0022-538X/97/$04.0010
Copyrightq1997, American Society for Microbiology
Characterization of T-Cell Response to Woodchuck Hepatitis Virus
Core Protein and Protection of Woodchucks from Infection by
Immunization with Peptides Containing a T-Cell Epitope
STEPHAN MENNE,1JAN MASCHKE,2TANJA K. TOLLE,3MENGJI LU,1
ANDMICHAEL ROGGENDORF1*
Institute of Virology1and Institute of Immunology,2University of Essen, D-45122 Essen, and
Institute of Medical Virology, University of Giessen, D-35392 Giessen,3Germany
Received 17 June 1996/Accepted 19 September 1996
Specific activation of T cells appears to be a prerequisite for viral clearance during hepatitis B virus (HBV) infection. The T-cell response to HBV core protein is essential in determining an acute or chronic outcome of HBV infection, but how this immune response contributes to the course of infection remains unclear. This is due to results obtained from humans, which are restricted to phenomenological observations occurring during the clinical onset after HBV infection. Thus, a useful animal model is needed. Characterization of the T-cell response to the core protein (WHcAg) of woodchuck hepatitis virus (WHV) in woodchucks contributes to the understanding of these mechanisms. Therefore, we investigated the response of woodchuck peripheral blood
mononuclear cells (PBMCs) to WHcAg and WHcAg-derived peptides, using our 5-bromo-2*-deoxyuridine
assay. We demonstrated WHcAg-specific proliferation of PBMCs and nylon wool-nonadherent cells from acutely WHV-infected woodchucks. Using a cross-reacting anti-human T-cell (CD3) antiserum, we identified nonadherent cells as woodchuck T cells. T-cell epitope mapping with overlapping peptides, covering the entire
WHcAg, revealed T-cell responses of acutely WHV-infected woodchucks to peptide1–20, peptide100–119, and
peptide112–131. Detailed epitope analysis in the WHcAg region from amino acids 97 to 140 showed that T cells
especially recognized peptide97–110. Establishment of polyclonal T-cell lines with WHcAg or peptide97–110
revealed reciprocal stimulation by peptide97–110 or WHcAg, respectively. We vaccinated woodchucks with
peptide97–110 or WHcAg to prove the importance of this immunodominant T-cell epitope. All woodchucks
immunized with peptide97–110or WHcAg were protected. Our results show that the cellular immune response
to WHcAg or to one T-cell epitope protects woodchucks from WHV infection.
The T-cell-mediated immune response to different hepatitis B virus (HBV) proteins may determine the course of HBV infection in humans (i.e., recovery from acute infection versus chronicity). Previous studies on cellular immune response to HBV have demonstrated the importance of virus-specific
Thelper(Th) cell and cytotoxic T-lymphocyte (CTL) responses
to HBV core (HBcAg) and surface protein (HBsAg) for re-covering from acute HBV infection and viral clearance (3, 9). Among the viral proteins, HBcAg has proven to be an impor-tant target of Th cells in patients with acute self-limiting HBV infection (9, 20, 21). The Th cell response to HBcAg appears to be low or absent in chronically HBV-infected patients (9, 16, 20, 21). It has been suggested that this weak immune response in chronically HBV-infected patients facilitates virus persis-tence by allowing the virus to evade immune surveillance and thereby permitting the development of chronic hepatitis B (reviewed in reference 12). However, the involvement of Th cell and CTL responses in the early phases of acute HBV infection resulting in either viral elimination or chronic disease remains unknown and requires studies in an animal model.
Woodchuck hepatitis virus (WHV) and its natural host, the eastern woodchuck (Marmota monax), constitute a useful an-imal model to investigate the pathogenesis of HBV infection in humans. WHV and HBV possess the same genomic organiza-tion and have a high homology in the amino acid sequences of
their proteins. Age dependence of chronicity after WHV in-fection and development of hepatocellular carcinoma in wood-chucks are similar to the situation in humans (5, 14, 22, 35, 36, 40).
Studies on immunization with core protein from HBV or WHV demonstrated that chimpanzees and woodchucks were partially or completely, respectively, protected from infection (17, 32, 33, 37, 39). The mechanism of such a protection after immunization with an internal virus component is unknown. Antibodies against core protein are always present at the onset of infection and reach high titers in the sera of humans or woodchucks chronically infected with HBV or WHV, but these antibodies presumably fail to neutralize the virus (1). It has been speculated that protection after immunization with these internal viral proteins is mediated by core protein-primed Th cells that may support the production of neutralizing antibod-ies against viral surface protein, as seen in the mouse model (30, 31). Likewise, it has been suggested that core protein-primed Th cells can support the induction of CTLs, which would require infection and replication of HBV or WHV to eliminate virus-infected cells (42, 43).
The aim of our study was to characterize the T-cell response to the WHV core protein (WHcAg) in the woodchuck model and its role in protection against WHV infection. The cellular response in the woodchuck was studied by establishing a pe-ripheral blood mononuclear cell (PBMC) proliferation assay with 5-bromo-29-deoxyuridine (BrdU) and with [2-3H]adenine
(25, 28). Using the BrdU assay, we investigated the response of woodchuck PBMCs to WHcAg in the acute, postacute, and chronic stages of WHV infection. A cross-reacting anti-human
* Corresponding author. Mailing address: Institut fu¨r Virologie, Uni-versita¨tsklinikum Essen, Hufelandstr. 55, D-45122 Essen, Germany. Phone: 49-201-7233550. Fax: 49-201-7235929. E-mail: roggendorf @uni-essen.de.
65
on November 9, 2019 by guest
http://jvi.asm.org/
intravenous (i.v.) inoculation with 10 woodchuck 50% infective doses (ID50) of a titrated WHV pool (39). The sera of acutely WHV-infected woodchucks, obtained 4 to 6 weeks after inoculation, were positive for WHV DNA and WHsAg. Sera obtained 10 to 20 weeks after inoculation (postacute phase of WHV infection) were negative for WHV DNA and WHsAg and positive for anti-WHs and anti-WHc.
Immunization with rWHcAg or WHcAg-derived peptides.Four WHV-nega-tive woodchucks were immunized three times subcutaneously with 100mg of recombinant WHcAg (rWHcAg) emulsified in incomplete Freund’s adjuvant (Sigma, Deisenhofen, Germany) at 2-week intervals. Immunization of four WHV-negative woodchucks with WHcAg-derived peptides (Genosys, Cam-bridge, England) was performed four times subcutaneously with either pep-tide97–110(100mg), peptide100–113(100mg), or both peptides (50mg of each) emulsified in incomplete Freund’s adjuvant at 2-week intervals. Two weeks after the last immunization, each animal was challenged i.v. with 105woodchuck ID
50 of the mentioned WHV pool (39). As a control, four nonimmunized woodchucks were infected i.v. with 105woodchuck ID
50of the same WHV pool. Blood samples were drawn weekly during follow-up.
