Copyright © 2000, American Society for Microbiology. All Rights Reserved.
CDP Is a Repressor of Mouse Mammary Tumor Virus
Expression in the Mammary Gland
QUAN ZHU, KEQIN GREGG, MARY LOZANO, JINQI LIU,ANDJAQUELIN P. DUDLEY*
Section of Molecular Genetics and Microbiology and Institute for Cellular and Molecular Biology, The University of Texas at Austin, Austin, Texas 78705
Received 7 January 2000/Accepted 19 April 2000
Mouse mammary tumor virus (MMTV) transcription is highest in the lactating mammary gland but is detectable in a variety of other tissues. Previous results have shown that MMTV expression is suppressed in lymphoid and other tissues through the binding of the homeodomain-containing repressor special AT-rich binding protein 1 to a negative regulatory element (NRE) in the MMTV long terminal repeat (LTR). Another homeoprotein repressor, CCAAT displacement protein (CDP), also binds to the MMTV NRE, but a role for CDP in MMTV transcriptional suppression has not yet been demonstrated. In this paper, we show that the level of CDP decreases during development of the mammary gland and that this decline in CDP level correlates with the known increase in MMTV expression observed during mammary gland differentiation. Moreover, CDP overexpression was able to suppress MMTV LTR-reporter gene activity up to 20-fold in transient-transfection assays of mouse mammary cells. To determine if this effect was due to direct binding of CDP to the
promoter-proximal NRE, we performed DNase I protection assays to map two CDP-binding sites fromⴙ835 toⴙ845 and
ⴙ920 toⴙ931 relative to the first base of the LTR. Mutations engineered into each of these sites decreased CDP
binding to the proximal NRE, whereas a combination of these mutations further reduced binding. Subse-quently, each of these mutations was introduced into the full-length MMTV LTR upstream of the luciferase reporter gene. Analysis of stable transfectants of LTR constructs showed that CDP binding site mutations in the proximal NRE elevated reporter gene expression two- to sixfold compared to wild-type LTR constructs. Thus, MMTV expression increases during mammary gland development, in part due to decreased CDP levels and CDP binding to the LTR. Together, these experiments provide the first evidence that CDP acts as a repressor of MMTV transcription in the mammary gland.
Mouse mammary tumor virus (MMTV) is a type B retrovi-rus that primarily induces mammary carcinomas and, at a lower frequency, T-cell lymphomas in mice (20, 33). Current data suggest that MMTV induces mammary tumors by the insertional activation of nearby cellular oncogenes (18, 50, 62). The disease specificity of MMTV appears to be linked directly to high viral expression in specific tissues (68). Milk-borne MMTV is expressed primarily in the lactating mammary gland (55). The high level of viral transcription increases MMTV insertions, leading to cell transformation in mammary tissue. A mutant form of MMTV (type B leukemogenic virus) that in-duces T-cell lymphomas shows high-level expression in T cells (4, 5, 17). Previous work showed that the tissue-specific expres-sion of the MMTV genome is governed by regulatory elements located in the long terminal repeat (LTR). These known ele-ments include a hormone response element (HRE), several negative regulatory elements (NREs), a mammary gland en-hancer, and NF-1, Oct-1, and TFIID binding sites (13, 14, 46–48, 52, 59).
