T Cells in Lymph Nodes of Rhesus Macaques after Experimental
Reactivation
Vicki Traina-Dorge,aLara A. Doyle-Meyers,bRobert Sanford,cJennifer Manfredo,dAnna Blackmon,dMary Wellish,dStephanie James,d Xavier Alvarez,eCecily Midkiff,eBrent E. Palmer,fEileen Deharo,aDon Gilden,d,gRavi Mahalingamd
Divisions of Microbiology,aVeterinary Medicine,band Comparative Pathology,eTulane University, Tulane National Primate Research Center, Covington, Louisiana, USA; Tulane Cancer Center, Tulane School of Medicine, New Orleans, Louisiana, USAc; Departments of Neurologydand Immunology & Microbiologygand Division of Allergy and Clinical Immunology, Department of Medicine,fUniversity of Colorado School of Medicine, Aurora, Colorado, USA
ABSTRACT
Like varicella-zoster virus (VZV), simian varicella virus (SVV) reactivates to produce zoster. In the present study, 5 rhesus
ma-caques were inoculated intrabronchially with SVV, and 5 months later, 4 monkeys were immunosuppressed; 1 monkey was not
immunosuppressed but was subjected to the stress of transportation. In 4 monkeys, a zoster rash developed 7 to 12 weeks after
immunosuppression, and a rash also developed in the monkey that was not immunosuppressed. Analysis at 24 to 48 h after
zos-ter revealed SVV antigen in the lung alveolar wall, in ganglionic neurons and nonneuronal cells, and in skin and in lymph nodes.
In skin, SVV was found primarily in sweat glands. In lymph nodes, the SVV antigen colocalized mostly with macrophages,
den-dritic cells, and, to a lesser extent, T cells. The presence of SVV in lymph nodes, as verified by quantitative PCR detection of SVV
DNA, might reflect the sequestration of virus by macrophages and dendritic cells in lymph nodes or the presentation of viral
antigens to T cells to initiate an immune response against SVV, or both.
IMPORTANCE
VZV causes varicella (chickenpox), becomes latent in ganglia, and reactivates to produce zoster and multiple other serious
neu-rological disorders. SVV in nonhuman primates has proved to be a useful model in which the pathogenesis of the virus parallels
the pathogenesis of VZV in humans. Here, we show that SVV antigens are present in sweat glands in skin and in macrophages
and dendritic cells in lymph nodes after SVV reactivation in monkeys, raising the possibility that macrophages and dendritic
cells in lymph nodes serve as antigen-presenting cells to activate T cell responses against SVV after reactivation.
P
rimary varicella-zoster virus (VZV) infection produces
chick-enpox (varicella), after which the virus becomes latent in
gan-glionic neurons along the entire neuraxis. As VZV-specific T cell
immunity declines with advancing age, VZV reactivates to
pro-duce zoster, which may be complicated by multiple neurological
and ocular disorders. Simian varicella virus (SVV) infection of
nonhuman primates has served as a good model with which to
study the pathogenesis of VZV because of the pathological,
immu-nological, and virological similarities of SVV to VZV (
1
,
2
). VZV
reactivation is increased in patients receiving chemotherapy or
after X-irradiation (
3
); similarly, SVV reactivates in latently
in-fected African green monkeys (AGM) and cynomolgus monkeys
(CM) after immunosuppression and environmental stress (
4
). In
rhesus macaques, intrabronchial inoculation with SVV produces
varicella, followed by the establishment of latency (
5
) and virus
reactivation after X-irradiation (
6
,
7
). At the time of SVV
reacti-vation in CM, expression of CXCL10 (a chemokine which recruits
activated T cells and NK cells) correlates with transient T cell
infiltration in ganglia (
8
). In the study described here, we
deter-mined if a combination of irradiation and treatment with
predni-sone and tacrolimus induces reactivation of SVV in latently
in-fected rhesus macaques to study the distribution of reactivated
SVV in ganglionic and nonganglionic tissues.
MATERIALS AND METHODS
Monkeys.Seven SVV-seronegative rhesus macaques were housed in the Tulane National Primate Research Center in Covington, LA, and used in
all experiments. The ages, sexes, and weights of the monkeys used in these experiments are presented inTable 1.
Establishment of latent SVV infection. SVV (a deltaherpesvirus strain) isolated from a naturally infected monkey (Erythrocebus patas) was propagated in Vero (African green monkey kidney) cells, and a virus stock was prepared as described previously (9). SVV-seronegative rhesus ma-caques (monkeys HB62, HI83, HF39, HC44, HA95, II49, and IK10) were inoculated intrabronchially with 104PFU of wild-type SVV (monkeys HB62, HI83, HA95, II49, and IK10) or SVV expressing enhanced green fluorescent protein (SVV-EGFP) (monkeys HF39 and HC44) as described previously (4,10). Earlier, we demonstrated that SVV-EGFP is pathogenic in African green monkeys and that SVV-EGFP is mildly attenuated com-pared to wild-type SVV (11,12). All monkeys were monitored by physical exams every 2 or 3 days, and blood samples were collected either weekly or biweekly. Monkeys HB62, HI83, HC44, HA95, II49, and IK10 developed
Received26 May 2015Accepted10 July 2015
Accepted manuscript posted online15 July 2015
CitationTraina-Dorge V, Doyle-Meyers LA, Sanford R, Manfredo J, Blackmon A, Wellish M, James S, Alvarez X, Midkiff C, Palmer BE, Deharo E, Gilden D, Mahalingam R. 2015. Simian varicella virus is present in macrophages, dendritic cells, and T cells in lymph nodes of rhesus macaques after experimental reactivation. J Virol 89:9817–9824.doi:10.1128/JVI.01324-15.