Serology and detection of WHV DNA.The serological status of woodchucks for WHsAg, anti-WHs, and anti-WHc was determined by enzyme-linked immu-nosorbent assay (ELISA) as described previously (37, 39). WHV DNA was detected by a dot blot technique: 5ml of serum was spotted on a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany), and the DNA was dena-turated and hybridized with a32P-labeled plasmid containing the entire WHV genome (37). Additionally, WHV DNA was detected by PCR using two WHV core gene-specific oligonucleotide primers (nucleotides [nt] 2015 to 2038 and 2570 to 2595 of WHcAg) after extraction from serum.
Recombinant WHV core protein.WHV DNA of a chronically WHV-infected woodchuck was extracted from serum. The 564-bp-long core gene coding for WHcAg without its pre-C region (nt 2022 to 2586) was amplified with two oligonucleotide primers (nt 2015 to 2038 [59TGGGGCCATGGACATAGATC CTTA 39] and nt 2570 to 2595 [59CATTGAATTCAGCAGTTGGCAGATGG 39]) containing NcoI and EcoRI restriction sites, respectively (underlined). The amplified fragment was cloned into the tac promoter pKK 233-2 vector (Clon-tech, Palo Alto, Calif.), resulting in vector pWc2, and transfected into Escherichia
coli JM109. Recombinant clones expressing the core gene were identified directly
by immunospot analysis with a monoclonal mouse anti-HBc antibody with high cross-reactivity to WHcAg (kindly provided by M. Kann, Gießen, Germany) after transfer to a nitrocellulose membrane and incubation on 5 mM isopropylthio-galactopyranoside (IPTG)-containing agar plates for 4 h at 378C. Following restriction enzyme analysis of positive colonies, the DNA sequences of the inserts were analyzed by the dideoxyribonucleotide chain termination method (38) with [35S]ATP, using a T7 polymerase-sequencing kit (United States Biochemical, Cleveland, Ohio). The expression of rWHcAg was also demonstrated after so-dium dodecyl sulfate polyacrylamide gel electrophoresis by a Western blot assay with the anti-HBc antibody. rWHcAg was purified from pWc2 to a purity of about 95% as described recently (37). The amount of rWHcAg was determined by a protein assay (Pierce, Rockford, Ill.). The particulate nature of the purified rWHcAg was analyzed by electron microscopy.
Isolation of PBMCs.Venous blood was drawn from anesthetized woodchucks via the vena saphena and collected in EDTA monovettes (Sarstedt, Nu¨mbrecht, Germany). PBMCs were separated by Ficoll-Paque (Pharmacia, Freiburg, Ger-many) density gradient centrifugation and suspended in 0.9% NaCl. Cell count-ing was performed in a Thoma hemocytometer or an electronic cell counter (Sysmex, Hamburg, Germany).
PBMC proliferation assay with BrdU.Triplicates of 53104PBMCs were cultured in flat-bottom 96-well microtiter plates (Falcon, Becton Dickinson, N.J.) at 378C in a humidified atmosphere containing 5% CO2. To each well was added 200ml of AIM-V medium (Gibco BRL, Eggenstein-Leopoldshafen, Germany) supplemented with 2% 0.2 ML-glutamine (Sigma), 1% 0.125 M gentamicin
sulfate (Sigma), and 10% fetal calf serum (Gibco).
PBMC proliferation to rWHcAg was determined by increasing concentrations (0.005, 0.01, 0.05, 0.1, 0.5, 1, 2, 5, and 10mg of rWHcAg per ml). In all of the following experiments, proliferation of PBMCs after stimulation with rWHcAg
gated anti-BrdU for 2 h at 378C. PBMCs were then washed three times with phosphate-buffered saline (PBS) and incubated with peroxidase substrate for 30 to 45 min. The enzymatic reaction was stopped with 1 M H2SO4, and absorption (optical density at 450 nm) was measured with a microtiter plate reader (Sorin, Du¨sseldorf, Germany).
Results for triplicate cultures (53104
PBMCs) are presented as mean stim-ulation index (SI) (mean total absorption for stimulated PBMCs divided by the mean total absorption for controls). SIs of$2.1 were considered to indicate specific PBMC proliferation in the BrdU assay. The stimulation experiments with PHA showed a good discrimination of specific and nonspecific PBMC prolifer-ation correlating to the excess of BrdU incorporprolifer-ation. On the basis of these results, we set the cutoff value at 2.1. In this assay, the determined standard deviations of the means were less than 10% of the mean (range, 5 to 20%). The mean total absorption for the controls (triplicates of 5 3104 unstimulated PBMCs) ranged from 0.15 to 0.39 (mean, 0.27).
Separation of PBMCs by nylon wool.Nylon wool columns (Polyscience, Inc., Warrington, Pa.) were packed and incubated with AIM-V medium for 1 h at 378C. PBMCs suspended in 2 ml of AIM-V medium were added to the prepared columns and incubated for 1 h at 378C. The nonadherent cells were collected by washing the columns with 100 ml of AIM-V medium. Collection of adherent cells was performed by adding AIM-V medium to fill the column and knocking the column three times to dislodge the cells. The separated adherent and nonadher-ent cells were pelleted by cnonadher-entrifugation (3003g, 10 min), the supernatant was
discarded, and the cell pellet was resuspended in the desired volume of AIM-V medium.
Immunofluorescence staining of woodchuck cells with anti-CD3 epsilon.
PBMCs, nonadherent cells, and adherent cells were fixed with 50% methanol. Cells were stained by incubation with rabbit anti-human CD3 epsilon (Dako, Copenhagen, Denmark) in PBS supplemented with 0.1% bovine serum albumin (BSA) and 0.1% sodium azide (P-B-A) for 1 h at 378C. Cells were then washed in P-B-A, incubated with fluorescein isothiocyanate-conjugated mouse anti-rab-bit immunoglobulin (Dako) for 1 h at 378C, and washed in PBS before immu-nofluorescence analysis.
Characterization of the proliferating cell type in PBMCs.PBMCs (63106) were stimulated with 1mg of rWHcAg or BSA per ml in a flat-bottom 24-well plate (106
PBMCs/well; Falcon) for 5 days. PBMCs were labeled with 10mM BrdU, and 43106PBMCs were then separated on nylon wool columns as described above. The separation procedure was done twice to obtain highly purified fractions of nonadherent and adherent cells. The separated adherent and nonadherent cells were counted and resuspended in AIM-V medium, and 53104
cells (triplicates) were added to each well of a 96-well microtiter plate. A total of 53104stimulated PBMCs (triplicates) were added as a control to each well of the same microtiter plate, and BrdU incorporation was measured as described.
