Beta and Gamma Interferons
Act
Synergistically
To
Produce
an
Antiviral State in Cells Resistant
to
Both
Interferons Individually
JOHN A. LEWIS,*AFROZA HUQ,AND BEI SHAN
Department ofAnatomyand Cell Biology, SUNYHealth Science CenteratBrooklyn, 450ClarksonAvenue,
Brooklyn, New York 11203
Received 26 April 1989/Accepted 5 July 1989
We showed previously thatthemousefibroblastoidcell line Ltk-aprt- is resistanttothe antiviraleffectsof betainterferon. This lackofresponsereflects apartial sensitivity tothe interferon that is accompanied bya
failuretoactivate expression ofseveralinterferon-regulated genes,althoughcertain othergenesrespondina
normal manner. We show here that Ltk-aprt- cells were also unableto establish anantiviral state and to activate expression of 2,5-oligo(A) synthetase when treated with gamma interferon. Strikingly, however,
treatmentwithacombination ofbetainterferonandgammainterferonprovided complete protection against viralreplication. Although thecellswerecompletely insensitivetoupto250 U of the interferonsperml added
singly, essentiallycomplete protection from viral cytopathiceffectswasachieved whenaslittleas10 U of each
of the interferons per ml were combined. Expression of 2,5-oligo(A) synthetase was also sensitive to this
synergistic effect. Activation ofanantiviral state could also be achieved by sequential treatment, first with
gammainterferon andthenwithbetainterferon. Partial protection against viral replication could be achieved
bypretreatmentwithgammainterferon foraslittleas 1hbeforeincubation with beta interferon and could be
blockedby the addition of specific antibodiesorbycycloheximide, indicating thatgamma interferon induces thesynthesis ofaprotein whichcanactsynergistically withasignal produced by the beta-interferonreceptor. We suggestthatLtk-aprt-cells sufferfrom defectsinone or morecomponentsofthegeneactivation pathways
for both type Iandtype IIinterferons. Nonetheless, gammainterferonisabletoactivate the expression ofa
gene encoding a protein required for signal transduction. This protein acts synergistically with a transient signal producedinresponsetobeta interferon, thereby activating theexpression ofafurthergroupofgenes. Interferons (IFNs) induce the production ofan antiviral
state by binding to high-affinity cell surface receptors and thereby activate the expression of several genes encoding
enzymes with antiviral capacities (25). Although several
IFN-responsivegeneshave been cloned and theirupstream
regulatoryelements have beendefined, little is knownabout
the process of signal transduction which couples the IFN
receptors with transcription activation factors.
Characteri-zation of these signals and the transcription factors with which they interact isamajor goal. Achieving this aim would
be facilitated by the availability of cell variants which are
defective in theirresponses toIFNs but, preferably, canbe
manipulatedtorespondunderappropriatestimuli. We have beenstudyingthe effectsofIFNson avariant cell line which fails toproduce an antiviral state when treated with either
betaorgammaIFN
(IFN-P
orIFN--y) (30, 31). Aswe showhere, however, a strongantiviral effect wasproduced when thecellswereexposedtoacombination of thesetwoIFNs. Our results suggest that IFN--y induces the synthesis ofa protein which acts synergistically with a signal induced by IFN-P to activate gene expression. This cell line may be
ideally suited for dissecting the pathways by which IFNs modulate gene expression, in order to identify the signals
andtranscriptionfactors involved.
To understand these pathways completely, it will be
necessaryto accountfortheeffects ofthe different classes of
IFNsonthe variousgeneswhichthey regulate.Three types of IFNs are recognized, according to the nature of the
producing cells and the stimulus for production. Thus,
IFN-a isproduced byvirus-infectedleukocyteswhileIFN-,
issynthesized byfibroblastoid cellsexposedeithertoviruses
*Correspondingauthor.
or to double-stranded RNA. These IFNs are very similar
chemically andgeneticallyandindeed bindtothesamecell
surface receptors in both human and mouse cells (5, 20).
IFN--y is quite distinct from these type I IFNs since it is
produced by a subpopulation of lymphocytes stimulated
with mitogens or specific antigens and appears to be
in-volved in immune andinflammatory responses. IFN--y not
only differs from IFN-a and
IFN-P
in its chemical andgenetic properties but also binds toa separate cell surface receptor (1, 3, 5, 20, 37, 41). Although all three species of IFNs induce theexpression ofasimilarsetofproteins,there
are several differences in the nature of the responses ob-served (48). Some proteins are induced preferentially by IFN--y (7, 38),whileotherproteinsareinducedbyIFN-a and
IFN-P
butnotby IFN--y (8, 21, 44). Moreover, therelativepotency of different IFNsinactivating particularresponses (e.g., activation ofmajorhistocompatibility antigen
expres-sion)varies (47). Forgenes regulated byall threeclasses of
IFNs,the mechanismsbywhich type I andII species bring
about induction may be somewhat different, since protein synthesisinhibitors block induction ofsomegenesbyIFN-y while theresponsetoIFN-a isnotaffected(11, 21-23).It has also been shown that the simultaneous addition of different IFNs can lead to synergistic effects (10, 13-15, 23, 52), suggesting that different mechanisms of action may be in-volved.
We havepreviously characterized a mutantmouse fibro-blastoid cell line, Ltk-aprt-, which is refractory to the antiviral effects ofIFN-P (30, 31)while stillexhibitingother
responses,includingcellgrowthinhibition and activationof
at least one gene, 1-8, to the same level as that seen in sensitive cells(42). By transfecting specificDNAsequences into these cellswe havebeen able to restore thecapacityof 4569
0022-538X/89/114569-10$02.00/0
Copyright© 1989,American Societyfor Microbiology
on November 10, 2019 by guest
http://jvi.asm.org/
IFN-, to activate antiviralresponses (26, 30,31).The lack of antiviraleffects inLtk-aprt- cellscorrelateswith afailure of IFN-,B to induce at least three enzymes with established antiviralproperties (2, 8, 31). Severalother geneswhichare usuallyregulated byIFN arealso refractorytoinduction in this cell line, including both positively (42) and negatively (B. Shan andJ. A. Lewis, manuscriptinpreparation) mod-ulated species. Since several genes are affected it is likely that some step in the signaling pathway between the cell surface receptor and the genome isinvolved. The fact that the cells are at least partially responsive to IFN-, (42)
indicates-thatfunctional cell surface receptors are present. As we show here, Ltk-aprt- cells are also resistant to the antiviral effects of murine
IFN-y.
Remarkably, however, treatment of these cells with acombinationoftype I and type IIIFNS provides completeprotectionagainst viralinfectionandinduces mRNAsin amannersimilar to that seen in cells whichrespond normally toIFNs.