Editor:R. M. Sandri-Goldin
Address correspondence to Ravi Mahalingam, ravi.mahalingam@ucdenver.edu.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
doi:10.1128/JVI.01324-15
on November 7, 2019 by guest
http://jvi.asm.org/
a mild varicella rash at 10 to 14 days postinoculation (dpi), while monkey HF39 did not develop a rash but seroconverted. Four months later, mon-keys II49 and IK10 were euthanized and lymph nodes and ganglia were collected. All procedures were performed following appropriate guide-lines and protocols approved by the Institutional Animal Care and Use Committee of the Tulane National Primate Research Center.
Immunosuppressive regimens.All immunosuppressive treatments were performed as described previously (4). Five months after primary infection, monkeys HB62, HI83, HF39, and HC44 were transported by van (2-h round trip) from the Tulane National Primate Research Center in Covington, LA, to the Tulane University Cancer Center in New Or-leans, LA, anesthetized, and exposed to a single dose of 200-cGy total body X-irradiation and then started on daily oral treatment with 500g (80
g/kg of body weight/day) of tacrolimus (Prograf) and 5 mg (2 mg/kg/ day) of prednisone until they were euthanized. Monkey HA95 was not irradiated or treated with drugs but was transported with the experimen-tal animals as described above (Fig. 1). All animals were monitored again by physical exams every 2 or 3 days, and blood samples were collected weekly until SVV reactivation.
Harvesting and processing of tissues.At 7 to 12 weeks, examination of the monkeys revealed a rash in all irradiated monkeys, including mon-key HA95, which was transported to the irradiation facility. They were all euthanized within 24 to 48 h after the appearance of zoster, and lymph nodes, skin, and ganglia were harvested and fixed as described below for immunological, virological, and pathological analyses. Areas of skin con-taining the zoster rash were punch biopsied while the monkeys were un-der anesthesia, fixed in 4% paraformaldehyde, and embedded in paraffin. Samples of lung and ganglionic tissue from each dermatome were har-vested from each monkey, fixed in 4% paraformaldehyde, and embedded in paraffin. A portion of the lymph node tissue samples was snap-frozen for DNA extraction, and the other portion was fixed in 4% paraformal-dehyde and embedded in paraffin.
Determination of anti-SVV antibody levels.AnSVV antibody ti-ters in serum obtained from all monkeys before and after varicella and at necropsy were determined using a plaque reduction assay as described previously (13). Briefly, dilutions of heat-inactivated sera from monkeys were mixed with an equal volume of Dulbecco modified Eagle medium containing 100 to 200 PFU of SVV. Serum-virus mixtures were incubated for 1 h at room temperature and plated onto a monolayer of CV-1 cells in duplicate petri dishes. SVV-infected cells were incubated at 37°C in CO2 for 4 to 5 days, and SVV plaques were fixed in methanol, stained with cresol violet, and counted. The titer of the antibody was determined on the basis of the dilution of serum that inhibited 80% or more of the plaques. DNA extraction.DNA was extracted from lymph node tissue samples as described previously (4,14,15).
Real-time qPCR.DNA extracted from triplicate lymph node tissue samples was analyzed by real-time quantitative PCR (qPCR) using SVV open reading frame 61 (ORF61)-specific primers as described previously (5).
Rabbit polyclonal antibodies against SVV IE63.Two synthetic pep-tides specific for the SVV IE63 protein were selected on the basis of their
antigenic potential, based on analysis of hydrophobicity and accessibility parameters, and synthesized by Alpha Diagnostic International (San An-tonio, TX). The two peptides, NH2-CIARASGRVTRDSRTLRR-COOH and NH2-CEHMAAKILTELRESVHNT-COOH, included amino acids 69 to 86 and 244 to 261, respectively. Rabbits were immunized three times with 5 mg of each peptide conjugated to a carrier protein. Antibody titers were determined by enzyme-linked immunosorbent assay.
Immunohistochemistry.Sections (5m) of skin, lung, ganglionic, or lymph node tissue were analyzed for the presence of SVV glycoprotein H (gH) and gL, the IE63 protein, or SVV nucleocapsids by immunohisto-chemistry as described previously (13). Normal rabbit serum (1:2,000 to 15,000 dilution) or a mixture of polyclonal antipeptide antibodies against SVV gH and gL (1:2,000 dilution) or polyclonal antipeptide antibodies against the SVV IE63 protein (1:15,000) raised in rabbits were used for analysis (28). Each experiment was repeated at least 3 times.