Virtually all MMTV proviruses acquired in mouse T-cell lymphomas contain LTR deletions or rearrangements encom-passing a 491-bp region (⫺655 to⫺165;⫹541 to⫹1031 rela-tive to the first base of the C3H LTR) (5, 33, 36, 45). These deletions and rearrangements result in higher levels of MMTV expression in T cells compared to endogenous wild-type MMTVs (13, 33). Transient and stable transfection experi-ments showed that this region contains negative regulatory
elements (NREs) (13, 33). Removal of NREs relieved the suppression of MMTV transcription in normally semipermis-sive or nonpermissemipermis-sive tissues. Transgenic mouse experiments with p1BCAT, a naturally occurring LTR deletion (⫺655 to
⫺165) mutant linked to the gene for chloramphenicol acetyl-transferase, revealed that this LTR deletion mutation allows high-level viral expression in semipermissive tissues (e.g., thy-mus) and lower expression in tissues that are normally non-permissive (brain, heart, and skeletal muscle) (55). Transient-transfection assays with sequential LTR deletion mutants have defined two NREs, promoter distal and promoter proximal (see Fig. 1) (13). Gel shift assays with these NREs detected binding of two major protein complexes identified as CCAAT displacement protein (CDP) and special AT-rich binding pro-tein 1 (SATB1) (13, 41). A substitution mutation (924) in the proximal NRE (pNRE) that decreased SATB1 binding in-creased basal expression ca. 2.5-fold compared with the wild-type promoter in transient-transfection assays with LTR-re-porter genes. The 924 mutant LTR showed a more dramatic elevation of reporter gene expression compared to wild-type LTR expression in the lymphoid tissues of transgenic mice (41). These data indicated that SATB1 functions as a suppres-sor of MMTV expression. However, the role of CDP in MMTV transcriptional control is unknown.
CDP (also known as Clox or Cux) is a homologue of the Drosophilaprotein Cut (2, 10, 61), which functions as a cell fate-determining factor during development (49). Elimination of Cut activity transforms external sensory organs into internal chordotonal organs (12), and ubiquitous Cut expression causes transformation of chordotonal organs into external sensory organs (11). When overexpressed in tissue culture cells, CDP was capable of repressing target gene expression (43).
More-* Corresponding author. Mailing address: Section of Molecular Ge-netics and Microbiology, 100 W. 24th St., The University of Texas at Austin, Austin, TX 78705. Phone: (512) 471-8415. Fax: (512) 471-7088. E-mail: jdudley@uts.cc.utexas.edu.
6348
on November 9, 2019 by guest
http://jvi.asm.org/
imply that CDP participates in cellular gene expression in the mammary gland. MMTV expression is suppressed in undevel-oped mammary tissue but is activated in the lactating mam-mary gland (34). CDP also binds to the MMTV transcriptional control region (40, 41). Therefore, it is possible that CDP functions as a repressor to suppress MMTV expression in undeveloped breast tissue, and viral suppression is relieved when mammary cells are fully differentiated.
Here we examined the role of the CDP homeoprotein in the control of MMTV expression. We have shown that the levels of full-length CDP decline during mammary gland differentia-tion, a period when MMTV transcription increases (25, 55). DNase I mapping experiments using the MMTV pNRE re-vealed the presence of two CDP binding sites. Mutations in either of these sites reduced CDP binding to pNRE probes, and reporter gene constructs carrying these mutations elevated MMTV expression in transient- and stable-transfection assays. Our data provide the first evidence that the homeoprotein CDP acts as a transcriptional repressor in the mammary gland.
MATERIALS AND METHODS