MATERIALSAND METHODS
Cells, cell growth, and IFNs. The origin of the
Ltk-aprt-cells and conditions of growth have been described previ-ously (30). Murine
IFN-P
(5.6 x107
U/mg of protein) waspurchased from Lee Biomolecular Research, San Diego, Calif., and the titer was determined against the National
Institutes of Health murine IFN-,B standard preparation (GbO2-902-511)onL-929 cells withvesicular stomatitisvirus (VSV) as described previously (27). Murine IFN--y was prepared fromsupernatants of aChinesehamsterovary cell line expressing a recombinant murine IFN-,y cDNA under control of the simian virus 40 late promoter (35). This cell line was a generous gift from Alan Morris (University of
Warwick, Coventry, England). Partial purification was achieved bychromatography onCibacron Blue-Agarose
and.
elution with 50% ethylene glycol in 2.0 M NaCl to give a
preparation with a specific activity of 5 x
10'
U/mg ofprotein. Titersarereportedwithrespectto themouse
IFN-1
standard and were determined by using the same assaydescribedforIFN-1,so theantiviral potenciesofourIFN-1
and IFN--y preparations were equivalent. Apolyclonal anti-IFN-3 serum was purchased from Lee Biomolecular
Re-search. Amonoclonal antibody, HB107, specific formurine
IFN--y was prepared from culture supernatants of a
rat-mouse hybridoma, HB107 (43).
Assays of antiviral activities. VSV was grown in L-929
cells, and the titer was determined by conventional plaque assayinthe same cells (27). Sensitivitytovirusinfectionwas
assayedbymeasuring cytopathiceffects, usingmethylviolet staining (27). Briefly, cells weregrown toconfluency in 24-or96-well dishes, treatedwithcombinations ofIFNs for the
times indicated, and then infected with VSV at 10 PFU per cell. After adsorption for 1 h, the virus inoculum was
removed and virus growth wasallowed toproceed for 24 to 48 h before staining with 0.25% methyl violet in 50% ethanol-0.9% NaCl-2% formaldehyde. The dishes were
thoroughly washed in H20, and the dye was eluted in50% ethanol-0.5MNaCl andquantitatedbyA570. Assaysof virus protein synthesis were performed in 24-well cultures. Cells were treated with IFNs, infected with 10 PFU of VSV per cell, and radiolabeled from 3.5 to 6 h postinfection with 10
pLCi
of[35S]methionine
and[35S]cysteine (35S-Translabel;
ICN) per ml in medium lacking methionine. The cells were
lysed in 1% Nonidet P-40-0.25% sodium deoxycholate-10
mM Tris hydrochloride (pH
7.5)-15
mM NaCl-1.5 mMMgCl2-1 mM phenylmethylsulfonyl fluoride-15 U of
Tra-sylolperml,andafterremovalof nucleiby centrifugationthe extractswereanalyzedby sodium dodecyl
sulfate-polyacryl-amidegel electrophoresis andautoradiography
(27).
Assay of 2,5-oligo(A) synthetase. 2,5-Oligo(A)synthetase
assays were performed by a modification of aprocedure
describedpreviously (31).Extractswerepreparedexactlyasdescribed(31), but synthesis of3H-labeled 2,5-oligo(A) was
performed in a solution assay. The extracts (25 ,ul) were
incubated in a final volume of 50 ,ul containing 10 mM HEPES
(N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic
acid)-KOH
(pH7.5)-90
mM KCl-10 mMmagnesium
ace-tate-7 mM 2-mercaptoethanol-1 mM [3H]ATP (40
Ci/mol)
with or without poly(I- C) (10,ug/ml).
Incubation was at30°C for60
min,
andreactionswereterminatedby heating
to90°C.
Denaturedproteinwasremovedby centrifugation,
and3H-labeled
2,5-oligo(A) was determined by binding toDEAE-cellulose and elution with 0.34 M KCl as described
previously
(J1).
Measurement of mRNA levels. Monolayers of
Ltk-aprt-cells (10-cm culture dishes) were treated with IFNs and washed with cold phosphate-buffered saline, and the cellswere lysed in 4 M guanidinium isothiocyanate-100 mM 2-mercaptoethanol. The extracts were layered overa
cush-ion of5.7 MCsCl-25mM sodium acetate and centrifugedat
35,000
rpminaBeckman SW50.1rotorfor18 hat20°C. Thepelleted RNA was dissolved in 0.3 M sodium acetate, ethanol precipitated, and analyzed by electrophoresis on
1.3% agarose gels after denaturation in
formamide-formal-dehyde. The RNA was transferred to Nytran paper
(Schle-icher & Schuell, Inc., Keene, N.H.) and hybridized with a
nick-translated
probe
as describedpreviously
(28).
RESULTSLtk-aprt- cells are resistant tothe antiviral effectsof
IFN-'Y.
We havedemonstrated previously thatLtk-aprt-cells failtoestablishanantiviralstatewhentreated withup to2,000 U of
IFN-P
per ml (30). The parental L-929 cell line is stronglyLtk-
aprt-VW- D
.:
e* hqt/,, ! ., l0 W., tr
L992
to
nU
of4v. - - go
N wwwww -F wVP
- *
M lw
FIG. 1. Ltk-aprt- cells are resistant to the antiviral effects of IFN--y. Ltk-aprt- cells were treated for 18 h with IFN-1 (lanes a to e) or IFN-,y (lanes f to j) at 0 (lanes a and f), 5 (lanes b and g), 25 (lanes c and h), 100 (lanes d and i), and 250 (lanes e andj) U/ml and then infected with VSV (10 PFU per cell). The cells were radiola-beled with a mixture of [35S]methionine and [35S]cysteine between 3.5 and 6 h after infection, and extracts were prepared for analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. The positions of viral proteins are indicated at the left. Extracts of uninfected cells are shown(ni).To demonstratethe efficacy of the IFNs, their effects on VSV protein synthesis in L-929 cells are shown: no addition (lane k), 100 U ofIFN-Pper ml(lane 1), 100 U ofIFN-1 per ml with polyclonal antibody toIFN-P(lane m), and 100 U of IFN--y per ml (lane n).
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.568.317.497.467.597.2]TABLE 1. Synergistic effects ofIFN-Pand IFN--y inpreventing VSV replicationa
TreatmentTreatment
~~~~(PFU/ml)
VSVyield reductionFoldNone 5.8 x 108
100 UofIFN-P/ml 5.6 x 108 1
100 Uof IFN--y/ml 1.4 x 108 4
100U ofIFN-P/ml + 100 U 1.6 x 106 363 ofIFN--y/ml
aLtk-aprt-cells weretreated withIFNs for 18 h andthen infected with
VSV (10PFU percell). After24 h themonolayerswerefrozenandthawedin
their medium and the yield of VSVwasdetermined by plaque assayonL-929 cells.
protected against VSV and mengo virus infection by 5 to 10 U of
IFN-P
per ml under the same conditions, with our laboratory endpoint being typically 3 reference units per ml. When Ltk-aprt- cells were treated with up to 250 U ofIFN-y
perml there wasonly a very slightreductionin the synthesisofVSVproteins (Fig. 1),indicatingthat these cells are also
essentially unable to respond to
IFN-y
by producing anantiviralstate.In contrast, 100 Uof
IFN-y
permlcompletelyabolished synthesis ofVSV proteins in the sensitive L-929
cell line. Measurements of virus yield also showed that
neither
IFN-P
norIFN--y was able to inhibitvirusreplicationsignificantly in Ltk-aprt- cells (Table 1). The very slight inhibition (two- to fourfold) of VSV production by IFN--y
(Table 1 and Fig. 1) is discussed below. This failure to
activate anappreciable antiviral state in Ltk-aprt- cellswas
also seen when the cells were challenged with a different virus such as Mengo virus (results not shown). As we have shownpreviously, IFN-,B also fails to activate the expression
of several genes in Ltk-aprt- cells, although at least one
gene, 1-8, is sensitiveto induction(42).