Immunofluorescence analysis.Fixed sections (5m) of lymph node tissues were deparaffinized in xylene for 5 min, followed by 3 rinses (5 min each) in 100% ethanol. Unless stated otherwise, all incubations were car-ried out at room temperature. Sections were dehydrated in graded ethanol and washed in phosphate-buffered saline (PBS) for 5 min. Antigen re-trieval was performed by incubating the sections in 10 mM sodium citrate, pH 6.0, in a vegetable steamer (vapor phase) for 10 min and cooling at room temperature for 5 min. After washing once for 5 min in PBS con-taining 0.05% Tween 20 followed by blocking in 10% normal horse or goat serum for 30 min, the sections were rinsed once, washed in PBS for 5 min, and incubated at 4°C for 16 h with primary antibody (normal rabbit serum [1:2,000 to 1:5,000 dilution] with or without mouse IgG1 [catalog no. X0931; Dako] or IgG2a [catalog no. 554527; BD Pharmingen], SVV IE63-specific rabbit polyclonal antiserum [1:15,000 dilution], or a mix-ture of rabbit polyclonal antibody specific for SVV gH and gL [1:2,000 dilution] with or without mouse anti-human CD163 [catalog no. MCA1853; AbD Serotec]). Sections were washed 3 times for 5 min each
[image:2.585.40.287.87.185.2]FIG 1Primary SVV infection and experimental reactivation in rhesus ma-caques. Seven Indian rhesus macaques (monkeys HB62, HI83, HF39, HC44, HA95, II49, and IK10) were inoculated intrabronchially with 104PFU of SVV or SVV-EGFP. With one exception, all monkeys developed varicella rash, and all became viremic at 10 to 14 days postinoculation. Two months later, two monkeys (monkeys II49 and IK10) were euthanized for analysis of latently infected tissues. Five months later, four monkeys (monkeys HB62, HI83, HF39, and HC44) were exposed to X-irradiation and treated orally with ta-crolimus and prednisone daily for the duration of the experiment. All four immunosuppressed monkeys developed a zoster rash at 7 to 12 weeks postim-munosuppression. The nonimmunosuppressed monkey (monkey HA95) de-veloped a zoster rash 6.5 months after virus infection. All five monkeys were euthanized 24 to 48 h after the appearance of the rash, and multiple tissue samples were analyzed for SVV antigens. m, months; w, weeks.
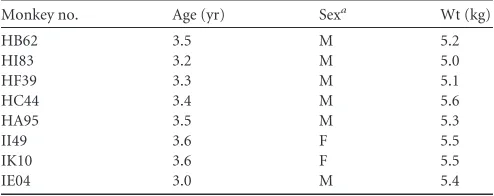
TABLE 1Age, sex, and weight of the Indian rhesus macaques used in this study
Monkey no. Age (yr) Sexa Wt (kg)
HB62 3.5 M 5.2
HI83 3.2 M 5.0
HF39 3.3 M 5.1
HC44 3.4 M 5.6
HA95 3.5 M 5.3
II49 3.6 F 5.5
IK10 3.6 F 5.5
IE04 3.0 M 5.4
a
M, male; F, female.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:2.585.299.542.407.593.2]time with PBS and further incubated for 2 h with secondary antibody (1:1,000 dilution of donkey Alexa Fluor 488-tagged anti-rabbit IgG [cat-alog no. A21206; Invitrogen] with or without a 1:1,000 dilution of goat Alexa Fluor 594-tagged anti-mouse IgG [catalog no. A11005; Invitro-gen]). After washing 3 times for 3 min each time with PBS, the sections were fixed in 1% paraformaldehyde in PBS for 1 min, briefly washed twice with PBS, incubated with DAPI (4=,6-diamidino-2-phenylindole; 1:4,000 dilution of 1 mg/ml) for 2 min, washed 3 times with PBS, and mounted on glass coverslips using Prolong Gold antifade reagent (catalog no. P36930; Invitrogen). Sections were visualized using either a Nikon E800 fluores-cence microscope or an Olympus FV1000 confocal microscope at the Advanced Light Microscopy Core Facility at the University of Colorado Anschutz Medical Campus.
RESULTS
Primary SVV infection, establishment of latency, and
experi-mental reactivation in monkeys.
Seven Indian rhesus macaques
inoculated with either wild-type SVV or SVV-EGFP developed
viremia and varicella (with one exception) at 4 to 14 days
postin-oculation (dpi) (
Fig. 1
and
Table 2
) and seroconverted at 28 dpi
(
Table 3
). SVV-specific antibody titers remained high (1:320) in
one monkey (monkey HI83) but were reduced (1:160) after dpi
142 in two monkeys (monkeys HB62 and HF39). In two monkeys,
SVV antibody titers were reduced to 1:40 by dpi 142. SVV viremia
peaked in all seven monkeys 4 to 7 dpi. Blood obtained from
monkeys at 4 to 7 dpi had lower numbers of copies of SVV DNA
than SVV-infected cells in culture, which required dilutions of up
to 10
12to detect 1,000 copies of DNA by qPCR (data not shown).
Five months later, after the establishment of latency, four
mon-keys (monmon-keys HB62, HI83, HF39, and HC44) were
immunosup-pressed; monkey HA95 was not immunosuppressed but had been
transported with the others. Before immunosuppression, white
blood cell (WBC) counts in all five monkeys were normal (7
⫻
10
3to 10
⫻
10
3cells per microliter). After immunosuppression, a
gradual decline in WBC counts was observed and was reduced to
1
⫻
10
3to 3
⫻
10
3cells per microliter by day 176, confirming
effective immunosuppression in all four treated monkeys. The
untreated control monkey (monkey HA95) had 7.3
⫻
10
3cells per
microliter by day 176. At 7 to 12 weeks after immunosuppression,
zoster lesions were observed in the left caudal abdomen in monkey
HB62; the thorax, abdomen, and scrotum in monkey HI83; the
thorax and the cranial and caudal abdomen in monkey HF39; the
right arm and right and left arm in monkey HC44; and in the axilla
(
Fig. 2
) and face in monkey HA95. Based on the findings of earlier
[image:3.585.41.545.79.181.2]studies, in which both immunosuppression and the stress of
transportation and isolation produced SVV reactivation (
4
,
13
),
we believe that the stress of transportation and isolation resulted
in SVV reactivation in monkey HA95. Neither viremia nor SVV
antibody status changed dramatically in any monkey at the time of
zoster (
Tables 1
and
3
).
TABLE 2Viremia after SVV inoculation and immunosuppression in rhesus macaques
Monkey no.
