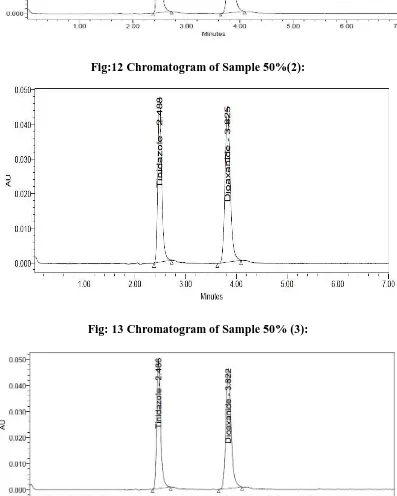
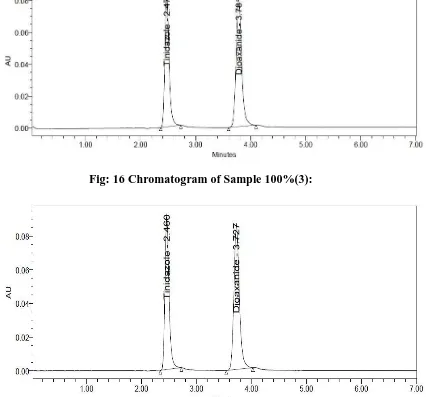
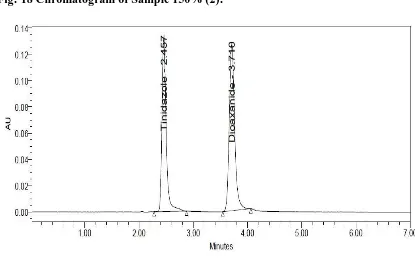
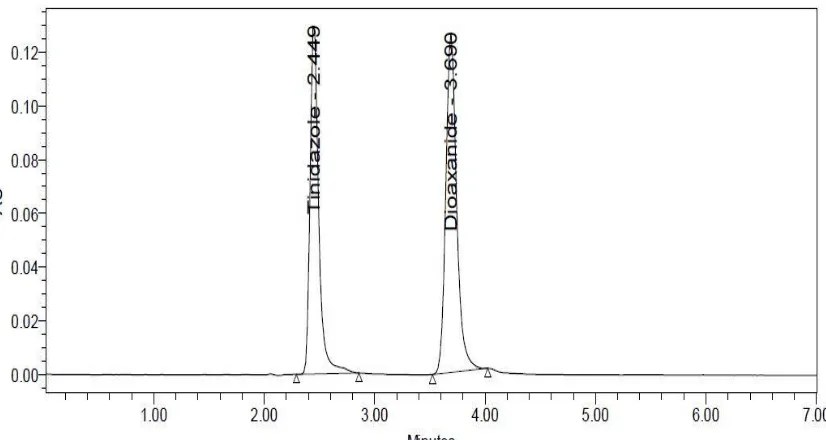
A RP-HPLC Method Development and Validation of Tinidazole and Diloxanide Furoate in Pharmaceutical Formulation and its Forced Degradation Studies
Full text
Figure
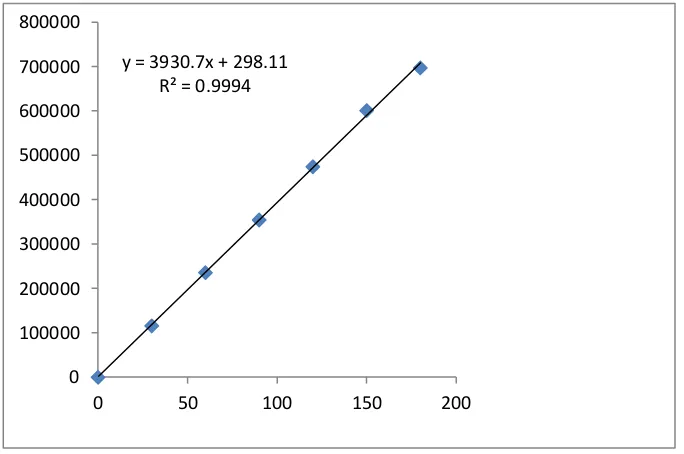
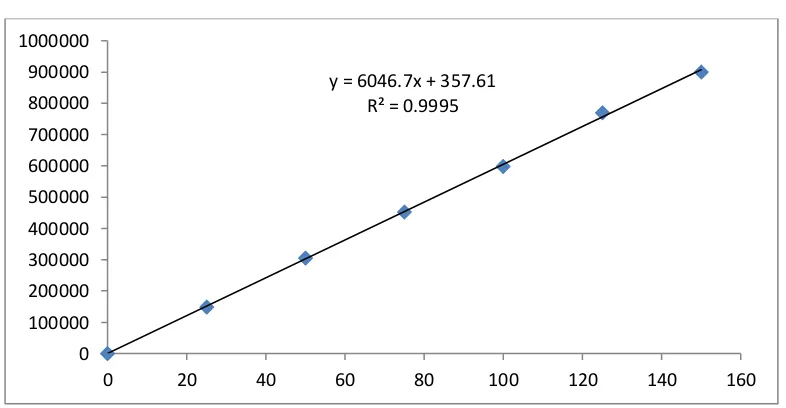
Related documents
supported, the B value of for previous experience of entrepreneur factor = .716> 0.01, which indicated that there is a positive and significant impact of previous
The aims and objectives of the study were to assess the nutritional status of children among 3-6 yrs in UHTC area of SRMC Nandyal and to identify the influence of
The research emphasizes on the contract as a reinforcement tool of project management practices and on CWM as one of the sustainability aspects in construction sector..For the
A decalcified section from the site of fracture (Fig. 2b,c) and a section from the surrounding muscle tissue showed metastatic, moderately differentiated adenocarcinoma with
Satoh describes that at the blastula stage all cells are of nearly equal size a n d enclose the blastocoel.. With the subsequent cell divisions the
The development of an agreed-upon set of foundational ethical values for the field of public health is ongoing. In this paper we outline key elements of recent convergence on some
The analysis of a larger number of cases of CMT involving GDAP1 mutations, along with detailed clinical, electrophysiological and pathological data, will be required to establish
Summarizing, the main contributions of our study are: (i) the design and implemen- tation of a system that uses big data gathered by in-vehicle sensors and components to