Synergistic effects ofIFN-,Iand IFN--y.Although Ltk-aprt-cells were resistant to the effects of IFN-,B and IFN--y when each was added singly, treatment with a combination of these agents brought about complete protection againstthe cytopathic effects ofVSV(Fig. 2). Ifcellsweretreatedwith
100Uof IFN-,B per mltogether with as little as 5 U ofIFN-y
per ml, partial protection against virus-induced cytopathic effects was observed, while treatment with 100 U of both IFNs per ml afforded complete protection. IFNs added
singlytothecellsataconcentration of100U/mlweretotally ineffectual (Fig. 2). A more detailed analysis ofthe dose
requirements for the two IFNs is shown in Fig. 3. When
equal concentrations ofthe two IFNs were seriallydiluted, 50% ofthecellswereprotected fromviralcytopathic effects
by approximately 6 U ofeach per ml (Fig. 3a). Dilutionof
oneofthe IFNs in the presenceofaconstant amountofthe
other indicated thata combinationof10 U ofIFN-, perml
and approximately 5 U of IFN--y per ml was sufficient to
protect50% ofthe cellsagainst the cytopathic effects of VSV (Fig. 3b). In the presence of 10 U of IFN--y per mlsignificant
protectionof cells could be achieved with less than 1.0 Uof
IFN-1
per ml (Fig. 3c) even though no protection wasaffordedby 100U of either of the IFNs per ml addedsingly
(Fig.3d). Thus, Ltk-aprt- cellscanbeprotected against viral
infectionby relativelylowconcentrationsof each of thetwo
2.5
2.0
i.5
0.5
0.0J
FIG. 2. Synergistic effectsofIFN-PandIFN-yonLtk-aprt-cells. Ltk-aprt-cellsweretreatedwith mixturesofIFN-1 andIFN--yfor 18 hasindicated beloweach bar. Numbers refertotheconcentration of eachIFNinunitspermilliliter. Cellsweretheninfected with VSV(10
PFU percell) and stained48hlater, andthedyewaseluted andquantitatedasdescribed inthetext. Aphotographof the wells is shownat
the top of thefigure, witheachwellcorrespondingtothe bar beneath it. The dataarefromatypicalexperiment.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.130.502.400.695.2]1.5
E
£ 1.0
0
I-._oZ
C)
5 0.5
0
0.0
FIG. 3. Synergistic effects of different combinations ofIFN-P andIFN--y. Ltk-aprt-cells cultured in a96-well dish weretreatedwith mixtures of IFN-,B and IFN--y for 18 h and then infected with VSV(1PFU percell).After 48hthe cellswerestained,and thedyewaseluted andquantitatedasdescribed inthetext.Resultsarethemeansofduplicatedeterminations,withreplicates generallywithin7% of themean.
Numbersrefertotheconcentrations ofeach IFNin unitspermilliliter.(a) Cells treated with serial twofold dilutions ofequaltiters(100U/ml)
of thetwoIFNs. Only dilutionsneartheendpointareshown. (b) Cells treated with twofold dilutions of IFN--yin mediumcontaining10U of IFN-,Bperml. (c)Cells treated with twofold dilutions ofIFN-P in mediumcontaining10 UofIFN--yperml. (d)Cells treated with100U of eitherIFN-P orIFN-yper ml ornottreated with eitherIFNsorVSV(cell control).
IFNstogether even though muchhigherdosesareineffectual when the IFN is addedindividually.
Analysis ofthesynthesis ofVSVproteinsshowed thatthis synergistic effect of type I and II IFNs is reflected in a
decreaseintheaccumulation of viralproteins,asexpectedif the translation of viral mRNAs were inhibited (Fig. 4). Although neither
IFN-P
nor IFN--y wasable to prevent thesynthesis of VSV proteins when added to Ltk-aprt- cells
alone, combined treatment with 100 U ofone type of IFN per ml withas little as 5 U ofthe other per ml resulted in stronginhibition ofVSVprotein production (Fig. 4). These results suggest that, as innormally sensitive cells, the IFN
treatmentresultedinactivation of mechanisms which selec-tively prevent viral mRNA translation (26). Addition of a
polyclonal antiserumspecificforIFN-Pwith the mixtureof IFNs abolished the protection against virus replication,
indicatingthat thesynergistic effect of theIFN-Ppreparation was indeed due to the IFN and not to impurities. Similarly,
additionof a monoclonal antibody to murine IFN--y (43) also abolished the synergistic effects, demonstrating that IFN--y andnot a contaminant in the partially purified preparation was the component responsible. In some experiments a
slight reduction of VSV protein synthesis was observed when cells were treated withIFN-y alone (Fig. 1 and Table 1), but this effect could be eliminated if polyclonal antibodies
specific forIFN-3 wereincludedin the medium (Fig. 4). This suggests that the Ltk-aprt- cells constitutively produce a
verysmallamountof
IFN-P
which iscapableofsynergizingwith the added IFN--y. Autocrine responses to endogenous IFNshave been reported previously (16, 49).Measurement of VSVyield furtherdemonstrated the synergistic capacity
of the two IFNs (Table 1). In the experiment for which resultsare shown,IFN-1 hadno effecton virusreplication while IFN--y alone produced a fourfold reduction in VSV
yield. Addition of 100 U of both IFNs per ml produced a
360-fold reduction in virusyield,and 100 U ofIFN-y perml
with 10 U ofIFN-3 per mllowered virusproductionatleast 50-fold.
PretreatmentwithIFN--ysensitizesLtk-aprt- cellstoIFN-,I. The synergistic actions of the two IFNs could also be observed by sequential addition of the individual prepara-tions. When cellswere pretreatedwith 100 U of IFN--y per ml for 2 h or more,complete protection againstviral
cyto-pathic effectswas rendered by subsequent incubation with 100 UofIFN-,B per ml alone (Fig.Sa). Preincubation for as
littleas1 hprovidedsubstantial butpartial protectionunder these conditions, suggesting that a period of 1 to 2 h is needed for the accumulation of sufficientamounts ofsome
IFN-y-induced signaltocomplementtheeffectsofseparate incubation with IFN-P. The effectiveness of the
pretreat-mentvaried somewhat fromoneexperimenttoanother,and in some cases only partial protection was achieved by preincubatingwith 100 U ofIFN--ypermlfor 4 h (seebelow).
With 10 U of IFN--y permllonger periods of preincubation
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.57.559.79.378.2]100
100 I 100 0
1001'
Of
IFNsandantibo..i..a.,indicae for--18...-h...Afe infetio with.VSV
L~~~-n w.- .- _ _ w.