SVV DNA copy no. on:
dpia⫺18 dpi 2 dpi 4 dpi 7 dpi 9 dpi 11 dpi 14 dpi 28 dpi 42 dpi 56 dpi 151b Day of zosterd
HB62 0 0 6 145 43 11 13 2 1 3 0 0
HI83 0 0 22 264 59 28 11 3 1 3 2 0
HF39 0 0 0 37 21 5 17 6 2 2 0 0
HC44 0 0 2 11 9 0 0 0 0 0 0 0
HA95c 0 0 8 69 6 6 4 2 0 2 0 0
II49 0 NDe 11 9 10 ND 4 ND ND ND ND NAf
IK10 0 ND 5 2 ND 2 1 ND ND ND ND NA
adpi, day postinoculation. b
One day after immunosuppression.
cThe monkey was not immunosuppressed. d
Zoster rash was observed on dpi 197 (monkey HB62), 197 (monkey HI83), 217 (monkey HF39), 238 (monkey HC44), and 190 (monkey HA95).
eND, not done. f
NA, not applicable.
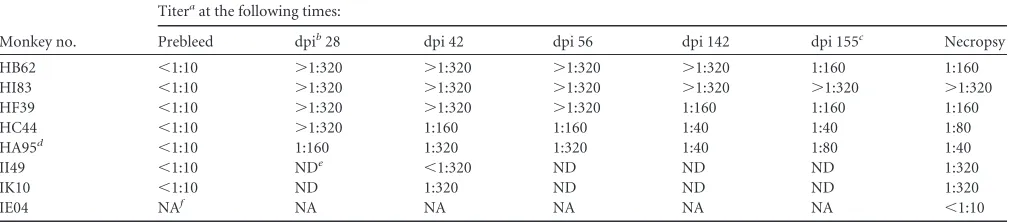
TABLE 3Antibody response to SVV infection and immunosuppression in rhesus macaques
Monkey no.
Titeraat the following times:
Prebleed dpib28 dpi 42 dpi 56 dpi 142 dpi 155c Necropsy
HB62 ⬍1:10 ⬎1:320 ⬎1:320 ⬎1:320 ⬎1:320 1:160 1:160
HI83 ⬍1:10 ⬎1:320 ⬎1:320 ⬎1:320 ⬎1:320 ⬎1:320 ⬎1:320
HF39 ⬍1:10 ⬎1:320 ⬎1:320 ⬎1:320 1:160 1:160 1:160
HC44 ⬍1:10 ⬎1:320 1:160 1:160 1:40 1:40 1:80
HA95d ⬍1:10 1:160 1:320 1:320 1:40 1:80 1:40
II49 ⬍1:10 NDe ⬍1:320 ND ND ND 1:320
IK10 ⬍1:10 ND 1:320 ND ND ND 1:320
IE04 NAf NA NA NA NA NA ⬍1:10
a
Anti-SVV antibody titers are expressed as the serum dilution that neutralized at least 80% of SVV plaques compared to the amount neutralized in control cultures.
bdpi, day postinoculation. c
Five days after immunosuppression.
dThe monkey was not immunosuppressed. e
ND, not done.
fNA, not applicable.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:3.585.41.546.559.670.2]Detection of SVV antigens in multiple tissues in monkeys
after SVV reactivation.
The SVV IE63 protein was detected in
the skin epithelium (not shown) and sweat glands of monkey
HB62 (
Fig. 3A
) and in all other monkeys (data not shown).
SVV gH and gL were detected in lung alveoli of monkey HB62
(
Fig. 3C
). SVV antigens were seen in both the nucleus and the
cytoplasm of neurons and, less frequently, in nonneuronal cells
in lumbar ganglia from monkey HC44 (
Fig. 3E
). SVV antigens
were also detected in the ganglia of all other monkeys (data not
shown). SVV antigens were detected in the medulla of the
in-guinal lymph nodes of monkey HC44 (
Fig. 3G
). In all five
monkeys, the SVV IE63 protein was found in multiple lymph
nodes, some of which were associated with areas of rash. While
SVV antigens were found in all tissues, the amount detected in
different monkeys was variable.
Detection of SVV DNA in lymph nodes from monkeys after
SVV reactivation.
On the basis of the findings of three
indepen-dent PCRs, an average of 30 copies of SVV DNA was detected in
100 ng of DNA extracted from snap-frozen axillary lymph node
from monkey HA95. No SVV-specific sequences were found in
axillary lymph node DNA from SVV-seronegative monkey
IE04.
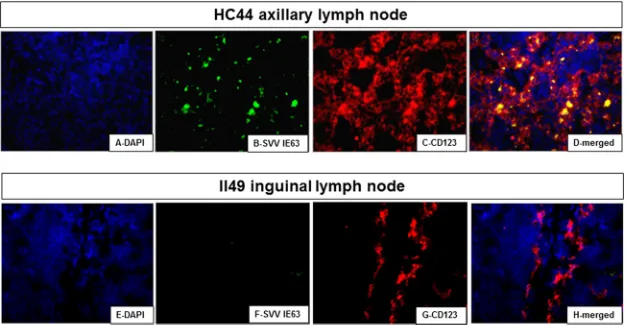
Detection of SVV IE63 antigen in macrophages and
den-dritic cells in lymph nodes from monkeys after SVV
reactiva-tion.
