Copyright © 1988,American Society for Microbiology
Gamma Interferon
Augments
FcY
Receptor-Mediated
Dengue
Virus
Infection of
Human
Monocytic
Cells
UDO KONTNY,t ICHIRO KURANE, ANDFRANCIS A. ENNIS*
Division of InfectiousDiseases, DepartmentofMedicine, University of Massachusetts Medical Center, Worcester, Massachusetts01605
Received 3 June 1988/Accepted 18 July 1988
It has been reported thatanti-dengue antibodiesat subneutralizing concentrationsaugmentdengue virus infectionof monocytic cells. Thisis duetothe increaseduptakeof denguevirus inthe form ofvirus-antibody complexes by cells via Fcl, receptors. We analyzed the effects of recombinant human gamma interferon
(rIFN-y) ondengue virus infection ofhumanmonocytic cells.U937 cells, a humanmonocytic cell line,were
infected with denguevirus in theform ofvirus-antibodycomplexes afterrIFN-y treatment. Pretreatment of U937 cells withrIFN-yresultedinasignificantincrease in the number ofdenguevirus-infected cells and inthe
yield ofinfectious virus.rIFN-ydidnotaugmentdenguevirus infectionwhencellswereinfected with virus in
the absence of anti-dengue antibodies. Gamma interferon (IFN--y) produced by peripheral bloodlymphocytes from dengue-immune donors after in vitro stimulation with dengue antigens also augmented dengue virus infection of U937 cells.IFN--y didnot augmentdengue virusinfectionswhen cellswereinfectedwithvirusin the presence of F(ab')2 prepared from anti-dengue immunoglobulin G. Human immunoglobulin inhibited
IFN-y-inducedaugmentation. IFN-yincreased the numberofFc,,receptorsonU937 cells.The increaseinthe percentage ofdengue antigen-positivecells correlated with theincrease inthe number of
Fcl,
receptors after rIFN--y treatment. These results indicate thatIFN-y-inducedaugmentation ofdengue virus infection is Fc,, receptormediated. Basedontheseresultsweconclude thatIFN-'y increases the number ofFc,,receptorsand that thisleadstoanaugmenteduptakeof dengue virusinthe form ofdenguevirus-antibody complexes,whichresultsin augmented denguevirusinfection.
Dengueisatropical illness causedbydengue virus
infec-tion of humans and is transmitted by mosquitoes. The
disease is endemicover a large part ofSoutheast Asia, the
Pacific, Africa, and Central America. Clinically, infection with dengue virus presents in twomajorforms,dengue fever
and dengue hemorrhagic fever-dengue shock syndrome (DHF-DSS). Dengue fever is a self-limited disease and represents most cases of dengue. In some cases, however, patients develop severe complications, DHF-DSS, which arelife threatening. From 1980through 1985, 500,000 cases
of DHF-DSS were reportedworldwide (16).
Human monocytes support active replication of dengue
virus in vivo (11). Studies done by Halstead et al. have shown that monocytesareinfectedto amuch greaterextent in vitro in the presenceofanti-dengue virus antibody (7, 10).
This isdue tothe increased uptake bymonocytes ofdengue virus in the form of dengue virus-antibody complexes via
FcY
receptors (4, 10). This phenomenon is referred asimmune enhancement. Interestingly, most cases of DHF-DSS occur during secondary dengue infections when there are dengue virus antibodies present (2, 8, 9), and immune
enhancement mayplayanimportantrole inthepathogenesis of DHF-DSS (18). We havereported that T
lymphocytes
ofdengue antibody-positive donors produce high titers of gamma interferon (IFN--y) after stimulation with dengue
antigens in vitro (I. Kurane, B. L. Innis, A. Nisalak, C.
Hoke, S. Nimmannitya, A. Meager, and F. A. Ennis,
submitted for publication). Therefore, it can be expected that IFN--y is produced in vivo during secondary dengue
infections. It has been reported that IFN--y increases the
*Correspondingauthor.
tPresent address: UniversityofTubingen MedicalSchool,7400 Tubingen,FederalRepublicofGermany.
numberof
FcY
receptorsonhuman monocytesandmonocy-tic celllines(6, 17). In thisstudyweinvestigatedthe effect of IFN--y on dengue virus infection of U937 cells, a human
monocyticcellline. IFN--y augmented denguevirus infection of U937 cells when cells were infected in the presence of
anit-dengue antibody. Theaugmenting effect of
IFN-y
wasFc,, receptordependent. Weconclude that IFN--yincreases the number of
FcY
receptors and that this results in theaugmented infection of the cells by dengue virus-antibody complexes.