~ ~ ~ ~
..-fIG.e 4.ll SynergistioweferctofpaiFN-,and tFN- ponsynthesisf ofV
VVproteinsar indiLtk-apt- chellst.Cellswere eihrutreated of
tFNsanedatbdeasindicatedwt 0Uofo 18Nh.yMter l nfectio VSV0o
(-)2
100 Uof IFN-13 permlorwith 100 UofIFN-Ppermland0, 1,5, 10,
or100U ofIFN-yperml. Cellswerealso treated with 100 U of both
IFN-Iiand IFN--y per ml togetherwith monoclonal antibodies to IFNs-y (Antib-y) or polyclonal antibodytoIFN-P(Anti-P).
werenecessary toachieve thesamelevelofprotection(Fig.
5a) and complete inhibition ofviral cytopathic effects was
only seen after a 24-h exposure. After pretreatment with IFN--y,inclusion of monoclonal antibodiesspecificforIFN--y
inthesubsequentincubationwithIFN-p didnotsignificantly
affect the development ofan antiviral state (Fig. 5b), indi-cating that the effects weredue to sequential activities and
not to carry-over of theIFN-ty intothe secondincubation.
Asexpected,theaddition ofmonoclonal antibodiesIFN-,yto during thepretreatmentdidblockdevelopmentofan
antivi-ral state (Fig. 5b).
IFN-yinduces thesynthesisofaprotein thatacts
synergis-tically with IFN-te. If the pretreatment with IFN-y was
carried out in the presence of cycloheximide for 4 h, the
synergistic effect was blocked (Fig. Sb), suggesting that
IFN-yinducesthesynthesisofaprotein(orseveralproteins)
which is required for the synergistic interaction with
re-sponsesproduced byIFN-13 in the secondincubation.Inthis
particular
experiment the 4-h pretreatment provided onlypartial protection against VSV, and this was completely
abolishedby cycloheximide. In otherinstances, more
com-pleteprotectionwasaffordedbythe shortpretreatment(Fig.
5a),butcycloheximide onlypartiallyinhibitedproductionof
the ensuing antiviral state (results notshown).Thisfinding is consistentwith the induced synthesis and accumulation of a specific mRNA during the incubation with IFN--y and cyclo-heximide and subsequenttranslation of the mRNA into the active protein during the second incubationwith
IFN-P
after removal of thecycloheximide. The ability of IFN--y toelicit synthesis of a protein when added to Ltk-aprt- cells alone indicates the presence offunctional receptors forIFN--y on the surface of these cells, as shown previously for IFN-, (42). Although IFN--y isunable to activate anantiviral state, the Ltk-aprt- cells express a partial response toitinvolving the production of some factor orsignal, either aprotein or a product of a newly synthesized enzyme, which permits synergistic interaction withsignals produced in response toIFN-P.
IFN--y elicits the production of a stable factor which sensi-tizes Ltk-aprt- cells to IFN-4. To obtainfurtherinformation on the nature of the factor produced by pretreatment with IFN--y, weperformed chase experiments (Fig. 6a). A strong antiviral effect could be achieved by treatment with
IFN-y
for 20 hfollowed bywithdrawal of thestimulus for 8 hbeforethe addition of IFN-,. The level of protection achieved against virus infection was only slightly lower than in cells
treated with IFN--y and then treated immediately with IFN-,B. Withdrawal for shortertimes, such as 6, 4, and 2h, hadno
effect on theantiviral state (results notshown). Remarkably,
pretreatment with
IFN-y
for 4 hfollowed by withdrawal for 24 h and thenincubation withIFN-P
gavethe same level of antiviral activity as did a 4-hpretreatment followed immedi-ately by the additionofIFN-P
(Fig. 6a). This indicates that the factorproducedin response to IFN--yisrelatively stable. Mostlikely thisreflects thesynthesis ofalong-livedprotein,
although we cannotexcludethesynthesis of astablemRNA. IFN-I8 acts synergistically with IFN-,y by producingatran-sient signal. In contrast to the results discussed above,
pretreatment with
IFN-P
for 24 h orfor shorter timesdid notpermit theestablishment of anantiviral state when cellswere
subsequently incubated with IFN-y (Fig. 6b). The distinct behaviour of IFN--y and
IFN-P
in this type of assay suggests differences in the nature of the signals induced bythesetwoagents and indicates that IFN-1 produces a transient signal
which can interact with a stable protein induced by IFN--y. Since double-stranded RNA has been reported to act as a
signal forinduction of genes regulated byIFNs (46, 54), we
tested its ability to actsynergistically with
IFN-P
andIFN--y.Partial protection against viral cytopathic effects could also beobtained by treating Ltk-aprt- cellswithpoly(I C)in the presence ofDEAE-dextran for 1 h followed by incubation
with IFN--y but not
IFN-P
(Fig. 6b). Since this effect ofpoly(I C)could be prevented by including
polyclonal
anti-bodies againstIFN-P
(Fig. 6b), the antiviral response wasalmost certainly due to induction of
IFN-P
production and not to a direct effect of double-stranded RNA. We have previously determined that the Ltk-aprt- cells used in ourlaboratory (30) secrete IFN in response to
poly(I.
C)treat-ment (J. A. Lewis, unpublished observations).
Induction of gene expression by combined treatment with
IFN-I and
IFN-y
Thelevel of2,5-oligo(A) synthetase activ-ity in extractsof Ltk-aprt-cells treated with thetwotypes of IFNs is shown in Table 2. Neither IFN-1 norIFN--y
wascapableofelicitingan increase in
expression
of this enzyme when added to the cellsindividually. The addition of 100 U of both types of IFN per ml together,however,
led to a significant increase in activity. A combination of 100 U of one IFN permlwith 10 U of the other per mlwas sufficient to produce a lower level of induction. The stimulation ofon November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.67.304.77.374.2]a.
b.
71
,&
~,
'r ' IL qa A. .J,r .. I.
-Q ,a,e
nfl1
[image:6.612.51.553.72.461.2]cJ
FIG. 5. Pretreatment ofLtk-aprt-cells with IFN--y permitsestablishment ofanantiviral state on subsequentexposure to IFN-1. (a) Ltk-aprt- cellswerepreincubatedwith 100 U(open bars)or10 U(hatched bars)ofIFN--yperml for the times indicated. The IFNwasremoved
andreplaced bymediumcontaining100 U ofIFN-,Bperml. After 18 h the cellswereinfectedwithVSV(10PFUpercell),and 48 h laterthey
werestained andthe dyewasquantitated. Each barrepresentsthemeanofduplicatedeterminations.(b)TreatmentofLtk-aprt-cellswas as
follows:pretreatmentfor 24 h with 10 U ofIFN--ypermlfollowedby100Uof IFN-1 perml without(10-y:100 1)orwith(10 -y:100 + Anti
y)monoclonal antibodiestoIFN--y;pretreatmentfor 24 h with 10 U ofIFN--yperml with monoclonalantibodies toIFN--yfollowedby100
U ofIFN-13perml(10 y+ Anti--y:100 1);pretreatmentfor 4 h with 100 U ofIFN--ypermlin theabsence(4h 100y:100 13)orpresence(4
h 100-y+ Cx:100 13)of 35,ugofcycloheximideperml followedby100 Uof IFN-1 perml;or nopretreatment(virus control). Incubations
with IFN-1 werefor18h, and cellsweretheninfectedwithVSV andcytopathiceffectsweredeterminedasdescribed in thetext.