The SVV IE63 protein was seen in the medulla of the
ingui-nal lymph node from monkey HC44 after reactivation (
Fig. 4B
)
but not in the inguinal lymph node from latently infected monkey
II49 (
Fig. 4F
). Macrophages were found in the medulla of lymph
nodes from both monkeys (
Fig. 4C
and
G
). The SVV IE63 protein
colocalized with macrophages after reactivation (
Fig. 4D
) and was
not seen in the lymph nodes from latently infected monkey II49
(
Fig. 4H
). The SVV IE63 protein was seen in the germinal center of
the axillary lymph node from monkey HC44 after reactivation
(
Fig. 5B
) and in dendritic cells in the germinal center (
Fig. 5C
).
The SVV IE63 protein colocalized with dendritic cells in the
axil-lary lymph node from monkey HC44 (
Fig. 5D
). Dendritic cells in
the germinal center of the lymph node from latently infected
monkey II49 (
Fig. 5G
) did not contain SVV IE63 (
Fig. 5F
).
Table 4
lists the results of analysis of multiple lymph nodes
from all five monkeys after SVV reactivation, three lymph nodes
from two monkeys latently infected with SVV (monkeys II49 and
IK10), and three lymph nodes from one SVV-seronegative
mon-key (monmon-key IE04) using antibodies specific for the SVV IE63
protein and SVV gH and gL. SVV IE63 was detected in 12 of 18
(66%) lymph nodes, and SVV gH and gL were detected in all 17
(100%) lymph nodes from five monkeys after reactivation. SVV
IE63, gH, and gL were not found in any lymph nodes from two
latently infected monkeys or in lymph nodes from one
SVV-sero-negative monkey. Either the SVV IE63 protein or SVV gH and gL
were detected in macrophages in 14 of 18 (77%) lymph nodes and
in dendritic cells in 12 of 18 (67%) lymph nodes from five
mon-keys after reactivation. SVV antigens were absent in macrophages
and dendritic cells in lymph nodes from two latently infected
monkeys and one seronegative monkey.
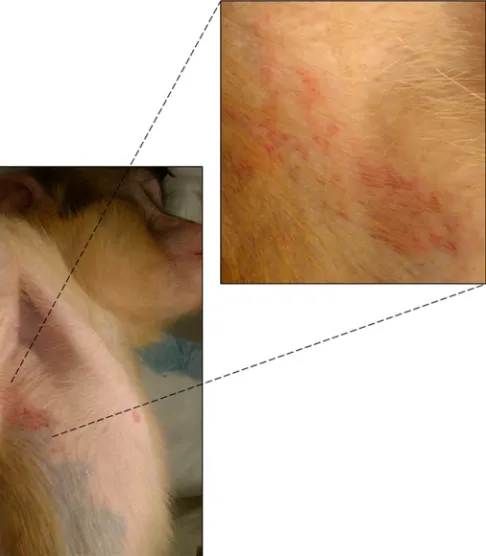
FIG 2Zoster rash in the axilla of monkey HA95 at 6 months after primary infection.
FIG 3Detection of SVV antigens in tissues from rhesus macaques after SVV reactivation. Paraformaldehyde-fixed, paraffin-embedded tissue sections were analyzed by immunohistochemistry. In monkey HB62, the SVV IE63 protein was detected in the sweat glands of skin at the site of zoster after immunostain-ing with rabbit polyclonal antibody directed against SVV IE63 peptides (A, arrow) and in lung alveoli immunostained with rabbit polyclonal antibody directed against SVV gH and gL (C, arrows). In monkey HC44, SVV antigen was seen in neurons and nonneuronal cells of lumbar ganglia (E, arrows) and in the medulla of inguinal lymph nodes (G, arrows) immunostained with rabbit polyclonal antibodies directed against SVV nucleocapsids. No staining was seen in the respective adjacent sections when preimmune rabbit serum was substituted for anti-SVV antibody (B, D, F, and H). Magnifications,⫻600 (A and B) and⫻200 (C to H).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:4.585.333.512.66.318.2] [image:4.585.42.285.68.346.2]Subcellular localization of SVV antigens in macrophages
and dendritic cells in lymph nodes from monkeys after SVV
reactivation.
Confocal microscopic analysis of lymph node
tis-sues after reactivation revealed the SVV IE63 protein in the
cyto-plasm of macrophages (
Fig. 6D
) and dendritic cells (
Fig. 7D
).
Detection of SVV IE63 protein in CD3
ⴙT cells in lymph
nodes from monkeys after SVV reactivation.
The SVV IE63
pro-tein was also seen in CD3
⫹T cells located in the lymphatic nodule
of the inguinal lymph node from monkey HC44 (
Fig. 8D
). Fewer
CD3
⫹T cells than macrophages and dendritic cells contained the
SVV ORF63 antigen. SVV antigens did not colocalize with B cells
in the inguinal lymph node from monkey HC44 (data not shown).
Overall, after experimental reactivation, SVV antigens were found
in the skin, lungs, and ganglia and in the cytoplasm of
macro-phages, dendritic cells, and T cells.