MATERIALS ANDMETHODS
Virus andantibody. Dengue virus type2, New Guinea C
strain, was used for infection. The virus was supplied by Walter E. Brandt of the Walter Reed Army Institute of
Research,Washington,D.C. The viruswaspassedinmouse brain and then propagated in mosquito cells (C6/36) as
previously described (14). The titerofthe viruspoolused in these experiments was 107 PFU/ml in Vero cells as
deter-mined by previously described methods (12). Ascitic fluid from mice hyperimmunized with dengue virus type 2 was usedas a source ofanti-dengue virus type 2antibody. This
antibodywasalsosupplied byWalter E. Brandt. Thetiter of thisantibodywas1:1,024asdeterminedbyaplaque neutral-ization test (14). Hyperimmune ascitic fluid was heated at
56°Cfor 30 minto destroy complementactivitybefore use. Cells cultures.U937,amonocyticcellline thatwasderived from a patient with histiocytic lymphoma (19), was used. Cellswere cultured inRPMI 1640medium (Flow Laborato-ries, McLean, Va.) supplemented with10%fetalcalfserum
(GIBCO Laboratories, Grand Island, N.Y.).
IFNs and anti-IFN antibodies. HumanrecombinantIFN-,y
(rIFN-y; Hoffman-LaRoche, Inc.,Nutley, N.J.) and human
lymphoblastoid IFN-a (Lee-Biomolecular Research, San 3928
on November 10, 2019 by guest
http://jvi.asm.org/
Diego, Calif.) were used. Forneutralization experiments, a
monoclonal antibody tohuman IFN--y (InterferonSciences, New Brunswick, N.J.) and a rabbit polyclonal antibody to IFN-a (Interferon Sciences) wereused.
Treatmentofcellswith human
y-globulin.
U937cellswereincubated with 10 mg of of human immunoglobulin
(-y-globulin) (Sigma Chemical Co., St. Louis, Mo.) per ml or
bovinealbumin fraction V powder (GIBCO) in RPMI for20
min at
4°C.
The cells were washed three times and theninfected by dengue virus-antibody complexes. The human
y-globulin
used in the experiments had no neutralizingactivity (<1:10) for dengue virustype2when itwastestedin aplaque-neutralizationassayataconcentrationof 10mg/ml.
Culture fluid of PBMC stimulated with dengue antigen. Peripheral blood mononuclear cells (PBMC) from a donor
whopreviously had been infected with denguevirus type 3 and subsequently developed antibodiestodengue virustype
3 were stimulated with a dengue virus type 3 antigen preparation or controlantigenfor7 days. Thisantigen was
prepared from dengue virus-infected Vero cells that were
treated with0.025% glutaraldehyde and sonicated. Control antigen was prepared from uninfected Vero cells, which were also glutaraldehyde treated and sonicated. Culture
fluidsweretestedfor IFN--y and IFN-aby AnthonyMeager,
London, byusinga radioimmunoassay withspecific
mono-clonalantibodies for human IFN--y and IFN-a(15). Infection of cells with dengue virus. U937 cells were
washed once in RPMI 1640containing 1% fetalcalfserum
and suspended to a concentration of 106 cells per 0.1 ml.
Theywerethen incubated with dengue virustype2or with
dengue virus type 2-antibody complexes for 2 h. These virus-antibody complexes were made before infection by
adding 10,ul ofanti-dengue virustype 2 mouse ascites fluid
atadilution of 1:200to5 x 106 PFUofdengue virus type2 in0.5mlandwereincubatedfor1h at4°C. Themultiplicity ofinfection(MOI)of thevirus inoculumwas5PFUpercell.
Cellswerewashed twice after 2 hofincubation withvirusor
virus-antibody complexes and thencultured at a
concentra-tion of2 x 105 cells per ml in RPMI 1640 containing 10%
fetal calf serum for 24 h. Cells were then stained for the presenceof dengue viral antigenby immunofluorescence as
previouslydescribed (14).The mouseanti-denguevirustype
2 serum described above and a fluorescein
isothiocyanate-conjugated sheepanti-mouse imtnunoglobulin G (IgG) anti-body (CappelLaboratories, Malvern, Pa.)were used.