2,5-oligo(A) synthetase expression, however, was weak when compared with the levels seenin L-929 cells treated
with either IFN alone. Combined treatment of L-929 cells
alsogave asynergisticeffectonthelevel ofenzymeactivity (Table 2).
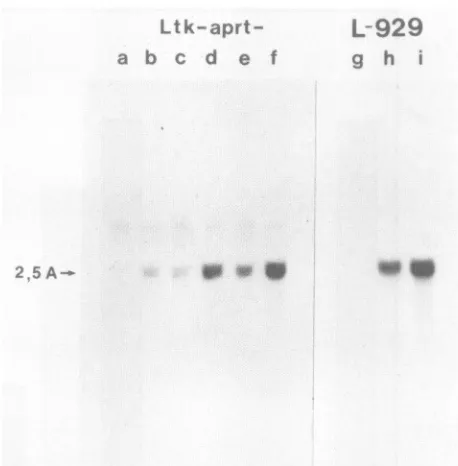
Northern (RNA) blot analysis of the effects of IFN on
gene expression is shown in Fig. 7. The mRNA for
2,5-oligo(A) synthetasewasundetectableinuntreated
Ltk-aprt-and L-929 cells. Only an extremely weak induction of the 1.8-kilobase mRNA could be detected in Ltk-aprt- cells
treated witheither
IFN-P,
asreported previously (28, 42),or IFN-y. However,asignificant level of inductionwas seeninLtk-aprt- cells treated simultaneously with both IFNs, al-thoughthe level ofaccumulation of 2,5-oligo(A) synthetase
mRNAwaslower than thatobserved in L-929 cells treated with IFN-P alone. This is in accord with the observations presented above for the level of enzyme activity. Blots
probedfor 1-actin showed nodifferencein signal intensity, indicating that the same amount of RNA was loaded in different lanes.
DISCUSSION
The mechanisms by which interaction ofIFNs with cell surfacereceptorsleadstomodulation ofgeneexpressionare poorly understood. After bindingtothe high-affinity recep-tors, IFNsare internalized (50, 51, 53),but definitive proof asto whetheruptake of IFN into the cell is necessary for further events has been elusive (2, 6, 17, 51, 53).
Microin-jection of IFN-cx and IFN-P into cells does not produce
antiviral effects(18, 19),but severalreportshave suggested
thatintracellular IFN-y may be capable ofactivating gene
expression and an antiviral state (12, 40). Several lines of
1.5
I .
a 1.0
0
0-in
%._
2:
o.o
C)
~05
0*
0.0
6t
-% 24 8 6 4 2 1 0 aPretreatment with IFN-y (hrs)
I I
on November 10, 2019 by guest
http://jvi.asm.org/
1.5
a.
b.
U-^.
A,o aC, ., o..
O V
QA
C.. A.
C,i e
0 %
.qCQq k-ir ~~~~~ ;:r(J
FIG. 6. Stability ofanIFN--y-inducedfactor which actssynergisticallywithIFN-Pand effects ofpretreatmentwithIFN-Porpoly(I C). (a) Ltk-aprt-cells weretreatedasfollows: pretreatmentfor 28 h with IFN--y followed by IFN-, (28 hy:Ochase:>);pretreatmentfor 20 h with IFN-,yfollowed byan8-hchaseingrowth medium and then an 18-h incubation withIFN-P(20 h y,8 hchase:
P);
pretreatmentfor 4 h with IFN--yfollowed bya24-hchase with growthmedium and then an 18-hincubation withIFN-P(4 h -y, 24 hchase:,P);pretreatmentfor 4 h with IFN-yfollowed immediately by incubation for18 hwithIFN-P(4 hy,0chase:>).CellswereinfectedwithVSV, andcytopathiceffects were assayedasdescribedin thetext.Thecell controlandviruscontrol and treatment witheitherIFN-PorIFN--yareshown.Inall cases the IFNs wereusedat aconcentration of100U/ml. (b)Ltk-aprt- cells were treated with 100 U ofIFN-Pper mlfor 24 hfollowed by 100 U ofIFN--yper ml(24hP, 24h -y)or withpoly(I *C)(50 ,ug/ml in50,ugofDEAE-dextranperml)for1hfollowedby a 24-hincubationwith 100 Uof
IFN-p
(polyI C,24h0)
orIFN--y(polyI.
C, 24 h -y)per ml or 100 U ofIFN-yper ml withpolyclonalantibodies toIFN-P(poly I C, 24 h -y + AntiP).
Cells were theninfectedwithVSV, andcytopathic effectswereassayedasdescribedin the text. Virus and cell controls are alsoshown.evidence suggest that IFN-a and IFN-P operate through somewhat different pathways thanIFN-y: the receptors of typeIand type IIIFNs are separate entities (1, 3, 5, 20, 37,
41), the kinetics of intracellular degradation of IFN-y in
mousecellsaremuch slower than thosefortype IIFNs(50), andinternalized IFN-yhasbeenreported tobe transported
to thenucleus (32). Differencesinthe sensitivity of various
genestoinduction by type I andIIIFNsand in theabilityof cycloheximide to block gene expression suggest that
dis-tinct mechanisms are involved in gene activation (11, 21, 22).
It is generally supposed that binding to the receptors is
followedby transmissionofasignalorsignalswhich insome
way modulate gene expression, perhaps by causing alter-ations intranscriptional activationfactors. Recently,protein factors which bind to upstream regulatory elements have been detected inextracts of cells treated with IFNs(9, 24,
36, 39). The IFN-induced appearance of suchtranscription
factorsmustresult fromactivationofpreexisting proteins by
posttranslational
modification(45)possibly accompanied
byde novo synthesis of additional factors (11, 21-23). The
differential effects ofcycloheximide on activation of some
genes suggest that the signals generated by occupancy of type I and type II IFN receptors may not be identical, consistentwith reportsof
synergistic
effects of the different typesofIFNs oncellgrowthinhibition andantiviralactivity
(10, 13-15, 52) and on the induction of 2,5-oligo(A) syn-thetase(13, 23). Also,morethanonepathway
islikelytobe involved in the activation ofdifferent genesbytypeIIFNs (21, 22,33,34,42),andthereforedifferentsetsofsignals
mayexist, with a degree of functional overlap between those
generated bytypeIand typeIIIFNs.Analysis ofvariantcell
linesand the effects of
cycloheximide
have shown thatsomegenescanbeactivatedwithoutaneed for
protein
synthesis,
1.0
0.5
0-1
4-,
%0
0
0.0
-U-v/I,
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.612.58.562.75.449.2]TABLE 2. Synergisticeffects ofIFN-PandIFN--y on2,5-oligo(A) synthetase levelsa
2,5-Oligo(A) synthetaseactivity Cells andtreatment (cpm/mgof protein per 60min) -Poly(I C) +Poly(I C)
Ltk-aprt-None 224 322
100 U ofIFN-,/ml 164 337
100 U ofIFN-y/ml 212 439
100 U ofIFN-P/ml + 100 U 369 3,590
ofIFN-,y/ml
100UofIFN-P/ml + 10 U 398 1,760
ofIFN--y/ml
100 Uof IFN--y/ml + 10 U 466 1,088
ofIFN-P/ml
L-929
None 665 385
100 UofIFN-,/ml 450 6,796
100UofIFN--y/ml 433 1,781
100UofIFN-13/ml + 100 U 554 15,985 ofIFN-y/ml
aLtk-aprt-andL-929 cellsweretreated withIFNs at theconcentrations
indicated for 18handharvested for enzymeassay asdescribed in thetext.