Establishment of WHV core protein-specific polyclonal T-cell lines.PBMCs of woodchucks in the acute phase of WHV infection were used to establish poly-clonal T-cell lines. A total of 63106PBMCs were cultured in tissue culture flasks (Falcon) in the presence of rWHcAg or peptide97–110at a concentration of 10mg/ml in AIM-V medium. After 1 week, stimulated PBMCs were expanded by adding 20 U of human interleukin-2 (IL-2; kindly provided by G. Lambrecht, Deutsches Rotes Kreuz, Springe, Germany) per ml for an additional week. Nonadherent cells were obtained by nylon wool separation and restimulated with 10mg of the desired WHV antigen per ml plus autologous irradiated (3,000 rads) PBMCs (5 3105
/ml) as antigen-presenting cells (APC) in AIM-V medium supplemented with 20 U of IL-2 per ml. Proliferating T-cell lines were restim-ulated weekly over a period of 5 to 6 weeks in AIM-V medium containing 20 U of IL-2 per ml.
For further characterization of the obtained polyclonal T-cell lines, the T cells were washed four times with 0.9% NaCl to remove IL-2. Subsequently, 53104 T cells (triplicates) were added to each well of a 96-well microtiter plate and incubated for 5 days with 105autologous irradiated (3,000 rads) PBMCs as APC in AIM-V medium and restimulated with 1mg of rWHcAg or peptides per ml. A BrdU proliferation assay was then performed.
on November 9, 2019 by guest
RESULTS
PBMC proliferation to WHcAg.PBMCs from acutely
WHV-infected woodchucks, from chronically WHV-WHV-infected wood-chucks, and from WHV-negative animals were tested for their proliferative responses to rWHcAg.
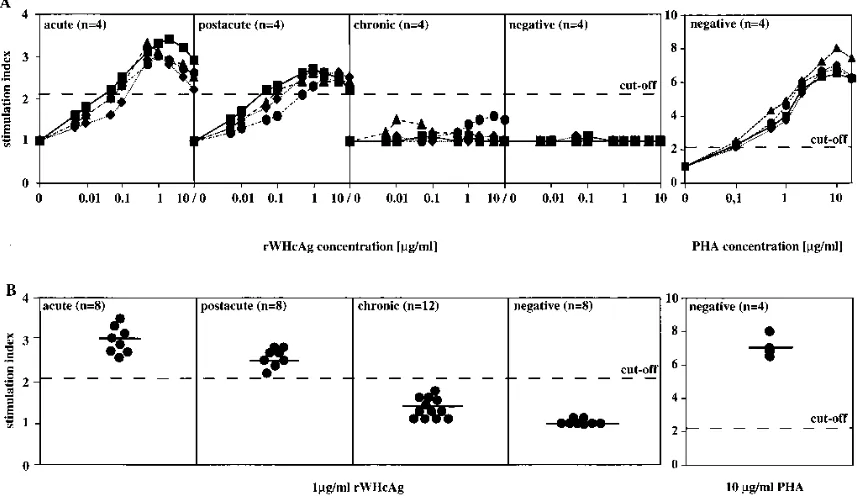
PBMCs were stimulated with rWHcAg at increasing concen-trations. rWHcAg induced a proliferative PBMC response in acutely and postacutely WHV-infected woodchucks. This pro-liferation was markedly dose dependent (Fig. 1A). In the BrdU assay, the rWHcAg concentration for optimal PBMC prolifer-ation was found to be 0.5 to 2.0 mg/ml. Kinetic experiments with rWHcAg revealed optimal culture conditions for detec-tion of PBMC proliferadetec-tion by incorporadetec-tion of BrdU on day 5 (data not shown). Stimulation with BSA at 1 mg/ml did not cause increased proliferation compared to spontaneous prolif-eration (data not shown), whereas stimulation with PHA at the optimal concentration of 10mg/ml resulted in SIs of between 6.5 and 8.0 (Fig. 1). The reactivity of PBMCs of postacutely WHV-infected woodchucks (10 to 20 weeks after inoculation) to rWHcAg was lower than the rWHcAg-specific proliferation of PBMCs observed in acutely WHV-infected woodchucks. PBMCs of chronically WHV-infected woodchucks and of WHV-negative woodchucks showed no proliferation after stimulation with rWHcAg (Fig. 1A).
The SIs for eight acutely WHV-infected woodchucks after stimulation of PBMCs with 1 mg of rWHcAg per ml ranged between 2.6 and 3.4; the mean SI was 3.0 (Fig. 1B). The reactivity of PBMCs to rWHcAg in the postacute phase was lower compared to the rWHcAg-specific proliferation of PBMCs from acutely WHV-infected woodchucks. The SIs of these eight woodchucks ranged between 2.3 and 2.7 (mean SI52.5). PBMCs from 12 chronically infected and 8
WHV-negative woodchucks did not proliferate after stimulation with rWHcAg (mean SIs51.3 and 1.0, respectively).
Using our [2-3H]adenine assay for woodchuck PBMCs, we
demonstrated comparable rWHcAg-specific PBMC prolifera-tion in acutely WHV-infected woodchucks (mean SI 5 7.1) (data not shown). PBMCs of chronically WHV-infected wood-chucks showed, in contrast to the BrdU assay, a weak specific proliferation (mean SI52.7) (25, 28).
Proliferation of nylon wool-separated cells in the presence
of WHcAg.Nylon wool separation of PBMCs was performed to
determine the type of cells responsible for the rWHcAg-spe-cific PBMC response. PBMCs from acutely WHV-infected woodchucks were cultured for 5 days in the presence of 1mg of rWHcAg or BSA per ml and labeled with BrdU. Subsequently, an aliquot was separated by nylon wool into nonadherent and adherent cells, and BrdU incorporation was assessed (Table 1). Nonseparated PBMCs and nonadherent cells responded spe-cifically to stimulation with rWHcAg, and similar mean SIs (3.2 and 3.6, respectively) were obtained. Adherent cells showed no rWHcAg-specific response (mean SI51.2). Stimulation with BSA did not lead to proliferation of PBMCs, nonadherent cells, or adherent cells. These differences in rWHcAg-specific proliferation indicate that the response of PBMCs detected in the BrdU assay is based on the proliferation of nonadherent cells.
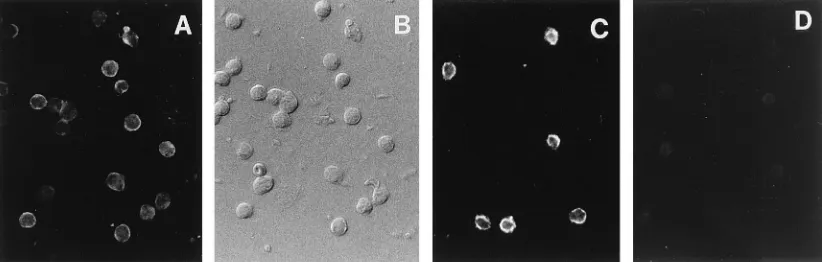
Identification of proliferating lymphocytes as T cells by
im-munofluorescence. PBMCs, nonadherent cells, and adherent
cells were stained with rabbit anti-human CD3 epsilon. Immu-nofluorescence analysis was performed after incubation of the labeled cells with fluorescein isothiocyanate-conjugated mouse anti-rabbit immunoglobulin (Fig. 2). Staining of nonseparated PBMCs showed that CD31cells are a major subpopulation of
FIG. 1. (A) PBMC proliferation (53104) after stimulation with rWHcAg at increasing concentrations. PBMC proliferation (53104) to different concentrations of PHA is shown as a positive control. Results of PBMCs (triplicates) from acutely (acute), postacutely (postacute), and chronically (chronic) WHV-infected woodchucks and WHV-negative animals (negative) are presented as mean SI. Each group contains four different animals (å,■,}, andF). (B) PBMC proliferation (53104) after stimulation with 1mg of rWHcAg per ml. PBMC proliferation (53104) to 10mg of PHA per ml is shown as a positive control. Results of PBMCs (triplicates) from 8 acutely (acute), 8 postacutely (postacute), and 12 chronically (chronic) WHV-infected woodchucks and 8 WHV-negative animals (negative) are presented as mean SI from a single woodchuck (F) and as mean SI from each group (2). SIs of$2.1 were considered specific. The value (optical density at 450 nm) for the controls was 0.2760.12.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:3.612.91.522.73.321.2]woodchuck PBMCs (60 to 70%). After separation of PBMCs by nylon wool, we could demonstrate that nonadherent cells (.90%) were CD31cells, i.e., woodchuck T cells, whereas no reactivity of anti-CD3 was observed in the adherent cells (Fig. 2).