DISCUSSION
The exact mechanism by which varicella reactivates from ganglia
and is transported to skin is unknown. SVV infection in
nonhu-man primates provides a model system with which to identify cells
involved in the dissemination of varicella to multiple organs
dur-ing primary infection as well as after reactivation. Durdur-ing
experi-mental primary SVV infection in AGM, alveolar macrophages and
dendritic cells become infected at 5 dpi and transfer virus to T cells
in lymph nodes (
12
). In the study described here, we extended
studies of SVV reactivation in rhesus macaques and demonstrated
FIG 4Detection of the SVV IE63 protein in macrophages in lymph nodes from rhesus macaques after virus reactivation but not during latency. Paraformal-dehyde-fixed, paraffin-embedded sections of inguinal lymph node tissue from monkey HC44 after zoster (A to D) and from latently infected monkey II49 (E to H) were analyzed by immunofluorescence. In monkey HC44, the SVV IE63 protein was detected in macrophages (B and C) in the cortical sinus immunostained with rabbit polyclonal antibodies directed against SVV IE63 peptides and with mouse anti-human CD163 (macrophages) antibody. Donkey Alexa Fluor 488-tagged anti-rabbit IgG (H⫹L) antibody and goat Alexa Fluor 594-tagged anti-mouse IgG (H⫹L) antibody were used as secondary antibodies. In latently infected monkey II49, the SVV IE63 protein was not detected (F), although macrophages were found (G). Nuclei were stained with DAPI (A and E) and visualized at 358 nm. (D and H) Merged images. Magnifications,⫻600.
FIG 5Detection of the SVV IE63 protein in dendritic cells in lymph nodes from rhesus macaques after virus reactivation but not during latency. Immunoflu-orescent staining of sections of paraformaldehyde-fixed, paraffin-embedded axillary lymph node tissue from monkey HC44 obtained 24 to 48 h after zoster (A to D) and inguinal lymph node tissue from latently infected monkey II49 (E to H). In monkey HC44, the SVV IE63 protein was detected in dendritic cells (B and C) in germinal centers immunostained with polyclonal antibodies directed against SVV IE63 peptides and mouse anti-human CD123 (dendritic cells). The fluorescence-tagged secondary antibodies used are described in the legend toFig. 4. In latently infected monkey II49, the SVV IE63 protein was not detected (F), although dendritic cells were found (G). Nuclei were stained with DAPI (A and E). (D and H) Merged images. Magnifications,⫻600.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:5.585.136.451.66.230.2] [image:5.585.136.450.503.666.2]the presence of SVV in the sweat glands of skin and in the
macro-phages and dendritic cells of lymph nodes. Compared to the low
levels seen in the ganglia and lungs, lymph nodes contained an
abundance of SVV antigen (
Fig. 3
), and this finding was validated
by the detection of SVV DNA. Importantly, in VZV-infected
neu-rons
in vitro
, VZV DNA is not abundant, despite extensive VZV
transcription and translation (
16
). While SVV antigens were seen
in the skin, lung, ganglionic, and lymph node tissues of all five
monkeys, a stronger signal was found in tissues from monkeys
HC44 and HB62 than in those from the other monkeys.
Detection of SVV antigens in macrophages that express both
CD163 and CD68 suggests phagocytosis, since CD163 is expressed
on activated macrophages, while CD68 is a marker for cells of the
macrophage lineage (
17
). Dendritic cells and macrophages take
up antigens in the periphery and display major histocompatibility
complex-peptide complexes to T cells in lymphoid organs (
18
).
Thus, detection of SVV antigens in both lymph node macrophages
and lymph node dendritic cells after reactivation is consistent with
the notion that these cells present antigens to T cells in lymph
nodes.
Since T cell numbers increase at the time of zoster in
[image:6.585.315.524.66.288.2]immuno-suppressed monkeys (
19
) and lymph nodes receive afferent neural
input from the ganglia (
20
,
21
), analysis of ganglionic and lymph
node tissues for virus DNA and antigens from monkeys within
hours after SVV-specific T cell levels in blood are increased will
TABLE 4Detection of SVV antigens in lymph nodes from rhesus macaques 24 h after appearance of zosterd
Monkey no.
Lymph node type
Detection of the following antigen:
SVV IE63
SVV gH
and gL CD163 (M) CD123 (DC)
HB62 Axillary ⫹ ⫹ ⫹ ⫹
Bronchial ⫺ ⫹ ⫺ ⫺
Inguinal ⫹ ⫹ ⫹ ⫹
Mandibular ⫺ ⫹ ⫹ ⫺
HI83 Axillary ⫹ ⫹ ⫹ ⫹
Bronchial ⫹ ⫹ ⫹ ⫹
Inguinal ⫹ ⫹ ⫹ ⫹
HF39 Axillary ⫹ ⫹ ⫹ ⫹
Bronchial ⫺ ⫹ ⫺ ⫺
Iliac ⫹ ⫹ ⫹ ⫹
Inguinal ⫺ ⫹ ⫹ ⫹
HC44 Axillary ⫹ ⫹ ⫹ ⫹
Bronchial ⫺ ND ⫺ ⫺
Inguinal ⫹ ⫹ ⫹ ⫹
HA95a Axillary ⫹ ⫹ ⫹ ⫹
Bronchial ⫺ ⫹ ⫺ ⫺
Inguinal ⫹ ⫹ ⫹ ⫹
Mandibular ⫹ ⫹ ⫹ ⫺
II49b Axillary ⫺ ⫺ ⫺ ⫺
Inguinal ⫺ ⫺ ⫺ ⫺
IK10b Axillary ⫺ ⫺ ⫺ ⫺
IE04c Axillary ⫺ ⫺ ⫺ ⫺
Bronchial ⫺ ND ⫺ ⫺
Inguinal ⫺ ⫺ ⫺ ⫺
a
Not immunosuppressed.
bThe monkey was latently infected. c
The monkey was SVV seronegative.
dND, not done; M, macrophages; DC, dendritic cells.
FIG 6Detection of the SVV IE63 protein in the cytoplasm of macrophages in lymph nodes from a rhesus macaque after virus reactivation. Confocal microscopy revealed the SVV IE63 protein in the cytoplasm of macrophages (D, arrow) im-munostained with the antibodies described in the legend toFig. 4in sections of inguinal lymph node tissue from monkey HC44 obtained 24 to 48 h after zoster. Nuclei were stained with DAPI (A). (D) Merged image. Magnifications,⫻630.