Preparation of F(ab')2 from anti-denguevirus type 2 IgG. F(ab')2 was prepared from anti-dengue virus type 2 mouse
ascites fluid with an Immuno Pure F(ab')2 preparation kit
(Pierce Chemical Co., Rockland, Ill.). Mouse ascites fluid
wasdiluted1:2with thebindingbuffer (pH8.2), and1 ml of
the dilutedfluidwasappliedtoa1-mlcolumnofimmobilized
proteinA.The columnwaswashedwith 15columnvolumes
of thebindingbuffer.IgGwaselutedwith5columnvolumes
of the elution buffer (pH 2.8). Eluted IgG was dialyzed
against20mMsodiumacetate atpH4.2andconcentratedto
an IgG concentration of 20 mg/ml with a minicon-A25
(Amicon Corp., Danvers, Mass.). Then 10 mg ofIgG was
treated with 12.5%immobilizedpepsin(Pierce)in 1mlof20
mMsodiumacetate(pH4.2)at37°Cfor 4h. Thecrudedigest
(10mgin3 ml)was appliedtoa3-mlcolumnofimmobilized
protein A, and F(ab')2 was eluted with 6 ml of the binding
buffer. IgG and F(ab')2 were dialized against
phosphate-buffered saline (pH 7.4)at4°Cfor 72 h. ThepurityofF(ab')2 and IgG was examined by using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. F(ab')2 did not contain
any detectable IgG. F(ab')2 and purified IgG were
concen-trated to aconcentration of1
mg/ml
withaminicon-A25.Theneutralizing
antibody
titers ofpurified
IgG
andF(ab')2
at 1mg/mlwere 1:320 and 1:160,
respectively,
determinedby
a plaque neutralizationtestfordengue virus type 2(14).
Detection of
Fc,,
receptors. Fcreceptorswereanalyzed
by
quantitative flow cytometry with a monoclonal
antibody,
MAb 32,
kindly
provided by
PaulGuyre
of Dartmouth MedicalSchool, Hanover,
N.H.(1).
MAb32 is anIgGl
antibody which reacts with the
high-affinity
FcreceptoronU937cellsand humanmonocytes
(1).
Cells(2.5
x106)
wereincubated with MAb32 at afinaldilution of1:3 in 150
RI
ofRPMI 1640
containing
40mgofhuman-y-globulin
permlat4°C for2 h. Cellswere washed twicein
phosphate-buffered
saline
containing
2% bovine serum albumin and incubated with 100 ,ul ofFITC-conjugated
goatF(ab')2
anti-mouseIgG
antibody (Caltag
Laboratories,
South SanFrancisco,
Calif.)
at a final dilution of 1:30 at
4°C
for 2 h. Cells were thenwashed two times in
phosphate-buffered
salinecontaining
2% bovine serumalbumin andfixedwith 2%paraformalde-hyde. Stained cells were
analyzed
with a fluorescence-activated cell sorter(Coulter
753;
Coulter,
Hialeah,
Fla.).
Quantitative fluorescein microbeads
(Flow
Cytometry
Stan-dards Corp., Research
Triangle
Park,
N.C.)
were used forcalibration to convert
the
fluorescenceintensity
values ob-tained fromtheflow cytometerintothe numberof molecules of secondantibody
bound per cell. Since the number of molecules ofsecondantibody
boundper cellisexpected
to beproportional
to the number of molecules of the firstantibody, which is the MAb32 tothe
high-affinity
Fc recep-tor, the numberof secondantibody
molecules boundpercellcan serve as a relative measurement ofthe number ofFc receptors percell.
RESULTS
IFN--yaugmentsdenguevirus infection of U937 cells inthe presence ofanti-dengue
antibody.
U937 cellswere incubatedwith 100 U ofrIFN--yper ml for 24 h and then infected with
denguevirusat an MOI of5 PFU percellin the presence of anti-dengue mouse serum. The percentage of
dengue
anti-gen-positive cells was determined
by
indirectimmunofluo-rescence
staining
24 hafterinfection.Anti-dengue
serum atfinal dilutions of
1:103,
1:104,
and 1:105augmented
infectionofnontreatedU937 cells and further
augmented
dengue
virus infection when U937 cellswerepretreated
withrIFN--y
(Fig.
1). Normal mouse serum, which did not contain detectable
levelsof
anti-dengue
antibody,
didnotaugmentdengue
virusinfection ofthe nontreated orthe
IFN--y-treated
U937 cells (datanotshown).
Based ontheseresults,
wedecided touseanti-dengue
serum at afinaldilutionof 1:104
inthefollowing
experiments.