Each extract wasincubated in the absenceorpresenceof10pLgofpoly(I C)
per ml.Levels ofradioactivity determined in control reaction mixtureslacking
extract wereapproximately 400cpmwithorwithoutpoly(I C).
i.e., a primary response (11, 21, 22). For other genes,
activationmayrequiretheproduction ofaprotein(s) induced by IFNs, i.e.,asecondaryresponse. Senandcolleagues (22,
23, 46) have shown thatinsomecells the activation of gene 561 expression by IFN-ot depends on a combination of multiple signals, including a protein that is synthesized in response to IFN-a. This protein can also be induced by IFN--y, whichalonefailstoactivateexpressionof gene 516.
SubsequenttreatmentwithIFN-a(22) orother agents such
asdouble-strandedRNA andgrowth factors (46)causesthe
activation ofgeneexpressioneven inthe presenceof cyclo-heximide. Thus, a protein induced by
IFN-at
and IFN--y interacts withvarioussignalsto promoteexpressionof gene 561.Ourresults with Ltk-aprt- cellshave established that this cellline ispartially sensitivetoIFNs. Although it lacks the
capacitytoestablishanantiviral stateandtoinduce
expres-sion of a particular set of genes (e.g., for 2,5-oligo(A) synthetase, eucaryotic initiation factor2 kinase, and major histocompatibility complex antigens) when treated with
IFN-P
andIFN--y, it stillrespondstoIFN-, byareductionin the rateof cellgrowth andby normal induction of gene I-8(42). These results cannot be explained on the basis of
defectivecellsurface receptors, and theinability of
Ltk-aprt-cellsto respondto added cadmiumbyincreasedexpression ofmetallothionein(29), the gene for which is also regulated
by IFN, lends further support to the idea that the defect is
not at the level of receptor functioning. The results pre-sented here support this hypothesis. The dramatic effect of
combinedtreatment with
IFN-P
and IFN-,y shows that both setsof receptors are indeedfunctional. Inan earlier report(42),weshowedthatIFN-,3failed to activate transcription of several genesin Ltk-aprt- cells, andthereforethedefective responses ofthis line are due either to alterations in
up-streamregulatory elements or to a failure to activate partic-ulartranscriptional activationfactors. Since many genes (at
least eight) are affected, the latter explanation seems most
likely. Theobservations presented here suggest that IFN--y
Ltk-
aprt-a b cd
ef
4,D A
-L'-- 929
g hI
OW *l
FIG. 7. IFN-Pand IFN-y act synergistically toinduce expres-sion of the 2,5-oligo(A) synthetase gene. RNA was extracted from cells treated with combinations of IFNs as described in the text. Northern blots were hybridized with a probe specific for 2,5-oligo(A) synthetase (2,5 A). Ltk-aprt- cells were untreated(lanea)
ortreated for16 h with 100 U ofIFN-Pper ml (lane b), 100 U of
IFN-yper ml (lane c), 100 U ofIFN-Pper mlplus 10 U ofIFN--yper ml (lane d), 100 UofIFN--y permlplus 10 U of IFN-,Bperml(lane e), or 100 U ofboth IFN-,BandIFN-yperml(lanef).As acontrol L-929 cells were treated with IFN-13 for 0(laneg), 8(lane h),or16 (lane i) h.
canovercome this failure to activate transcription
factor(s)
in Ltk-aprt- cells treated withIFN-P.
These cells therefore provide an excellent system forstudying the mechanism by which signal transduction is coupled to gene activation.FIG. 8. Schematic model for activation ofgene expression of
IFN-P andIFN-,y. Receptors forIFN-,3 (square)andIFN--y(circle) in the cellmembraneareshown;uponactivation bybinding of the IFNs, they give rise to signals (denoted by the arrows) which interact with transcription activation factors (TFs) causing alter-ations in gene expression. Potential blocks in these pathways are
shownbyX's. Althoughsignalsareshownassinglelines, multiple steps may be involved in fulfilling any path. eIF-2, Eucaryotic initiation factor2.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.612.57.297.102.298.2] [image:8.612.318.556.482.648.2]To accountfor the insensitivity of Ltk-aprt- cells to IFNs, we propose that a defect in signal processing exists for both type I and II IFNs, resulting in an inability to effect certain of the normalresponses, although some signaling pathways areapparently intact and thus able to activate expression of cytostatic responses and induction of gene 1-8 (42). A schematic model is shown in Fig. 8 that has some similarities to one proposed by Kusari and Sen (22) based on their studies of gene 561 induction in HeLa cells. Both models propose that IFNs activate multiple signals and that IFN--y exertsits effect in partby inducingthesynthesisof aprotein.
We suggest that type I and II IFNs activate separate but
functionally overlapping sets of signals and the absence of a required component in one set may be complemented by the other whenboth types of IFNs are present. Activation of a
particular gene or group of genes by a single type of IFN
requirestheinteractionof one or more signals with aspecific transcription factor. In Ltk-aprt- cells at least one ofthese components, a signal or a transcription factor, is absent and
hence expression ofthedependent genesisnotpossible.The
complementaryIFNhasasimilarly defective but distinctset ofsignals, one of which can replace the defective compo-nent.
Our results indicate that IFN-y produces at least one
signal (GlinFig. 8)which induces the synthesisof a protein
(Sg, for IFN--y-induced synergistic factor). This protein is
relativelystable, as seen in the pretreatment and withdrawal
experiments, and its production is blocked by
cyclohexi-mide. We propose that protein Sg is able to interact with
signal Sb produced bythe IFN-, receptor and thusactivate expression of the 2,5-oligo(A) synthetase gene and other
genes,with theproductionofanantiviralstate.Activation of
anantiviralstatebyIFN-y normally dependsonthe produc-tion of signal G2, which activatestranscription factor TFg2, butoneof these is defective inLtk-aprt- cells. Induction of 2,5-oligo(A) synthetase and major histocompatibility
com-plex gene expression by IFN--y has been shown to be
sensitive tocycloheximide in othercells (4, 11), in contrast to inductionby IFN-a. Thiscould reflectaneed to synthe-size TFg2, which is activatedbyasecond signal (G2) from the
IFN-y
receptor. IfLtk-aprt- cells failtogenerateG2, it is possible that Sg and TFg2areidentical and thusSg
may be atranscription factor analogoustoTFbl.The
IFN-P
receptorinLtk-aprt- cells activatestranscrip-tion ofgene I-8 through signal B2, which does not need
complementation. Induction ofan antiviral state
by
IFN-P
requires
activation ofa constitutively expressed transcrip-tionfactor, TFbl, by signal Bi.Signals
Bi,
B2,and Sb may beidenticalifLtk-aprt-cellsfailto synthesize TFbl. Inthis case atransient signal (B1 = B2 = Sb) wouldbegeneratedbut couldonly activate those genes regulated by TFb2and thus an antiviral state would not be produced. In the presenceofIFN-y,however,Sg is produced andthis can be activatedby Sb, leadingtoexpression ofgeneproductswith
antiviral capacities. If Sg is the same
protein
as TFg2, Sb would effectively replacesignalG2.Whether thesignals whicharedefective in
Ltk-aprt-
cellsare intermediates in the
pathway
or are factors which interactdirectlywithgeneregulatoryelements remainstobeestablished, and the Ltk-aprt- cells
provide
an excellent system forstudying
the nature of these factors. We arecurrently
attempting
toidentify Sg
and determine whetherfactors capable of
binding
to upstreamregulatory
elementsare induced in Ltk-aprt- cells after treatment with various combinations of IFNs.