FIG 7Detection of the SVV IE63 protein in the cytoplasm of dendritic cells in lymph nodes from a rhesus macaque after virus reactivation. Confocal microscopy revealed the SVV IE63 protein in the cytoplasm of dendritic cells in sections of axillary lymph node tissue from monkey HC44 (D, arrow) obtained 24 to 48 h after zoster and immunostained with the same antibodies described in the legend toFig. 5. Nuclei were stained with DAPI (A). (D) Merged image. Magnifications,⫻600.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:6.585.38.284.87.436.2] [image:6.585.317.524.437.656.2]help to determine whether reactivated SVV is transported
transax-onally from the ganglia to the lymph nodes. Indeed, detection of
SVV antigens in sweat glands (
Fig. 3A
) in the absence of viremia
most likely reflects the transaxonal transport of reactivated SVV
from the ganglia to the skin.
Although focal necrosis, multinucleated epithelial cells, and
viral infection of the sweat glands are seen in the affected skin of
humans during zoster (
22
,
23
), we did not find these pathological
features in the skin of monkeys with zoster. The absence of SVV
viremia during zoster in monkeys parallels the findings in humans
with varicella and zoster (
24–26
).
Finally, since VZV infection of dendritic cells in culture is
pro-ductive (
27
), it will be interesting to examine possible SVV
infec-tion of lymph node macrophages and dendritic cells from
mon-keys after zoster.
ACKNOWLEDGMENTS
We thank Wayne Gray for providing the SVV gH and gL antibodies, the Tulane National Primate Research Center Veterinary Medicine staff for excellent animal care, Georges Verjans and Werner Ouwendijk for useful discussions, Marina Hoffman for editorial assistance, and Cathy Allen for manuscript preparation.
This work was supported in part by Public Health Service grants AG032958 (to D.G., R.M., and V.T.-D.) and AG006127 (D.G.) from the National Institutes of Health. This work was also supported in part with federal funds from the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Insti-tutes of Health through grant number P51 RR00164 to the Tulane Na-tional Primate Research Center (to V.T.-D.). Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Ad-vanced Light Microscopy Core Facility, supported in part by NIH/NCRR Colorado CTSI grant number UL1 RR025780.
REFERENCES
1.Haberthur K, Messaoudi I.2013. Animal models of varicella zoster virus infec-tion. Pathogens2:364–382.http://dx.doi.org/10.3390/pathogens2020364. 2.Mahalingam R, Messaoudi I, Gilden D. 2010. Simian varicella virus
pathogenesis. Curr Top Microbiol Immunol342:309 –321.http://dx.doi
.org/10.1007/82_2009_6.
3.Mandal BK.1987. Herpes zoster and the immunocompromised. J Infect
14:1–5.http://dx.doi.org/10.1016/S0163-4453(87)90652-9.
4.Mahalingam R, Traina-Dorge V, Wellish M, Lorino R, Sanford R, Ribka EP, Alleman SJ, Brazeau E, Gilden DH.2007. Simian varicella virus reactivation in cynomolgus monkeys. Virology368:50 –59.http://dx
.doi.org/10.1016/j.virol.2007.06.025.
5.Messaoudi I, Barron A, Wellish M, Engelmann F, Legasse A, Planer S, Gilden D, Nikolich-Zugich J, Mahalingam R. 2009. Simian varicella virus infection of rhesus macaques recapitulates essential features of vari-cella zoster virus infection in humans. PLoS Pathog5:e1000657.http://dx
.doi.org/10.1371/journal.ppat.1000657.
6.Schoeb TR, Eberle R, Black DH, Parker RF, Cartner SC.2008. Diag-nostic exercise: papulovesicular dermatitis in rhesus macaques (Macaca mulatta). Vet Pathol45:592–594.http://dx.doi.org/10.1354/vp.45-4-592. 7.Kolappaswamy K, Mahalingam R, Traina-Dorge V, Shipley ST, Gilden DH, Kleinschmidt-DeMasters BK, Mcleod CG, Hungerford LL, De-Tolla LJ.2007. Disseminated simian varicella virus infection in an irradi-ated rhesus macaque (Macaca mulatta). J Virol81:411– 415.http://dx.doi
.org/10.1128/JVI.01825-06.
8.Ouwendijk WJD, Abendroth A, Traina-Dorge V, Getu S, Steain M, Wellish M, Andeweg AC, Osterhaus ADME, Gilden D, Verjans GMGM, Mahalingam R.2013. T-cell infiltration correlates with CXCL10 expres-sion in ganglia of cynomolgus macaques with reactivated simian varicella virus. J Virol87:2979 –2982.http://dx.doi.org/10.1128/JVI.03181-12. 9.Mahalingam R, Clarke P, Wellish M, Dueland AN, Soike KF, Gilden
DH, Cohrs R.1992. Prevalence and distribution of latent simian varicella virus DNA in monkey ganglia. Virology188:193–197.http://dx.doi.org
/10.1016/0042-6822(92)90749-F.
10. Mahalingam R, Wellish M, Soike K, White T, Kleinschmidt-DeMasters BK, Gilden DH.2001. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology279:339 –342.http://dx.doi
.org/10.1006/viro.2000.0700.
11. Mahalingam R, Wellish M, White T, Soike K, Cohrs R, Kleinschmidt-DeMasters BK, Gilden DH.1998. Infectious simian varicella virus ex-pressing the green fluorescent protein. J Neurovirol4:438 – 444.http://dx
.doi.org/10.3109/13550289809114543.