U937 cells were incubated with variable amounts of rIFN-y for24 hand infected with
dengue
virusatanMOI of5 PFU per cell in the presence of the
anti-dengue
mouse serum at afinaldilutionof1:104.
Pretreatment of U937 cells with rIFN--y at concentrations from 1 to10,000
U/ml
in-creasedthepercentage of
dengue
antigen-positive
cells.Thepercentage of
antigen-positive
cells reached a maximum levelbypretreatment withIFN--y
at 100U/ml
(Fig.
2).
When U937 cellswereinfected withdengue
virus in the absence ofanti-dengue
antibody,
pretreatment withrIFN--y
did not increase the percentage ofantigen-positive
cells(1
to 3%without rIFN--y treatment and 1 to 4% with rIFN-y
treat-ment). Pretreatment of U937 cells with 100 Uof
rIFN--y
per ml also increaseddengue
virus titers in the culture fluids when cells were infected with virus in the presence ofon November 10, 2019 by guest
http://jvi.asm.org/
C1)
C-)
0)
0._ a)
01) 0
0)
0) CY)
102
lo,
lo4105
106
No serumDilution of anti-dengue 2 serum
FIG. 1. Effect of dilution of anti-dengueserum ondengue virus
infection of U937 cells. U937 cellswereincubated withorwithout
100U ofrIFN-yperml for 24 h and infected with dengue virusatan
MOI of 5 PFU/ml in thepresenceof variable dilutions of anti-dengue
mouse serum.Thepercentageof dengue antigen-positive cells was
determined by indirect immunofluorescence staining 24 h after infection. Symbols: 0, U937 cells pretreated with rIFN-,y; 0,
nontreated U937 cells. Thepercentageof antigen-positive cellswas
compared between IFN-_y-pretreated cells and nontreated cellsat
samedilutions of anti-dengue virustype2serum.P<0.001atserum
dilutionsoflo-3, 10-, and i0-5;P<0.05atserumdilution of 10-6.
P> 0.05(not significant) at aserum dilution of 10-2 and withno serum.
antibodybutnot when cellswereinfectedin theabsence of
antibody (Table 1).
IFN--y producedbydengue-immunelymphocytesafter stim-ulationwith dengue antigen augments dengue virus infection of U937 cells. IFN--y produced by human lymphocytes in vitro wasused in the following experiments. PBMC from a
dengue antibody-positive donorwere cultured with dengue
antigen for 7 days, and the culture fluid was collected. The
[image:3.612.71.284.68.241.2]culture fluid contained 650 U of IFN-y per ml and no
TABLE 1. Virus titers in the culture fluids of dengue virus-infected U937 cells pretreated with IFN-ya
Virus titer(PFU/ml)
IFN-y(U/ml) With anti- Without
dengueantibody antibody
0 5.0 x 103 3.5 x 102
1 4.5 x 104 4.0x 102
10 6.0 x 104 6.5 x 102
100 9.0 x 104 7.0 x 102
1,000 1.0 x 105 6.0 x 102
aU937cellswereincubated withvarious concentrations ofrIFN-,y for 24 h andinfected withdengue virusat anMOIof5 PFUpercell inthe presenceof anti-dengue mouse serum at final dilution of l0-4 or in the absence of
antiserum. Cells wereculturedat3 x 105cellsper ml in RPMI 1640containing
10% fetal calfserumfor 24 h.Titers ofdengue viruscontained in the culture
fluidsweredetermined byusingaplaquetitrationassay.
detectableIFN-aasdeterminedbyradioimmunoassays spe-cific for humanIFN--yandIFN-ao. U937 cellswereincubated for 24 h with various dilutions of this culture fluid and infectedwithdengue virus-antibody complexes. Thediluted culture fluids fromlymphocytes of the dengue-immune
do-nor that had been stimulated with dengue antigens and
contained 10or 100 U ofIFN--y per ml augmented dengue virus infection of U937 cells aswell as recombinant IFN--y
(Table 2).
