA is synthesized in cells of strain 2355pol
III" at 48 C. Accordingly, no progeny phage is produced at this temperature. Under similar conditions, both DNA synthesis and phage production were normal in
oe-infected
cells ofstrain2344polIII+ (fromwhich 2355polIIr has
been derived) and in 4e-infected cells of the revertant strain R2355pol III+. These results indicate that the failure of ke-infectedcells of
strain 2355pol IIPO to support the synthesis of
phage DNA is due exclusively to the inactiva-tion of the thermolabile DNA polymerase III. We have obtained similar results with phage SPOl (datanotshown).
Inhibition ofbacterial DNA synthesis per se
cannot account for the arrest of phage-DNA
synthesis. This isborneoutby the finding that phage DNA synthesis is normal in
thymine-requiringhostcellsthat are starved for thymine
(unpublished data), as well as in HPUra-treated cells (18). The temperature-shift-down experiment reported here indicates that, in
4e-infected
2355pol
IIPcells,
thethermolabile DNA polymerase III is irreversibly inactivated at48C and hastobe synthesized denovobefore phage DNA synthesis can begin. This experi-mentalso showsthat theinabilityofoe
todirect the synthesis ofits own DNA in 2355pol IIP cellsat48 C is notduetothehightemperatureperse,buttotheinactivation of DNA
polymer-ase III. The immediate arrest of phage-DNA
synthesis in
oe-infected
2355polIIP' cellsupontransfer from 37 to 48C indicates that DNA
polymerase III is required for phage-DNA syn-thesis throughout thestage ofphage replication. The requirement for DNA polymeraseIII by phage
oe
isunexpectedin view oftheresistance ofphage-DNA synthesis toHPUra. Theexpla-nation ofthese conflicting datamaylie with the
phage-induced bacterial-DNA-arrest protein which is responsible for host-DNA arrest. We
proposethat thisproteinmodified DNA
polym-erase III in such a way that the enzyme stops
replicating bacterial DNA and starts to repli-catephage DNA. Thesamemodification should also account for the loss of sensitivity to HPUra
by the enzyme. The connection between DNA
polymerase III and the new DNA polymerase whichappears inphage-infectedcells(33)is not clear.
48C at 10min;U,2355pol IIPstransferredto 48 C at
25 min; V, 2355pol IIPs transferred to 48 C at 35
min;*,
2355polIII's transferred to 48 C at 45min.VOL.14,1974 1341
on November 10, 2019 by guest
http://jvi.asm.org/
1342 LAVIET AL.
We are now studying in vitro the interaction
between a purified bacterial-DNA-arrest
pro-tein and DNA polymerase III.
ACKNOWLEDGMENTS
We wish tothankJ. Grossforthe giftofB.subtilisstrains 2344and 2355; R. Losick for thegiftofphageSPO1;and B. W. Langley of I.C. I.forthe giftofthedrug HPUra.
This work wassupported by grantstoM.Marcusfromthe Authority for research and development, Hebrew University (34876) and from the Leslie Lavi research fund(057330).
LITERATURE CITED
1. Aposhian, H.V. 1965. A dTMPase found after infection with phageSP5C. Biochem. Biophys. Res. Commun. 18:230-235.
2. Bazill, G. W., and J. D. Gross. 1972. Effert of 6-(p-Hydroxyphenyl)-azo uracilonB. subtilis DNA polym-erases. NatureN.Biol.240:82-83.
3. Bazill, G. W., and J. D. Gross. 1973. Mutagenic DNA polymeraseinB.subtilis. NatureN.Biol. 243:240-243. 4. Brown, N. C. 1971.Inhibitionofbacterial DNA replica-tionby6-(p-Hydroxyphenylazo)-uracil: differential ef-fect onrepair andsemi-conservativesynthesisin Bacil-lussubtilis. J. Mol. Biol. 59:1-16.
5. Cozzarelli, N. R., and R. L. Low. 1973. Mutational alterationofBacillus subtilisDNA polymerase IIIto hydroxyphenylazo-pyrimidine resistance: polymerase IIIisnecessaryfor DNAreplication. Biochem. Biophys. Res. Commun. 51:151-157.
6. DeWaard, A., A. V. Paul, and I. R. Lehman. 1965. The structural genefordeoxyribonucleic acid polymerasein
bacteriophagesT4 and T5.Proc.Nat. Acad.Sci. U. S. A. 54:1241-1248.
7. Dumas, L. B., and C. A. Miller. 1973. Replication of bacteriophage kX174DNAinatemperature sensitive dnaE mutant of Escherichia coli C. J. Virol. 11:848-855.
8. Ganesan, A. T., C. 0. Yehle, and C. C. Yu. 1973. DNA replicationinapolymerase I deficient mutant and the identification of DNA polymerase II and III in Bacillus subtilis. Biochem. Biophys. Res. Commun. 50:155-163. 9. Gass, K. B., and N.R. Cozzarelli. 1973. Further genetic and enzymological characterizationofthe three Bacil-lus subtilis deoxyribonucleic acid polymerases. J. Biol. Chem.248:7688-7700.