ACKNOWLEDGMENTS
WethankM.Esteban,R.Bablanian,R.Janeczko,and M.A.Q. Siddiqui for generous support and for critical analysis of the
manuscript. The recombinant CHO cell line expressing mouse IFN-y was a generous gift from A. G. Morris (University of
Warwick,Coventry, England). PlasmidpSP65-J2containinga par-tial cDNA for mouse 2,5-oligo(A) synthetase was a kind gift of B. R. G. Williams (Hospitalfor SickChildren, Toronto, Ontario, Canada).
LITERATURE CITED
1. Aguet, M., F. Belardelli, B. Blanchard, F. Marcucci, and I. Gresser.1982.Highaffinity bindingof125I-labeledmouse inter-feronto aspecificcell-surfacereceptor.IV. Mouse-yinterferon andcholera toxin donotcompetefor thecommonreceptorsite of
a/p
interferon. Virology117:541-544.2. Anderson,P.,B.Tycko,F.Maxfield,andJ. Vilcek.1982.Effect ofprimaryaminesoninterferon action.Virology117:510-515. 3. Anderson, P.,Y. K.Yip,andJ.Vilcek.1982. Specificbindingof
125I-human interferon-y to high affinity receptors on human fibroblasts. J. Biol. Chem. 257:11301-11304.
4. Blanar, M. A.,E. C. Boettger, and R. A.Flaveli. 1988.
Tran-scriptionalactivation of HLA-DRa by interferon yrequires a
trans-acting protein.Proc.Natl. Acad. Sci. USA 85:4672-4676. 5. Branca,A.A.,andC.Baglioni. 1981. Evidence that typesIand IIinterferons have different receptors. Nature (London) 294: 768-770.
6. Branca, A. A., C. R. Faltynek, S. B. D'Alessandro, and C.
Baglioni.1982. Interaction ofinterferonwithcellular receptors. Internalization anddegradationofcell-bound interferon. J. Biol. Chem.257:13291-132%.
7. Caplen,H. S.,and S. L.Gupta.1988. Differentialregulationof
acellulargenebyhuman
interferon--y
andinterferon-a.J.Biol. Chem. 263:332-339.8. Cheng,Y.-S.E.,R.J.Colonno,and F. H. Yin.1983.Interferon induction of fibroblastproteinswithguanylatebindingactivity.
J. Biol. Chem. 258:7746-7750.
9. Cohen, B., D.Peretz, D.Vaiman, P. Benech, andJ. Chebath. 1988. Enhancer-like interferon responsive sequences of the human and murine (2'-5') oligoadenylate synthetasegene
pro-moters. EMBO J. 7:1411-1419.
10. Czarniecki,C.W.,C. W.Fennie,D. B.Powers,and D. A. Estell. 1984. Synergistic antiviral and antiproliferative activities of Escherichiacoli-derivedhumanalpha, beta, and gamma inter-ferons. J. Virol.49:490-496.
11. Faltynek, C.R., S. McCandless, J. Chebath,and C. Baglioni.
1985. Different mechanisms for activation of genetranscription byinterferonsaand-y.Virology144:173-180.
12. Fidler,I.J.,W.E.Fogler,E. S.Kleinerman,and I.Saiki. 1985.
Abrogationofspecies specificity for activation of tumoricidal
properties in macrophages by recombinant mouse or human
interferon--y encapsulatedinliposomes.J. Immunol. 135:4289-4296.
13. Fish, E. N., G. E. Hannigan, K. Bannerjee, and B. R. G. Williams.1988. The interaction of interferonaand fy:
regulation
of(2-5)A synthetaseactivity. Virology165:87-94.
14. Fleischmann,W. R.1982.Potentiation of the direct anticellular
activityofmouseinterferons: mutualsynergismand interferon concentrationdependence. CancerRes.42:869-875.
15. Fleischmann,W.R.,J.A.Georgiades,L.C.Osborne,and H. M. Johnson. 1979. Potentiation of interferon activity by mixed
preparations of fibroblastoid and immune interferon. Infect. Immun.26:248-253.
16. Friedman-Einat, M., M. Revel, and A. Kimchi. 1982. Initial characterization of a spontaneous interferon secreted
during
growthanddifferentiation of Frienderythroleukemiacells.Mol. Cell. Biol.2:1472-1480.17. Hannigan, G., and B. R. G. Williams. 1986.
Transcriptional
regulation ofinterferon-responsive genes is
closely
linked tointerferonreceptor occupancy. EMBOJ.5:1607-1613. 18. Higashi, Y.,andY.Sokawa.1982. Microinjectionofinterferon
and2',5'-oligoadenylateintomouseL-cells and their effectson virusgrowth. J.Biochem.91:2021-2028.
on November 10, 2019 by guest
http://jvi.asm.org/
19. Huez, G., M.Silhol, and B. Lebleu. 1983. Microinjected inter-feron does not promote an antiviral response in HeLa cells. Biochem. Biophys. Res. Commun. 110:155-160.
20. Joshi, A. R., F. H. Sarkar, and S. L. Gupta. 1982.Interferon receptors. Cross-linking of humanleukocyte interferon-a2to its receptor on human cells. J. Biol. Chem. 257:13884-13887. 21. Kelly, J. M., C. S. Gilbert, G. R. Stark,andI.M. Kerr. 1985.
Differential regulation of interferon-inducedmRNAsand c-myc mRNAby a and -y interferons. Eur.J. Biochem. 153:367-371. 22. Kusari, J., and G. C. Sen. 1986. Regulation ofsynthesis and
turnover of an interferon-inducible mRNA. Mol. Cell. Biol. 6:2062-2067.
23. Kusari, J., R. K. Tiwari, R. Kumar, and G. C. Sen. 1987. Expression of interferon-inducible genes in RD-114 cells. J. Virol. 61:1524-1531.
24. Levy, D. E., D. S. Kessler, R. Pine, N. Reich, and J. E.Darnell. 1988. Interferon-induced nuclear factors that bind a shared promoter element correlate withpositive and negative transcrip-tional control. Genes Dev. 2:383-393.