Anti-IFN-y antibody inhibits IFN-y-inducedaugmentation ofdengue virus infections. To confirm that the IFN--y
con-tained in the culture fluid is responsible for theaugmented denguevirus infection shown in Table2,culture fluid which contained 10 U of IFN--y per ml was incubated with a
monoclonalanti-IFN--y antibody and then usedtotreatU937 cells. Culture fluid pretreated with an anti-IFN--y antibody
didnot augmentdengue virusinfection,but the culture fluid pretreated with an anti-IFN-cx antibodydid augment infec-tion(Table 3). We also triedtoblock the effect ofrIFN--yby usingamonoclonalanti-IFN--y antibody toconfirm that the
rIFN-y-inducedaugmentation of infectionwasduetoIFN--y and not toother substances derived from the productionof this rIFN-yinEscherichia coli (3). U937 cellswere
pretrea-8
50-g40
-Q
4) 30
-X
20-cr
CD1
0 0.01 0.1 1 10 100 1000 10000
Conc. of IFN-t (U/ml)
FIG. 2. Dengue virus infection of U937 cells pretreated with IFN--y. U937 cells were incubated with variable concentrations of
IFN-y for 24 h and then infected with dengue virus-antibody complexesatanMOIof 5 PFUpercell. Thepercentageof dengue antigen-positive cells was determined by indirect
immunofluores-centstaining 24 h after infection. Thepercentageof antigen-positive
cellswascompared between IFN--y-pretreated cellsand nontreated
cells. P < 0.001 at IFN-y concentration of1, 10, 100, 1,000, and
10,000 U/ml.P>0.05 (not significant) at0.01and0.1 U/ml.
TABLE 2. Culturefluidofdengue-immune donorPBMC stimulated withdengue antigenaugmentsdengue
virus infection of U937 cells
Source ofIFN-y Titer(U/mI) antigen-positive cells"% of dengue
None 0 11.5
Dengueculturefluid" 1 13.2'
10 21.6
100 32.6e
RecombinantIFN--y 100 42.6e
"U937cells were incubated withrIFN-yorwithdenguevirus-stimulated culturefluidfor24h andtheninfectedwithdengue virus-antibody complexes
atanMOIof5PFUpercell.The percentage ofdengue antigen-positivecells was determined byindirect immunofluorescence 24 h after infection. The
percentage ofantigen-positivecells wascomparedbetweenIFN-y-pretreated cellsand nontreated cells.
bThe PBMC from adengueantibody-positivedonor were cultured with dengue antigenfor 7days,andculturefluidwascollected. This culturefluid, which contained IFN-y at atiter of 650 U/mland nodetectable IFN-a as
determinedbyradioimmunoassay, wasdiluted tocontain 1, 10, or 100 Uof
IFNper mlforpretreatment of U937 cells. ' P>0.05(notsignificant).
dp<0.01.
eP<0.001.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.319.558.93.182.2] [image:3.612.70.290.502.643.2] [image:3.612.317.557.539.612.2]TABLE 3. IFN--y contained in the culture fluid of PBMC is responsible for augmentingdengue virusinfectionof U937cells
SourceofIFN--y Antibodies' %ofdengue
antigen-positivecells'
Dengue culturefluidc None 66.0
Anti-IFN-y 20.5
Anti-IFN-a 59.6
Control culture fluidd None 20.9
None None 15.0
Anti-IFN-y 16.4
Anti-IFN-ot 12.1
a Dengueculturefluid which containedIFN-y wasdulutedto 10U/ml and thenincubated with1,000Uof monoclonal anti-IFN-yper mland2,000 Uof anti-IFN-otat4'C for2h.
b U937 cellswereincubated with culture fluids for24 handinfectedwith
denguevirus-antibody complexesat anMOI of5PFUpercell.The percent-ageof dengueantigen-positive cellswasdetermined by indirect
immunofluo-rescentstaining24hafter infection.
' Dengue culture fluidwasobtainedasdescribed in footnotebof Table 2 and wasdilutedtocontain10 Uof IFN-yperml.
dPBMCof thesamedonorwerecultured withacontrolantigen for7days,
and a culture fluid was collected. This culture fluid, which contained no
detectableIFN-yorIFN-ca,wasdilutedsimilarly.
ted with 10 U of
rIFN-y
which had been incubated with amonoclonal IFN--y antibody or a polyclonal anti-IFN-ax antibodyper ml. An
anti-IFN-y
antibody inhibited theaug-menting effect ofrIFN--y, but
anti-IFN-ao
antibody had noeffect(datanotshown). These results confirmthat IFN--yis
responsible forthe augmentation of dengue virus infection shownin Table 2 and Fig. 1 and 2.