10. Gass, K. B., R. L. Low, and N. R. Cozzarelli. 1973. InhibitionofaDNApolymerasefromBacillussubtilis by hydroxyphenylazopyrimidines. Proc. Nat. Acad. Sci.U.S. A.70:103-107.
11. Green, D. M. 1964. Infectivity of DNA isolated from Bacillus subtilis bacteriophage, SP82. J. Mol. Biol.
10:438-451.
12. Grippo, P.,andC. C. Richardson.1971.Deoxyribonucleic acidpolymerase ofbacteriophage T7. J. Biol. Chem. 246:6867-6873.
13. Haslam, E. A., D. H. Roscoe, and R. G. Tucker. 1967. Inhibition ofthymidylate synthetase inbacteriophage infected Bacillus subtilis. Biochim. Biophys. Acta
134:312-326.
14. Kallen, R. G., M. Simon, and J. Marmur. 1962. The occurence of a newpyrimidine basereplacing thymine
J. VIROL.
in abacteriophage DNA: 5-Hydroxymethyluracil. J. Mol.Biol.5:248-250.
15. Laipis, P. J.,and A. T. Ganesan. 1972. Adeoxyribonucleic acid polymeraseI-deficient mutant of Bacillus subtilis. J.Biol. Chem. 247:5867-5871.
16. Lavi, U., and M. Marcus. 1972. Arrest of host DNA synthesis in Bacillus subtilis infected withphage 4e. Virology 49:668-674.
17. Mackenzie, J. M., M. M. Neville, G. E.Wright, and N. C. Brown. 1973. Hydroxyphenylazopyrimidines: char-acterization of the active forms and theirinhibitory action on a DNA polymerase from Bacillus subtilis. Proc. Nat.Acad. Sci. U. S. A. 70:512-516.
18. Marcus, M., and M. C. Newlon. 1971. Controlof DNA synthesis in Bacillus subtilis by phage ke. Virology 44:83-93.
19. Mitra,S., and D. R. Stallions. 1973.Role of dna genes of Escherichia coli in M13 phage replication. Virology 52:417-424.
20. Neville, M. M., and N. C. Brown. 1972. Inhibition of a discrete bacterial DNApolymerase by 6-(p-Hydroxy-phenylazo)-uracil and 6-(p-Hydroxyphenylazo)isocyto-sine.Nature N. Biol. 240:80-82.
21. Okuho, S., B. Strauss, and M. Stadolsky. 1964. The possible role of recombination in the infection of competent Bacillus subtilisby bacteriophage
deoxyri-bonucleic acid. Virology 24:552-562.
22. O'Sullivan, A., and N. Sueoka. 1967.Sequential replica-tion ofthe Bacillus subtilischromosome. IV. Genetic mappingby density transfer experiment. J. Mol. Biol. 27:349-368.
23. Price, A., and S. J. Cook. 1972. New deoxyribonucleic acidpolymerase inducedby Bacillus subtilis bacterio-phage PBS2. J. Virol. 9:602-610.
24. Roscoe, D. H. 1969. Thymidine triphosphate nu-cleotidohydrolase. A phage induced enzyme in B. subtilis.Virology38:520-523.
25. Roscoe, D.H.1969.SynthesisofDNA inphage-infected Bacillus subtilis. Virology 38:527-537.
26. Roscoe, D.H., and R. G. Tucker.1966.Thebiosynthesis
of 5-Hydroxymethyldeoxyuridylic acid in bacterio-phage-infectedBacillus subtilis. Virology 29:157-166. 27. Rutberg, L., R. W. Armentrout, and J. Jonasson. 1972.
UnrelatednessoftemperateBacillus subtilis bacterio-phageSP02 and0105,J.Virol. 9:732-737.
28. Schaller, H., B. Otto, V. Nuslein,J. Huf, R. Herrman,
and F. Bonhoeffer. 1972.Deoxyribonucleic acid replica-tionin vitro. J. Mol. Biol. 63:183-200.
29. Sonenshein,A.L., and D. H. Roscoe. 1969. Thecourseof phage 1e infection in sporulating cells of Bacillus subtilisstrain3610.Virology 39:265-276.
30. Speyer, J. F. 1965. Mutagenic-DNA polymerase. Bio-chem.Biophys. Res. Commun. 21:6-8.
31. Warner, H.R.,and J. E.Barnes. 1966. Deoxyribonucleic acid synthesis inEscherichia coli infected with some deoxyribonucleicacidpolymerase-less mutants of bac-teriophage T4. Virology 28:100-107.
32. Yehle, C. O.,andA. T.Ganesan. 1972.Deoxyribonucleic acidsynthesisinbacteriophage SPOl-infected Bacillus subtilis.J. Virol. 9:263-272.
33. Yehle, C. O., and A. T. Ganesan. 1973. Deoxyribonucleic acidsynthesisinbacteriophage SPOl-infected Bacillus subtilis. J. Biol. Chem.248:7456-7463.
on November 10, 2019 by guest
http://jvi.asm.org/