PBMC proliferation to WHcAg-derived peptides. Previous
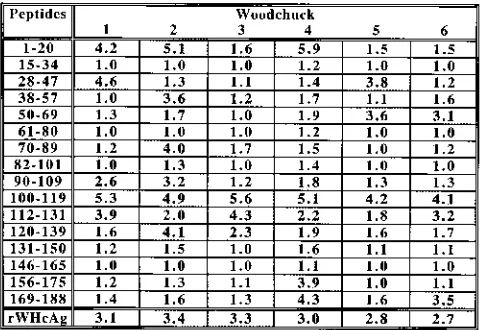
studies have shown that peptides derived from HBcAg con-taining Th cell epitopes can stimulate human PBMCs (8, 20, 29). For epitope mapping, a set of 16 overlapping peptides, 20 amino acids (aa) in length, covering the entire WHcAg was synthesized (Fig. 3). Epitope mapping was performed by stim-ulation of PBMCs from acutely WHV-infected woodchucks with 1mg of each peptide per ml. This approach revealed a specific PBMC response to peptide1–20(three of six animals),
peptide100–119(six of six), and peptide112–131(four of six). The
other peptides induced PBMC proliferation in only two or fewer woodchucks or showed no proliferative effect (Fig. 4).
To exclude suboptimal conditions for peptide-specific PBMC proliferation, we stimulated the PBMCs of acutely WHV-in-fected woodchucks with peptide100–119 and peptide112–131 at
concentrations from 0.5 to 10 mg/ml. Stimulation with
pep-tide100–119and peptide112–131induced PBMC proliferation in
acutely WHV-infected woodchucks in a dose-dependent man-ner (Fig. 5). Specific recognition of peptides by PBMCs was observed at a concentration of 1mg/ml. Kinetic experiments determined optimal response to these peptides at day 5 fol-lowing stimulation (data not shown). WHV-negative wood-chucks showed no PBMC proliferation with either peptide at any concentration (Fig. 5).
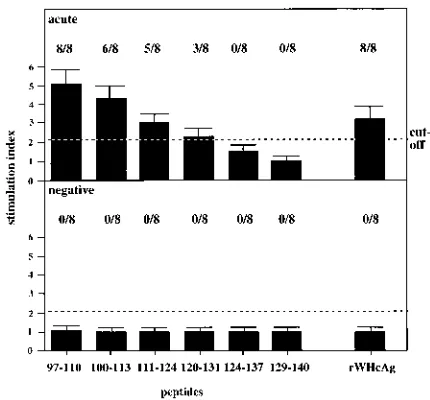
For the detailed analysis of T-cell epitopes located in the
was weak but specific and observed only in five and three, respectively, of eight acutely WHV-infected woodchucks. No specific PBMC proliferation was seen after stimulation with peptide124–137and peptide129–140(Fig. 6).
Characterization of WHV core protein-specific polyclonal
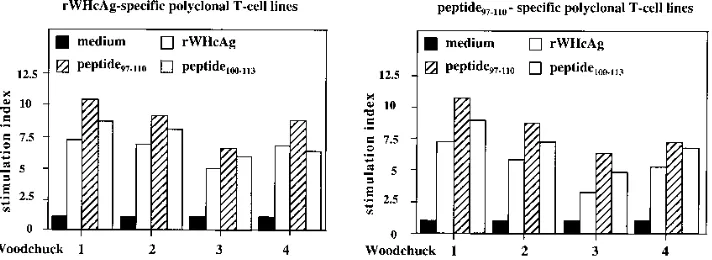
T-cell lines.Polyclonal T-cell lines were established to confirm
the antigen-specific PBMC proliferation after stimulation with rWHcAg or peptide97–110. PBMCs of acutely WHV-infected
woodchucks were continuously stimulated with 10 mg of rWHcAg or peptide97–110per ml plus irradiated autologous
PBMCs in the presence of IL-2 (Fig. 7). T-cell lines established by stimulation with rWHcAg (rWHcAg-specific polyclonal T-cell lines) showed specific proliferation to peptide97–110(mean
SI58.6). The presence of the overlapping peptide100–113
re-sulted in a lower proliferative response (mean SI57.1). In the reciprocal experiment, T-cell lines established with peptide97–110
(peptide97–110-specific polyclonal T-cell lines) proliferated
spe-cifically after stimulation with the entire rWHcAg (mean SI5 5.3). A proliferative response to peptide100–113was also found
(mean SI56.9). No stimulatory effects were induced by
pep-tide129–140or BSA (data not shown). The observed stimulatory
effects of rWHcAg and peptide97–110to these T-cell lines
con-firmed that the amino acid sequence represented by peptide97–110
contains a T-cell epitope which is presumably presented by APC after processing the native WHcAg.
Immunization of woodchucks with WHcAg-derived peptides
and rWHcAg.The relevance of the T-cell response to epitopes
of the HBV or WHV core protein for immune defense mech-anisms in viral infections is poorly understood. Therefore, we immunized four WHV-negative woodchucks with either
pep-tide97–110(100mg), peptide100–113(100 mg), or both peptides
[image:4.612.57.298.100.163.2](50mg of each) and tested whether a T-cell response to the
FIG. 2. Immunofluorescence staining of methanol-fixed woodchuck PBMCs, nonadherent cells, and adherent cells with anti-human CD3 epsilon antiserum. (A) Immunofluorescence of anti-human CD3-stained PBMCs; (B) the same cell population shown in phase contrast; (C and D) immunofluorescence of nonadherent cells (C) and adherent cells (D).
on November 9, 2019 by guest
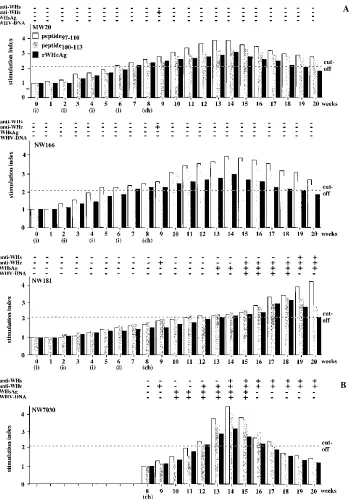
[image:4.612.102.513.568.699.2]immunodominant T-cell epitope (aa 97 to 110 of WHcAg) may provide protection from WHV infection. The extent of the PBMC response to peptide97–110, peptide100–113, and rWHcAg
during immunization and after challenge was analyzed (Fig. 8A). As a control for the immunization experiments, we in-fected four WHV-negative, nonimmunized woodchucks and monitored the PBMC response to rWHcAg and peptides dur-ing the natural course of disease (Fig. 8B).