Human
y-globulin
blocksIFN-ly-induced
augmentation of dengue virus infection. It has been reported that IFN--yincreases Fcy receptors on
U937
cells (6, 17). We tried todeterminewhether theIFN--y-induced
augmentation
of den-gue virus infection was Fc receptor mediated. U937 cells that had been treated with 100 UofrIFN-yper ml for24 h were incubatedwith-y-globulin
at4°C for20 min. Cellswerethen infected with dengue
virus-antibody
complexes.-y-Globulin inhibited infection by dengue virus-antibody
com-plexes of U937 cells that were pretreated with IFN--y,
whereas bovine serum albumin at the same concentration
had no effect (Table 4). These results suggest that the
IFN-y-induced augmentation of dengue virus infection is
mediated by Fcy receptors.
[image:4.612.56.298.95.205.2]IFN-,y does not augmentdengue virus infectionofU937 cells in the presence of the
F(ab')2
fraction of anti-dengue IgG antibody. We then used theF(ab')2
fraction ofanti-dengue IgG toconfirmthatIFN--y-induced augmentation ofdengueTABLE 4.
Inhibition
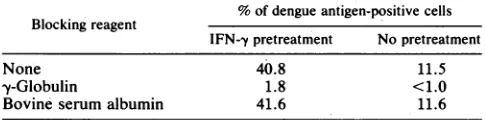
ofIFN--y-induced augmentation of dengue virus infection by human-yglobulina% ofdengueantigen-positivecells Blockingreagent
IFN-ypretreatment No pretreatment
None 40.8 11.5
y-Globulin 1.8 <1.0
Bovineserumalbumin 41.6 11.6
aU937cells thathad been treatedwith 100 UofrIFN--yper mlfor24 hwere
incubated with humany-globulin (10mg/ml) orbovineserumalbumin(10 mg/ml)at 4'Cfor20 min. Cellswere infected withdengue virus-antibody
complexesat anMOI of 5PFU per cell. The percentage ofdengue
antigen-positive cells was determined by indirect immunofluorescence 24 h after
[image:4.612.314.557.103.218.2]infection.
TABLE 5. F(ab')2 preparedfrom anti-dengue IgG does not augment dengue virus infection of U937 cells
pretreatedwithIFN--ya
% ofdengue antigen-positive cells Final concn of
IgG and F(ab')2 IFN-y pretreatment No treatment (,ug/ml)
IgG F(ab')2 IgG F(ab')2
0 1.0 1.0 1.4 1.4
0.001 1.3 1.3 1.0 0.5
0.01 6.2 0.9 3.5 1.5
0.1 36.5b 2.1 9.8b 1.2
1 73.7b 0.4 34.2b 1.2
10 36.5 <0.3 16.8 <0.3
100 11.6' 0.4 7.0c <0.3
a U937 cells were incubated with or without100U/ml ofrIFN-y for24h and infected with dengue virus at an MOI of 5 PFU/ml in the presence of various concentrations of purifiedanti-dengue IgG or F(ab')2 prepared fromIgG. The percentageof dengueantigen-positive cellswasdetermined24 h after infec-tion.The percentage of antigen-positivecellswascompared between IFN-y-pretreated cells and nontreated cells at same concentrations of IgG and
F(ab')2-bp< 0.001.
'P<0.005. Results were notsignificant (P>0.05)exceptwhereindicated.
virus infection is
FcY
receptor mediated. Pretreatment of U937 cells withIFN--ydidnotaugmentinfection when cells were infected with dengue virus in the presence ofF(ab')2prepared from anti-dengue IgG, but
IFN-y
pretreatment augmented infection when cells were infected with virus in the presence of purified anti-dengue IgG at0.1 to 10,ug/ml (Table 5). This result confirms that the IFN-y-induced aug-mentation of dengue virus infection is mediated by Fcy receptors on U937 cells.Augmentation of dengue virus infection
correlates
with increase in the numberof Fc,, receptors. We tried to deter-mine whether there isa correlation between the number ofFcY
receptors and the percentageof dengueantigen-positivecells. U937 cells were incubated with variable
concentra-tions of
IFN-y
for 24 h and examined forFcY
receptorexpression by quantitative fluorescence-activatedcellsorter
analysis after exposure to MAb32, which is specific forthe human Fc
YR1.