25. Lewis, J. A. 1982. The mechanism of action of interferon, p. 357-384. In L. Kohn and R. Friedman (ed.), Horizons in biochemistry and biophysics. John Wiley & Sons, Inc., New York.
26. Lewis, J. A. 1986.Induction of interferonsensitivity in resistant cellsby specific gene sequences. UCLASymp. Mol. Cell. Biol. 50:77-85.
27. Lewis, J. A. 1987. Biological assays for interferons, p. 73-87. In M.J.Clemens, A. Morris, andA.Gearing (ed.), Interferonsand
lymphokines-apractical approach.IRL Press Ltd., Oxford. 28. Lewis, J.A.1988.Induction ofanantiviralstateby interferonin
the absence of elevated levels of 2,5-oligo(A) synthetase and eIF-2kinase. Virology 162:118-127.
29. Lewis, J. A., and A. Bendicenti di Girolamo. 1987.Activation of metallothionein expression is potentiated by DNA sequences present in the herpes simplex virus thymidine kinase gene. FEBSLett.217:292-296.
30. Lewis, J. A., and M. Esteban. 1984. Inductionofan antiviral response and2',5'-oligo Asynthetase by interferon in several thymidine kinase deficient cell lines. Virology 133:464-469. 31. Lewis, J. A., E. Mengheri, and M. Esteban. 1983. Induction of
anantiviral response by interferon requires thymidine kinase. Proc. Natl.Acad. Sci. USA 80:26-30.
32. MacDonald, H. S., V.M.Kushnaryov, J. J. Sedmak, and S.E. Grossberg. Transport of -y-interferon into the cell nucleus may be mediated by nuclear receptors. Biochem. Biophys. Res. Commun. 138:254-260.
33. McMahon, M., G. R. Stark, and I. M. Kerr. 1986. Interferon-induced gene expression inwild-type and interferon-resistant humanlymphoblastoid (Daudi) cells. J. Virol. 57:362-366. 34. Mills,G.B.,G. E.Hannigan, J.F.Stanley,S.Grinstein,E. W.
Gelford,and B.R. G.Williams. 1988. Pertussis toxin prevents interferon-induced expression of 2-5 A synthetase but not growth inhibition. Eur. J. Immunol. 18:917-922.
35. Morris,A.G., andG. Ward. 1987. Production of recombinant interferons by expressioninheterologous mammalian cells, p. 61-71. In M. J. Clemens, A. Morris, and A. Gearing (ed.), Interferons and lymphokines-a practical approach. IRL Press Ltd.,Oxford.
36. Porter,A. C. G., Y. Chernajovsky, T. C. Dale, C. S. Gilbert, G. R.Stark,andI.M.Kerr. 1988. Interferon response element ofthehuman gene6-16. EMBO J. 7:85-92.
37. Raziuddin, A., F. H. Sarkar, R. Dutkowski, L. Shulman, F.H. Ruddle, and S. L.Gupta. 1984. Receptors for human a and a
interferon but notforyinterferon arespecified by chromosome 21. Proc.Natl. Acad. Sci. USA 81:5504-5508.
38. Rosa,F.M.,M. M.Cochet, and M. Fellous. 1986. Interferon and major histocompatibility complex genes: a model to analyse eukaryotic gene regulation?, p. 47-87. In I. Gresser (ed.), Interferon 7. AcademicPress, Inc., New York.
39. Rutherford, M. N., G. E. Hannigan, and B. R. G. Williams. 1988. Interferon-inducedbinding of nuclear factorstopromoter elements of the 2-5 Asynthetase gene. EMBO J. 7:751-759. 40. Sanceau, J., J. A. Lewis, P. Sondermeyer, F. Beranger, R.
Falcoff,andC.Vaquero. 1986. Expression of extracellular and intracellular human IFN-y inmouse L-cells transformed with the human IFN-y cDNA gene. Biochem. Biophys. Res. Com-mun. 135:894-901.
41. Sarkar, F. H., and S. L. Gupta. 1984. Receptors for human
y-interferon: binding and cross-linking of '25I-labeled recombi-nanthuman y interferon to receptors on WISH cells. Proc.Natl. Acad. Sci. USA 81:5160-5164.
42. Shan, B.,andJ. A.Lewis. 1989.Interferon-induced expression of different genes is mediatedby distinctregulatory pathways. Virology 170:277-281.
43. Spitalny,G.L.,and E. A. Havell. 1984.Monoclonalantibodyto murine gamma interferon inhibitslymphokine-induced antiviral andmacrophage tumoricidal activities. J. Exp. Med. 159:1560-1565.
44. Staeheli, P., M. A. Horisberger, and 0. Hailer. 1984. Mx-dependent resistance toinfluenza viruses is inducedbymouse interferonsaand ,B butnoty. Virology 132:456461.
45. Tiwari,R.K., J. Kusari,R.Kumar,andG. C. Sen. 1988. Gene activation by interferons and double-strandedRNA: selective inhibition by2-aminopurine. Mol. Cell. Biol. 8:4289-4294. 46. Tiwari, R. K., J. Kusari, and G. C. Sen. 1987. Functional
equivalents of interferon-mediated signals needed for induction ofan mRNA can begenerated by double-stranded RNA and growth factors. EMBO J. 6:3373-3378.
47. Wallach, D.,M.Fellous,and M.Revel.1982.Preferential effect of -y interferon on the synthesis of HLA antigens and their mRNAsinhumancells. Nature(London)299:833-836. 48. Weil, J., C. J.Epstein,L. B.Epstein,J.J.Sedmak, J.L.Sabran,
and S. E. Grossberg. 1983. A unique set of polypeptides is inducedbyyinterferon in additiontothose inducedincommon with a and 13interferons. Nature (London) 301:437-439. 49. Wells, V.,and L.Mallucci.1985.Expression of the 2-5A system
during thecell-cycle. Exp. CellRes. 159:27-36.
50. Wietzerbin, J., C. Gaudelet, M. Aguet, and E. Falcoff. 1986. Binding andcross-linking of recombinantmouseinterferon-yto receptorsinmouseleukemia L-1210 cells:interferon-y internal-ization and receptor down-regulation. J. Immunol. 136:2451-2455.
51. Yonehara, S., A. Ishii, and M. Yonehara-Takahashi. 1983. Cell-surfacereceptor-mediated internalization of interferon: its relationshiptotheantiviralactivity of interferon.J.Gen. Virol. 64:2409-2418.
52. Zerial, A., A. G. Hovanessian, S. Stefano, K. Huygen, G. H. Werner, and E. Falcoff. 1982. Synergistic activities of type I (alpha, beta)andtypeII(gamma) murine interferon. Antiviral Res. 2:227-239.
53. Zoon, K. C.,H.Arnheiter, D. zur Nedden, D. J. P. Fitzgerald, and M. C. Willingham. 1983. Human interferon alphaenters cellsby receptor-mediated endocytosis. Virology 130:195-203. 54. Zullo, J. N.,B. H.Cochran, A. S.Huang, and C.D.Stiles. 1985. Platelet-derived growth factor and double-stranded ribonucleic acids stimulateexpression ofthesame genesin3T3cells. Cell 43:793-800.