The woodchuck immunized with peptide97–110(MW20) and
two woodchucks immunized with both peptides (data shown for NW166) were protected from WHV infection. No WHV DNA was detectable by dot blot hybridization and PCR in the sera after challenge. These woodchucks were also negative for WHsAg and anti-WHs during the follow-up (12 weeks). Assays for anti-WHc, which is regarded as the most sensitive marker of WHV infection, even after a low-dose inoculation, were also negative during follow-up (12 weeks) except at week 9 (1 week after challenge). This positive result for anti-WHc at week 9 may be explained by the inoculum, which contained a high titer of anti-WHc.
A specific PBMC response to peptide97–110, peptide100–113,
and rWHcAg was observed 6 to 7 weeks after the first immu-nization. The detection of this specific proliferative response could be also confirmed by the [2-3H]adenine assay (data not
shown). The maximum PBMC response was detected between week 13 to 15 (5 to 7 weeks after challenge).
The woodchuck immunized with peptide100–113 (NW181)
was not protected from WHV infection and became posi-tive for WHV DNA, WHsAg, anti-WHc, and anti-WHs after challenge. This woodchuck showed no specific PBMC re-sponse after stimulation with peptide97–110, peptide100–113, or
rWHcAg up to week 10 (2 weeks after challenge). A specific PBMC response to peptide97–110, peptide100–113, and rWHcAg
became detectable 11 to 14 weeks after first immunization (3 to 6 weeks after challenge), suggesting that proliferation was due to WHV replication after challenge and not to vaccination with peptide100–113. The PBMC response to rWHcAg, peptide97–110,
and peptide100–113increased up to weeks 18 to 20 (10 to 12
weeks after challenge), correlating with the presence of anti-WHs and anti-WHc and clearance of the virus.
As a control, the PBMC response was investigated in four nonimmunized woodchucks experimentally infected with the high dose of 105woodchuck ID
50. The animals developed an
acute self-limiting WHV infection. Woodchuck NW7030 is included in Fig. 8B as an animal representative of the non-immunized controls that became positive for WHV DNA,
WHsAg, anti-WHc, and anti-WHs after inoculation. A specific PBMC response to rWHcAg, peptide97–110, and peptide100–113
was observed beginning at week 4 after inoculation. The max-imum PBMC response was seen at week 6. The decrease of PBMC response correlated with the detection of anti-WHc and anti-WHs, and clearance of the virus.
In summary, these experiments indicated that immunization with peptide97–110was effective to achieve protection against
WHV infection, whereas vaccination with peptide100–113
seemed not to be protective.
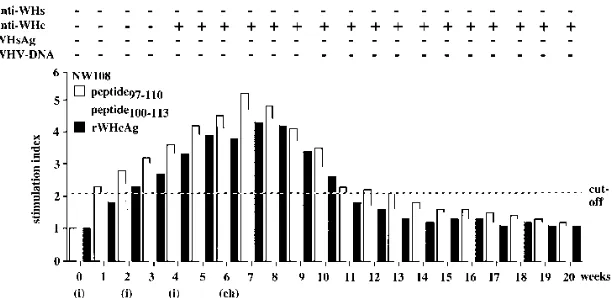
In additional control experiments, four WHV-negative woodchucks were immunized with 100mg of rWHcAg, and the PBMC responses to rWHcAg, peptide97–110, and peptide100–113
were characterized. The PBMC responses to WHV antigens of one woodchuck (NW108), representative of all four rWHcAg-immunized animals, are shown in Fig. 9.
This woodchuck developed anti-WHc 4 weeks after first immunization. The first specific PBMC response to peptide97–110,
peptide100–113, or rWHcAg was detectable 1 to 2 weeks after
[image:5.612.139.474.69.224.2]the first immunization and before seroconversion to anti-WHc occurred. The maximum PBMC response to all WHV antigens was observed 7 weeks after first immunization (1 week after
FIG. 3. Schematic representation of 16 synthetic peptides covering the entire core protein. The locations of six additional peptides used for detailed analysis of WHcAg region from aa 97 to 140 are illustrated.
FIG. 4. PBMC proliferation (53104) after stimulation with 1mg of WHcAg-derived peptides or rWHcAg per ml. Results of PBMCs (triplicates) from six acutely WHV-infected woodchucks are presented as mean SI. SIs of$2.1 were considered specific and are indicated as stippled squares. The value (optical density at 450 nm) for the controls was 0.2760.07.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:5.612.317.558.518.683.2]challenge). All rWHcAg-immunized woodchucks were pro-tected from WHV infection, as neither WHV DNA, WHsAg, nor anti-WHs was detectable in the sera.
DISCUSSION
Characterization of the cellular immune response to WHV proteins in the woodchuck model is a prerequisite for under-standing the immunological mechanisms mediating the patho-genesis of HBV infection. The outcome of HBV infection essentially depends on Th cell and CTL responses to viral proteins that are adequate in acute self-limiting HBV infection or may be defective in chronic HBV infection. An animal model is urgently needed to determine the role of HBcAg-sensitized T cells as the main cell population responsible for HBV elimination. Studies on the cellular immune response in the woodchuck by proliferation assays based on the incorpo-ration of [3H]thymidine (6, 24) failed to detect PBMC
prolif-eration due to low thymidine kinase activity (unpublished re-sults). In this study, we initially established a proliferation assay with BrdU. To prove the specificity of the BrdU assay, we determined the distribution of incorporated3H-labeled BrdU
([19,29-3H]BrdU) in proliferating PBMCs after stimulation
with the mitogen PHA or concanavalin A and demonstrated that 97% of BrdU was incorporated into DNA (28). Using this assay, we demonstrated PBMC proliferation after stimulation with rWHcAg and WHcAg-derived peptides in acutely WHV-infected woodchucks. PBMC proliferation was also observed following immunization with rWHcAg or peptides. Identical results were obtained in our previously described [2-3
H]ade-nine assay (25, 28).