The percentage ofantigen positive cells wasdetermined24hafterinfection.Therewas agood correlation
between the percentageofdengueantigen-positive cells and
the number of
FcY
receptors(Fig. 3).This resultisconsistent with those shown in Tables 4 and 5 and indicates thataugmentation ofdengue virus infection induced by
IFN-y
is mediated byFcY
receptors.DISCUSSION
In this report we demonstrate that IFN--y augments den-gue virus infection of U937 cells in the presence of
anti-dengue antibodies. This effect is Fcy receptor mediated,
because (i) IFN--y had noaugmenting effectonthe infection of U937 cells when cells were infected with dengue virusin
the absence of anti-dengue antibody, (ii)
IFN-y
did not augment dengue virus infection when cells were infected with virus complexed to the F(ab')2 fraction prepared fromanti-dengue IgG, (iii) IFN--yhad no
augmenting
effect whenFcy
receptors onU937cellswereblockedby-y-globulin,and (iv) there was agood correlation between the percentage of dengue antigen-positive cells and the number ofFcy recep-tors on U937 cells. We observed that IFN--y increased the number ofFcy
receptors on U937 cells, aspreviously
reported by other investigators (6, 17). Based on theseon November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.57.300.618.678.2]50
M.
W0
12.Ox140
°-CM
C30
"O*0~~~~~~~~~~~~~~~~~
.0 CD
-10
0 0.1 1 2 5 10 100
Conc. of IFN-7 (U/ml)
FIG. 3. Correlationbetween IFN--y increasedFcY receptorsand
dengue virus infection. U937 cells were incubated with variable titers of IFN-y for 24 h. Cells were infected with dengue
virus-antibody complexes at an MOI of5 PFU/cell. The percentage of
dengue antigen-positive cellswas determined by indirect
immuno-fluorescentstaining24 hafterinfection. The relative numbers ofFcY
receptors wasmeasuredby quantitativefluorescence-activated cell
sorteranalysis asdescribedin Materials andMethods.
observations,weconcludethat theincrease in the numberof
Fc,, receptors on U937 cells induced by IFN-y leads to an
augmented uptake of dengue virus in the form of dengue virus-antibodycomplexes, whichresults inahigher
percent-age of dengue virus-infected cells and in higher yields of infectiousdengue virus. IFN--yalsoaugmented dengue virus
infection of human monocytes enriched from peripheral blood mononuclear cells, when monocytes were infected
withvirus inthepresence ofanti-dengue antibodies. IFN--y didnotaugmentdengue virus infection of humanmonocytes whencellswereinfectedinthe absence of antibody (datanot
shown).
It has been reported that IFN-a has no or little effect on
thenumberofFc, receptorson monocyticcells (6, 17). We
foundthatIFN-o suppresseddengue virusinfection of U937 cells at doses higher than 1 U/ml even when cells were
infected in the presence of anti-dengue antibody (data not
shown). We attribute this suppressive effectto the antiviral activity of IFN-a. We recently reported that high levels of IFN-a were produced by dengue virus-infected monocytes
(13) andby HLA DRantigen-positive, non-T lymphocytes culturedwithdengue virus-infectedmonocytes(12); further-morethe IFN-a producedwasactive in limiting infection of human monocytes bydengue virus (12, 13).
Ithas been hypothesized that increased infection of
mo-nocytes with dengue virus in the form of dengue virus-antibody complexesmay occurin vivoand playanimportant
role inthe pathogenesis of DHF-DSS (8). This is supported by epidemiological studies, which reported that most cases
of DHF-DSS occur during secondary dengue infections when anti-dengue antibodies arepresent(2, 9, 18). We have reported that IFN--y is produced by previously sensitized
humanTlymphocytes aftersecondaryantigenic stimulation (5, 20). We have also found that the T lymphocytes of
individualswhohave antibodiestodengueviruses
prolifer-ate andproduce high titers ofIFN-y after stimulation with dengue antigens in vitro (Kurane et al., submitted). The IFN--y produced bydengue virus stimulation of immune T
cells was active in augmenting dengue virus infection of
U937 cells andhuman monocytes. Therefore, we
hypothe-size thatIFN--y is producedby dengue-specific T
lympho-cytes during secondary dengue infections after stimulation
with conserved dengue antigens and that the IFN--y
pro-ducedmightcontributetothepathogenesisof DHF-DSSby
enhancing
Fc,,
receptor expression on human monocytes, thereby increasing the number of infected monocytes andthe yields of infectious dengue virus in the presence of anti-dengue virus antibodies.
ACKNOWLEDGMENTS
We thank Paul Guyre of Department of Physiology, Dartmouth Medical School, for providing monoclonal antibodies, Marcia Mc-Fadden for fluorescence-activated cell sorteranalysis, Jurand Janus for technical assistance, and Judy Gully for editorialassistance.