12. Ouwendijk WJ, Mahalingam R, de Swart RL, Haagmans BL, van Am-erongen G, Getu S, Gilden D, Osterhaus AD, Verjans GM.2013. T-cell tropism of simian varicella virus during primary infection. PLoS Pathog
9:e1003368.http://dx.doi.org/10.1371/journal.ppat.1003368.
13. Mahalingam R, Traina-Dorge V, Wellish M, Deharo E, Singletary ML, Ribka EP, Sanford R, Gilden D.2010. Latent simian varicella virus reacti-vates in monkeys treated with tacrolimus with or without exposure to irradi-ation. J Neurovirol 16:342–354.http://dx.doi.org/10.3109/13550284.2010
.513031.
FIG 8Detection of the SVV IE63 protein in CD3⫹T cells in lymph nodes from rhesus macaques after virus reactivation but not during latency. Sections of inguinal lymph node tissue from monkey HC44 obtained 24 to 48 h after zoster (A to D) and from latently infected monkey II49 (E to H) were analyzed by immunofluorescence. In monkey HC44, the SVV IE63 protein was detected in CD3⫹T cells after immunostaining with rabbit polyclonal antibodies directed against SVV IE63 peptides (B) and mouse anti-human CD3 (T cells) antibody (C). The fluorescence-tagged secondary antibodies used are described in the legend
toFig. 4. In latently infected monkey II49, the SVV IE63 protein was not detected (F), although CD3⫹T cells were found (G). Nuclei were stained with DAPI (A
and E) and visualized at 358 nm. (D and H) Merged images. Magnifications,⫻600.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:7.585.137.451.66.229.2]14. White TM, Mahalingam R, Traina-Dorge V, Gilden DH.2002. Persistence of simian varicella virus DNA in CD4(⫹) and CD8(⫹) blood mononuclear cells for years after intratracheal inoculation of African green monkeys. Virol-ogy303:192–198.http://dx.doi.org/10.1006/viro.2002.1664.
15. White TM, Mahalingam R, Traina-Dorge V, Gilden DH.2002. Simian varicella virus DNA is present and transcribed months after experimental infection of adult African green monkeys. J Neurovirol8:191–203.http:
//dx.doi.org/10.1080/13550280290049705.
16. Baird NL, Bowlin JL, Yu X, Jonjic S, Haas J, Cohrs RJ, Gilden D.2014. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology458-459:1–3.http://dx.doi.org/10.1016/j.virol.2014.04.014. 17. Kazankov K, Barrera F, Moller HJ, Bibby BM, Vilstrup H, George J,
Gronbaek H.2014. Soluble CD163, a macrophage activation marker, is in-dependently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology60:521–530.http://dx.doi.org/10.1002/hep.27129. 18. Banchereau J, Steinman RM.1998. Dendritic cells and the control of
immunity. Nature392:245–252.http://dx.doi.org/10.1038/32588. 19. James SF, Traina-Dorge V, Deharo E, Wellish M, Palmer BE, Gilden D,
Mahalingam R.2014. T cells increase before zoster and PD-1 expression increases at the time of zoster in immunosuppressed nonhuman primates latently infected with simian varicella virus. J Neurovirol20:309 –313.
http://dx.doi.org/10.1007/s13365-014-0237-7.
20. Kurkowski R, Kummer W, Heym C.1990. Substance P-immunoreactive nerve fibers in tracheobronchial lymph nodes of the guinea pig: origin, ultrastructure and coexistence with other peptides. Peptides11:13–20.
http://dx.doi.org/10.1016/0196-9781(90)90103-C.
21. Nance DM, Sanders VM.2007. Autonomic innervation and regulation of
the immune system (1987–2007). Brain Behav Immun21:736 –745.http:
//dx.doi.org/10.1016/j.bbi.2007.03.008.
22. Sangueza OP, Gordon MD, White CR, Jr. 1995. Subtle clues to the diagnosis of the herpesvirus by light microscopy. Herpetic syringitis. Am J Dermatopathol17:163–168.
23. Rinder HM, Murphy GF. 1984. Eccrine duct involvement by herpes zoster. Arch Dermatol120:261–262.http://dx.doi.org/10.1001/archderm
.1984.01650380121025.
24. Mainka C, Fuss B, Geiger H, Hofelmayr H, Wolff MH. 1998. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol56:91–98.http://dx.doi.org/10.1002/(SICI)1096
-9071(199809)56:1⬍91::AID-JMV15⬎3.0.CO;2-Z.
25. Kimura H, Kido S, Ozaki T, Tanaka N, Ito Y, Williams RK, Morishima T.2000. Comparison of quantitations of viral load in varicella and zoster. J Clin Microbiol38:2447–2449.
26. Bezold G, Lange M, Pillekamp H, Peter RU. 2002. Varicella zoster viraemia during herpes zoster is not associated with neoplasia. J Eur Acad Dermatol Venereol 16:357–360. http://dx.doi.org/10.1046/j.1468-3083
.2002.00448.x.
27. Abendroth A, Morrow G, Cunningham AL, Slobedman B.2001. Vari-cella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J Virol75:6183– 6192.http://dx.doi.org/10.1128/JVI.75.13.6183-6192.2001.
28. Ashburn CV, Gray WL.2002. Expression of the simian varicella virus glycoprotein L and H. Arch Virol147:335–348.http://dx.doi.org/10.1007
/s705-002-8323-6.
on November 7, 2019 by guest
http://jvi.asm.org/