After stimulation with rWHcAg, we observed a dose-depen-dent proliferative response and found an rWHcAg concentra-tion of 1mg/ml to be optimal for PBMC proliferation in acutely WHV-infected woodchucks (Fig. 1). This rWHcAg concentra-tion correlates to HBcAg-induced PBMC proliferaconcentra-tion in hu-mans (20). The rWHcAg-specific PBMC response decreased during the postacute phase of WHV infection.
the site of liver cell injury and viral replication, as described for HBV infection (10). The lack of a PBMC response to WHcAg could also be a consequence of antigen-specific suppressor mechanisms that downregulate the generation of these effector cells, as seen in HBV-infected patients (2, 11; for a review, see reference 12) or a consequence of inadequate T-cell activation (i.e., anergy or apoptosis).
Using nylon wool separation, we demonstrated that the non-adherent cells are responsible for rWHcAg-induced PBMC response (Table 1). Studies of humans and mice have identi-fied the nylon wool-nonadherent cells (e.g., PBMCs, splenic mononuclear cells, and tonsil-, thymus-, and lymph node-de-rived lymphocytes) as T cells (15, 19, 27). Additional evidence that T cells proliferate after stimulation with rWHcAg has been obtained by staining of cells with an anti-human CD3 antiserum (Fig. 2) that has a high affinity to the most conserved subunit epsilon of CD3 molecules from mammals (rats and mice) and ducks (4, 18). Therefore, we conclude that the rWH-cAg-induced PBMC response in the woodchuck was mainly achieved by T-cell proliferation. This view is further supported by the observation that polyclonal T-cell lines could be estab-lished from nonadherent cells by stimulation with rWHcAg or peptide97–110over 6 weeks in the presence of IL-2.
[image:6.612.60.298.71.271.2]Antigen-specific proliferation of PBMCs and nonadherent cells was
FIG. 5. PBMC proliferation (53104
) after stimulation with peptide110–119 or peptide112–131at increasing concentrations. Results of PBMCs (triplicates) from acutely WHV-infected woodchucks (acute) and WHV-negative animals (negative) are presented as mean SI. Each group contains four different animals (å,■,}, andF). The value (optical density at 450 nm) for the controls was 0.2560.05.
FIG. 6. PBMC proliferation (53104) after stimulation with 1mg of rWHcAg or various peptides covering aa 97 to 140 of the WHcAg per ml. Results of PBMCs (triplicates) from eight acutely WHV-infected woodchucks (acute) and eight WHV-negative animals (negative) are presented as mean SI. The value (optical density at 450 nm) for the controls was 0.2760.1.
on November 9, 2019 by guest
[image:6.612.327.542.483.683.2]observed, as stimulation with rWHcAg plus BSA did not differ from stimulation with rWHcAg alone (data not shown). The nylon wool-adherent cells contain the B-cell fraction (45, 46). Adherent cells could not be stained with anti-human CD3, and the undetectable proliferation of these cells to rWHcAg sug-gests that the B cells were not involved in the observed PBMC proliferation.
To identify T-cell epitopes, we analyzed the T-cell response of acutely WHV-infected woodchucks to peptides covering the entire WHcAg (Fig. 4 to 6). A striking observation was that the sequence from aa 100 to 119 of WHcAg was recognized by the T cells from all six woodchucks tested. This finding suggests that the peptide can be presented by different major histocom-patibility complex (MHC) molecules. Two additional peptides containing aa 1 to 20 or 112 to 131 led to proliferation in three or four, respectively, of six animals. Additional amino acid sequences were recognized by T cells from two or fewer wood-chucks. The individual pattern of peptide recognition could be caused by MHC restriction, as described for HBcAg in HBV-infected patients (8, 20). The cellular response to these pep-tides that typically stimulate Th cells in humans (8, 20) suggests that the proliferating woodchuck cells are Th cells. Surface markers to determine this T-cell population more directly have yet to be identified.
The WHcAg region from aa 100 to 131 was characterized in detail by a set of six overlapping peptides, ranging from aa 97 to 140. Peptide97–110induced T-cell proliferation in all eight
acutely WHV-infected woodchucks, and peptide100–113 did
so in six out of eight animals (Fig. 6). The T-cell response to peptide97–110and peptide100–113may be caused by linear
fea-tures of these peptides, 12 to 14 aa in length. These feafea-tures may allow the linear peptides to be processed more efficient-ly or presented more directefficient-ly in the MHC groove of APC than the same peptides derived after processing of the entire rWHcAg. This explanation is confirmed by our observation that in the presence of these peptides, T-cell proliferation was increased compared to stimulation with rWHcAg (Fig. 6). Ad-ditional experiments showed that peptide97–110also stimulated
PBMCs from some chronically WHV-infected woodchucks (data not shown). PBMC proliferation to peptide97–110of all
acutely WHV-infected woodchucks (n58) and also of some chronically WHV-infected animals so far tested suggests that this amino acid sequence could be promiscuous and therefore able to bind different MHC molecules. This peptide could also contain more than one T-cell recognition site, animals may possess similarities in the locus of their MHC II molecules, or
animals were outbred but had some kind of immunological relationship.
The characterized T-cell epitope of WHcAg, represented by peptide97–110and peptide100–113, is probably similar to the
mu-rine T-cell HBcAg epitope within residues 100 to 120 (29) and to human T-cell HBcAg epitopes within residues 81 to 105, 100 to 119, and 101 to 125 (8, 20). The finding that residues 1 to 20 represent an additional HBcAg determinant was confirmed by our data from the WHcAg-derived peptide1–20,
which induced T-cell proliferation in three of six wood-chucks.
Polyclonal T-cell lines were established to confirm that the amino acid sequence of peptide97–110 is presented by APC
after intracellular processing of the native WHcAg and recog-nized by T cells during natural WHV infection. We demon-strated that polyclonal T-cell lines established by PBMC stim-ulation with rWHcAg could be restimulated not only with the entire rWHcAg but also with peptide97–110(Fig. 7). The
reciprocal experiment in which peptide97–110-specific
poly-clonal T-cell lines proliferated after stimulation with the entire rWHcAg showed evidence that peptide97–110contains an
im-munodominant T-cell WHcAg epitope.
Subsequently, we asked whether the in vitro response of woodchuck T cells to rWHcAg and WHcAg-derived peptides is relevant to in vivo defense mechanisms in WHV infection. To prove the importance of T-cell response to the identified T-cell epitope, we immunized woodchucks with peptide97–110,
peptide100–113, or both peptides (Fig. 8A).
Woodchucks immunized with peptide97–110alone or in
com-bination with peptide100–113 were protected from WHV
in-fection, as no WHV DNA was detectable. Protection was achieved despite the absence of anti-WHc and anti-WHs after challenge. In contrast to the absent humoral response, we observed a specific and increasing T-cell response to
pep-tide97–110, peptide100–113, and rWHcAg following
[image:7.612.131.487.73.203.2]immuniza-tion. The predominant T-cell response to WHV antigens during immunization and after challenge and the simultaneous absence of a humoral response suggest that protection was based on the cellular response (Th cells and/or CTLs). Like-wise, immunization of mice with peptides derived from the nucleoprotein from lymphocytic choriomeningitis virus re-sulted in protection without detectable virus neutralizing anti-bodies (23, 34). Protection from foot-and-mouth disease virus, herpes simplex virus, and lymphocytic choriomeningitis virus infection after peptide immunization that was dependent on a
FIG. 7. T-cell proliferation (53104) after stimulation with 1mg of rWHcAg, peptide
97–110, or peptide100–113per ml. T cells were obtained from eight polyclonal T-cell lines established from four acutely WHV-infected woodchucks by continuous stimulation with rWHcAg or peptide97–110. Results of T cells (triplicates) are presented as mean SI. The value (optical density at 450 nm) for the controls was 0.3460.07.
on November 9, 2019 by guest
http://jvi.asm.org/
protective T-cell response and induced virus neutralizing anti-bodies was observed in mice and cattle (34, 44, 47, 48).