This research has been supported by grant DAMD 17-86-C-6208 from the Department of Army and Medical Research and Develop-ment and by Public Health Service grant NIH-T32-AI 07272 from the National Institutes of Health.
LITERATURE CITED
1. Anderson, C. L.,P. M.Guyre, J. C. Whitin, D. H.Ryan, R.J. Looney, and M. W. Fanger. 1986. Monoclonal antibodies to Fc receptors forIgG on human mononuclear phagocytes: antibody characterization and induction of superoxide production in a monocyte cell line. J. Biol. Chem. 261:12856-12864.
2. Burke, D. S., A. Nisalak, D. E. Johnson, and R. M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38:172-180.
3. Caray, P. W., D. W. Geungi, D. Pennica, R. Yelverton, R. Narajan, C. C. Simonsen, R. Derynck, P. J. Sherwood, D.M. Wallace, S. L. Berger, A. D. Levinson, and D. V. Goeddel. 1982. Expression of human immune interferon cDNA in E. Coli and monkey cells. Nature (London) 295:503-508.
4. Daughaday, C. C., W. E. Brandt, J. M. McCowan, and P. K. Russell. 1981. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistent immune complex receptors. In-fect. Immun. 32:469-473.
5. Ennis, F. A., and A. Meager. 1981. Immune interferon produced to high levels by antigenic stimulation of human lymphocytes with influenza virus. J. Exp. Med. 154:1279-1289.
6. Guyre, P. M., P. Morganelli, and R.
Miller.
1983. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J. Clin. Invest. 72: 393-397.7. Halstead, S. B. 1979. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J. Infect. Dis. 140:527-533.
8. Halstead, S. B. 1981. The pathogenesis of dengue: molecular epidemiology in infectious disease. Am. J. Epidemiol. 114:632-647.
9. Halstead, S. B., S.Nimmannitya, and S. N. Cohen. 1970. Obser-vations related to pathogenesis of dengue
hemorrhagic
fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J. Biol. Med. 42:311-328.10. Halstead, S. B., E. J. O'Rourke, and A. C. Allison. 1977. Dengue viruses and mononuclear phagocytes.
I.
Infection enhancement by non-neutralizing antibody. J. Exp. Med. 146:201-217. 11. Halstead,S. B., E. J. O'Rourke, and A. C. Allison. 1977. Dengueviruses and mononuclear phagocytes.
II.
Identity of blood and tissue leukocytes supporting in vitro infection. J. Exp. Med. 146:218-229.12. Kurane, I., and F. A. Ennis. 1987. Induction of interferon-a from human lymphocytes by autologous, dengue virus-infected monocytes. J. Exp. Med. 166:999-1010.
13. Kurane, I., and F. A. Ennis. 1988. Production of interferon alpha by dengue virus-infected human monocytes. J. Gen. Virol. 69: 445-449.
14. Kurane, I., D. Hebblewaite, W. E. Brandt, and F. A. Ennis. 1984. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-cell-mediated cytotoxicity. J. Virol. 52:223-230.
15. Kurane, I., A. Meager, and F. A. Ennis. 1986. Induction of interferon alpha and gamma from human lymphocytes by den-gue virus-infected cells. J. Gen. Virol. 67:1653-1661.
.1-0
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.64.305.70.200.2]16. Monath, T. B. 1985. Flaviviruses,p.955-1004.InB. W. Fields (ed.), Virology. Raven Press, Inc., New York.
17. Perussia, B., E. T. Dayton, R. Lazarus, V. Fanning, and G. Trinchieri. 1983. Immune interferon induces the receptor for monomeric IgGl on human monocytic and myeloid cells. J.
Exp. Med. 158:1092-1113.
18. Sangkawibha, N., S. Rojanasuphot, S. Ahandrik, S.
Viriya-pongse,J. Jatanasen, V.Salitul, B. Phanthumachinda, and S. B. Halstead. 1984. Risk factors in dengue shock syndrome: a
prospective epidemiologic study in Rayond, Thailand. Am. J. Epidemiol. 120:653-669.
19. Sundstroem, C., and K. Nilsson. 1976. Establishment and char-acterizationofahuman histiocytic lymphoma cell line (U937).
Int. J.Cancer 17:565-577.
20. Yamada, Y. K., A. Meager, A. Yamada, andF. A.Ennis. 1986. Humaninterferon alpha andgammaproduction by lymphocytes during the generation of influenza virus specific cytotoxic T
lymphocytes. J. Gen. Virol. 67:2325-2334.