The woodchuck vaccinated with peptide100–113was not
pro-tected and developed a delayed, acute WHV infection which was similar in terms of SI to nonimmunized, experimentally infected woodchucks (Fig. 8). The mechanism of delayed WHV replication compared to nonimmunized acutely
WHV-infected woodchucks is not known and needs to be investi-gated.
Immunication of woodchucks with rWHcAg was performed to confirm that protection from WHV infection depends on the T-cell response to the entire rWHcAg, especially to the characterized immunodominant T-cell epitope (aa 97 to 110). In addition to this cell epitope, the existence of further
T-FIG. 8. (A) Humoral and cellular response kinetics to peptide97–110, peptide100–113, and rWHcAg during immunization with peptides (i) and after challenge with 10 5 woodchuck ID50(ch). Woodchuck MW20 was immunized with peptide97–110, animal NW166 was immunized with peptide97–110and peptide100–113, and animal NW181 was immunized with peptide100–113. (B) Humoral and cellular response kinetics to peptide97–110, peptide100–113, and rWHcAg after experimentally infec-tion of nonimmunized, WHV-negative woodchuck NW7030 with 105
woodchuck ID50(ch). Viremia was proved by DNA dot blotting and PCR. The presence of WHsAg, anti-WHs, and anti-WHc in the sera was detected by ELISA. PBMC proliferation (53104
) after stimulation with 1mg of peptide97–110, peptide100–113, or rWHcAg per ml was analyzed weekly. Results of PBMCs (triplicates) are presented as mean SI. The value (optical density at 450 nm) for the controls was 0.2760.05.
on November 9, 2019 by guest
[image:8.612.134.483.69.569.2]cell epitopes was apparent since T cells from various wood-chucks were able to be stimulated by additional WHcAg-derived peptides (Fig. 4). For this reason, we expected to observe differences in the T-cell responses from peptide-and rWHcAg-immunized woodchucks; the T-cell response of rWHcAg-immunized woodchucks to WHV antigens was in-creased and occurred earlier during immunization and before seroconversion to anti-WHc (Fig. 9). The requirement of T cells for protection from rabies virus infection after immuni-zation with nucleoprotein despite undetectable virus neutralizing antibodies has also been described for mice and dogs (7, 41).
In conclusion, our results show that immunization with a single peptide containing an immunodominant T-cell epitope was as effective as immunization with the entire rWHcAg. We demonstrate here that protection of woodchucks from WHV infection is dependent less on a humoral response than on a specific T-cell response to WHV antigens, espe-cially to the immunodominant T-cell epitope (aa 97 to 110 of WHcAg).
ACKNOWLEDGMENTS
The generous gifts of the anti-HBc antibody from M. Kann (Gießen, Germany) and IL-2 from G. Lambrecht (Springe, Germany) are grate-fully acknowledged. We grategrate-fully acknowledge the helpful discussions and critical comments of M.-C. Jung (Munich, Germany) and J. P. Kruppenbacher (Essen, Germany).
This work was supported by Deutsche Forschungsgemeinschaft grant Ro 687/2-2.
REFERENCES
1. Beasly, R. P., L. Trepo, C. E. Stevens, and W. Szmuness. 1977. The e-antigen and vertical transmission of hepatitis B surface antigen. Am. J. Epidemiol.
105:914–918.
2. Bertoletti, A., A. Sette, F. V. Chisari, A. Penna, M. Levrero, M. De Carli, F.
Fiaccadori, and C. Ferrari.1994. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature (London)
369:407.
3. Bertoletti, A., C. Ferrari, F. Fiaccadori, A. Penna, R. Margolskee, H. J.
Schlicht, P. Fowler, S. Guilhot, and F. V. Chisari.1991. HLA class-I human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nu-cleocapsid antigen. Proc. Natl. Acad. Sci. USA 88:10445–10449.
4. Bertram, E. M., R. G. Wilkinson, B. A. Lee, A. R. Jilbert, and I. Kotlarski. Identification of duck T lymphocytes using an anti-human T cell (CD3) antiserum. Vet. Immunol. Immunopathol., in press.
5. Buendia, M. A. 1994. Hepatitis B viruses and liver cancer: the woodchuck model, p. 183–187. In A. Mirson, J. Neil, and M. McCrae (ed.), Viruses and cancers. Symposium for General Microbiology. Cambridge University Press, Cambridge.
6. Cote, P. J., and J. L. Gerin. 1995. In vitro activation of woodchuck
lympho-cytes measured by radiopurine incorporation and interleukin-2 production: implications for modelling immunity and therapy in hepatitis B virus infec-tion. Hepatology 22:687–699.
7. Fekadu, M., J. W. Sumner, J. H. Shaddock, D. W. Sanderlin, and G. M.
Baer.1992. Sickness and recovery of dogs challenged with a street rabies virus after vaccination with a vaccinia virus recombinant expressing rabies virus N protein. J. Virol. 66:2601–2604.
8. Ferrari, C., A. Bertoletti, A. Penna, A. Cavalli, A. Valli, G. Missale, M. Pilli,
P. Fowler, T. Giuberti, F. V. Chisari, and F. Fiaccadori.1991. Identification of immunodominant T cell epitopes of the hepatitis B virus nucleocapsid antigen. J. Clin. Invest. 88:214–222.
9. Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. Degli Antoni, T. Giuberti, A.
Cavalli, M. A. Petit, and F. Fiaccadori.1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 145:3442–3449.
10. Ferrari, C., A. Penna, P. Sansoni, T. Giuberti, T. M. Neri, F. V. Chisari, and
F. Fiaccadori.1986. Selective sensitization of peripheral blood T lympho-cytes to hepatitis B core antigen in patients with chronic active hepatitis type B. Clin. Exp. Immunol. 67:497–506.
11. Ferrari, C., A. Penna, T. Giuberti, M. J. Tong, E. Ribera, F. Fiaccadori, and
F. V. Chisari.1987. Intrahepatic, nucleocapsid antigen-specific T cells in chronic active hepatitis B. J. Immunol. 139:2050–2058.
12. Franco, A., C. Ferrari, A. Sette, and F. V. Chisari. 1995. Viral mutations, TCR antagonism and escape from the immune response. Curr. Opin. Im-munol. 7:524–531.
13. Galibert, F., T. N. Chen, and E. Mandart. 1982. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J. Virol. 41:51–65.
14. Gerin, J. L., P. J. Cote, B. E. Korba, R. H. Miller, R. H. Purcell, and B. C.
Tennant.1991. Hepatitis B virus and liver cancer: the woodchuck as an experimental model of hepadnavirus induced liver cancer, p. 556–559. In F. B. Hollinger, S. M. Lemon, and H. Margolis (ed.), Viral hepatitis and liver disease. The Williams & Wilkins Co., Baltimore, Md.
15. Greaves, M. F., and G. Brown. 1974. Purification of human T and B lym-phocytes. J. Immunol. 112:420–423.
16. Inoue, M., S. Kakumu, K. Yoshioka, Y. Tsutsumi, T. Wakita, and M. Arao. 1989. Hepatitis B core antigen specific IFN-gamma production in peripheral blood mononuclear cells in patients with chronic hepatitis B virus infection. J. Immunol. 142:4006–4012.
17. Iwarson, S., E. Tabor, H. C. Thomas, P. Snoy, and R. J. Gerety. 1985. Protection against hepatitis B virus infection by immunization with hepatitis B C-antigen. Gastroenterology 88:763–767.
18. Jones, M., J. L. Cordell, A. D. Beyers, A. G. D. Tse, and D. Y. Mason. 1993. Detection of T and B cells in many animal species using cross reactive anti-peptide antibodies. J. Immunol. 150:5429–5435.
19. Julius, M. H., E. Simpson, and L. A. Herzenberg. 1973. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur. J. Im-munol. 3:645–649.
20. Jung, M.-C., H. M. Diepolder, U. Spengler, E. A. Wierenga, R. Zachoval,
R. M. Hoffmann, D. Eichenlaub, G. Fro¨sner, H. Will, and G. R. Pape.1995. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD41T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J. Virol. 69:3358–3368.
21. Jung, M.-C., U. Spengler, W. Schraut, R. Hoffmann, R. Zachoval, J.
[image:9.612.155.461.69.218.2]Eisen-burg, D. Eichenlaub, G. Riethmu¨ller, G. Paumgartner, H. W. L. Ziegler-Heitbrock, H. Will, and G. R. Pape.1991. Hepatitis B virus antigen specific FIG. 9. Humoral and cellular response kinetics of woodchuck NW108 to peptide97–110, peptide100–113, and rWHcAg during immunization with rWHcAg (i) and after challenge with 105
woodchuck ID50(ch). Viremia was proved by DNA dot blotting and PCR. The presence of WHsAg, anti-WHs, and anti-WHc in the sera was detected by ELISA. PBMC proliferation (53104
) after stimulation with 1mg of peptide97–110, peptide100–113, or rWHcAg per ml was analyzed weekly. Results of PBMCs (triplicates) are presented as mean SI. The value (optical density at 450 nm) for the controls was 0.2460.06.
on November 9, 2019 by guest
http://jvi.asm.org/
hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology 22:61–68.
27. Lowry, R. P., A. E. Person, J. E. Goguen, C. B. Carpenter, and M. R.
Garovoy.1978. Technical modifications for antibody-dependent cell-medi-ated cytotoxicity versus separcell-medi-ated T and B lymphocytes: T- and B-cell sep-aration by nylon wool columns. Transplant. Proc. 10:833–837.
28. Menne, S., J. Maschke, T. K. Tolle, E. Kreuzfelder, H. Grosse-Wilde, and M.
Roggendorf.Determination of peripheral blood mononuclear cell responses to mitogens and woodchuck hepatitis virus core antigen in woodchucks by 5-bromo-29-deoxyuridine or 2[3
H]adenine incorporation. Arch. Virol., in press.
29. Milich, D. R., A. McLachlan, A. Moriarty, and G. B. Thornton. 1987. Im-mune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J. Immunol. 139:1223–1231. 30. Milich, D. R., A. McLachlan, G. B. Thornton, and J. L. Hughes. 1987.
Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature (London) 329:547–549. 31. Milich, D. R., J. L. Hughes, A. McLachlan, G. B. Thornton, and A. Moriarty. 1988. Hepatitis B synthetic immunogen comprised of nucleocapsid T-cell sites and an envelope B-cell epitope. Proc. Natl. Acad. Sci. USA 85:1610– 1614.
32. Murray, K., S. A. Bruce, A. Hinnen, P. Wingfield, P. M. C. A. van Erd, A. de
Reus, and H. Schellekens.1984. Hepatitis B antigens made in microbial cells immunize against viral infection. EMBO J. 3:645–650.
33. Murray, K., S. A. Bruce, P. Wingfield, P. M. C. A. van Erd, A. de Reus, and
H. Schellekens.1987. Protective immunization against hepatitis B virus in-fection by immunization with an internal antigen of the virus. J. Med. Virol.
23:101–107.
34. Oldstone, M. B. A., A. Tishon, M. Eddleston, J. C. de la Torre, T. McKee,
41. Sumner, J. W., M. Fekadu, J. H. Shaddock, J. J. Esposito, and W. J. Bellini. 1991. Protection of mice with vaccinia virus recombinants that express the rabies nucleoprotein. Virology 183:703–710.
42. Sylvan, S. P. E., U. B. Hellstro¨m, and B. Flehming.1987. Characterization of cell mediated immune responses to the hepatitis B core protein in man. Clin. Exp. Immunol. 68:233–241.
43. Vitiello, A., G. Ishioka, H. M. Grey, R. Rose, P. Farness, R. LaFond, L. Yuan,
F. V. Chisari, J. Furze, R. Bartholomeuz, and R. W. Chesnut.1995. Devel-opment of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J. Clin. Invest. 95:341–349.
44. Watari, E., B. Dietzschold, G. Szokan, and E. Heber-Katz. 1987. A synthetic peptide induces long-term protection from lethal infection with herpes sim-plex virus 2. J. Exp. Med. 165:459–470.
45. Werner, C., P. T. Klouda, M. C. Corre´a, P. Vassalli, and M. Jeannet. 1977. Isolation of B and T lymphocytes by nylon fiber columns. Tissue Antigens
9:227–229.
46. Wong, D. M., and K. K. Mittal. 1981. HLA-DR typing: a comparison be-tween nylon wool adherence and T cell rosetting in the isolation of B cells. J. Immunol. Methods 46:177–186.
47. Zamorano, P., A. Wigdorovitz, M. Perez-Filgueira, C. Carrillo, J. M.
Escri-bano, A. M. Sadir, and M. V. Borca.1995. A 10-amino-acid linear sequence of VP1 of foot and mouth disease virus containing B- and T-cell epitopes induces protection in mice. Virology 212:614–621.
48. Zamorano, P., A. Wigdorovitz, M. T. Chaher, F. M. Fernandez, C. Carrillo,
F. E. Marcovecchio, A. M. Sadir, and M. V. Borca.1994. Recognition of B and T cell epitopes by cattle immunized with a synthetic peptide containing the major immunogenic site of VP1 FMDV 0 1 Campos. Virology 201:383– 387.