Cell culture and transfections.HC11 normal mouse mammary cells (6) from BALB/c mice were obtained from Jeff Rosen (Baylor College of Medicine, Houston, Tex.). Cells were grown in RPMI medium (GIBCO BRL, Gaithers-burg, Md.) containing 10% fetal bovine serum (FBS; Hyclone, Logan, Utah), insulin (Sigma Chemical Co., St. Louis, Mo.) at 10g/ml, epidermal growth factor (GIBCO BRL) at 0.5g/ml, and gentamicin (Elkins-Sinn, Inc., Cherry Hill, N.J.) at 40g/ml. NMuMG cells (51) were grown in Dulbecco’s high-glucose minimal essential medium (GIBCO BRL) containing 10% FBS, 10 mM HEPES (pH 7.4), insulin at 10 g/ml, and antibiotics. On the day prior to transfection, cells were treated with trypsin and replated in growth medium at approximately 5⫻106/100-mm-diameter plate. Single cells were obtained with trypsin, growth medium was added, and the cell count was determined. For transient transfections, cells were pelleted and resuspended to 107/200l in medium (lacking FBS) containing 40g of pLC-LUC DNA (13) and 40g of DNA with various ratios of a CDP expression vector (pRc/CMV CDP) (39) or an expression vector lacking the CDP insert (pcDNA3) (Invitrogen, Carlsbad, Cal-if.). To normalize for DNA uptake in transient-transfection assays, 5g of pRSV/lacZor up to 0.7g of pRL-TK (Promega, Madison, Wis.) was added to all samples in the same transfection. Cells were transfected by electroporation using a BTX electroporator (BTX, San Diego, Calif.) at 1,750F and 150 V. Each electroporation was plated into a 100-mm-diameter tissue culture dish and incubated for 48 h in growth medium prior to preparation of cytoplasmic and/or nuclear extracts. Transfections were normalized for DNA uptake by measure-ment of-galactosidase activity (pRSVlacZ) (65) orRenillaluciferase (pRL-TK) or by hybridization analysis of nuclear DNA from transfected cells with a probe for firefly luciferase and quantitation using a PhosphorImager. Assays for firefly and Renillaluciferase were performed using the Luciferase Reporter Assay System (Promega).-Galactosidase activity was measured using standard meth-ods as described previously (13). Transfections for each DNA sample were performed in triplicate and pooled for assays. For stable transfections, 2⫻105 HC11 cells were plated in each well of six-well plates and incubated overnight until the cells were ca. 50% confluent. Subsequently, 4g of the test plasmid and 1g of pcDNA3 containing the geneticin resistance gene in 0.5 ml of RPMI medium without serum were added to 12l of DMRIE-C reagent (GIBCO BRL) in an equal amount of medium, mixed, and incubated at room temperature for 45 min. The cells were washed with RPMI medium, and then the DNA solution was incubated with the cells for 7 h at 37°C prior to the addition of 1 ml of complete RPMI medium with 20% FBS. After further incubation for 48 h, cells were selected in geneticin (GIBCO BRL) at 1 mg/ml for 3 weeks. All of the
mice. Tissues were ground to a fine powder under liquid nitrogen and then resuspended in 10 ml of hypotonic buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 10% glycerol, 2 M sucrose, 1 mM dithio-threitol (DTT), 1.25 mM phenylmethylsulfonyl fluoride, pepstatin A at 0.5g/ml, and a 1⫻protease inhibitor cocktail [Sigma; P9599]). Inhibitors were added to the buffer just prior to use. The samples were incubated on ice for 20 min and then homogenized using 20 to 30 strokes with a glass Dounce homogenizer pestle A. The supernatant was removed by centrifugation at 21,000 rpm in a Sorvall SS-34 rotor at 4°C for 30 min. The nuclear pellets were resuspended in 2 ml of high-salt buffer (400 mM KCl, 25 mM HEPES [pH 7.9], 25% glycerol, 1 mM DTT, and the same protease inhibitors as in buffer A) and homogenized for 20 strokes with a Dounce homogenizer pestle B. The nuclear suspension was then incubated on ice for 1.5 h with stirring and subjected to centrifugation at 21,000 rpm in a Sorvall SS-34 rotor for 30 min at 4°C. The supernatant was adjusted to 60% ammonium sulfate using a saturated solution in water and incubated on ice for 2.5 h. The precipitated proteins were sedimented at 40,000 rpm for 30 min at 4°C using a Beckman SW55Ti rotor. The pellet was resuspended in 1 ml of buffer D (20 mM HEPES [pH 7.9], 100 mM KCl, 20% glycerol, 2 mM EDTA, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, pepstatin A at 0.5g/ml) and dia-lyzed thrice against 200 volumes of buffer D for 16 h at 4°C. The dialysate was subjected to centrifugation at 10,000⫻gat 4°C for 15 min to remove precipi-tates, and the protein concentration was determined using the Bio-Rad protein assay system (Bio-Rad Laboratories, Hercules, Calif.). Protein extracts were stored in aliquots at⫺80°C.
EMSAs.Electrophoretic mobility shift assays (EMSAs) were performed as described by Liu et al. (41). The 22-bp sequence spanning the imperfect inverted repeat in the pNRE was multimerized, and four copies of this sequence were cloned in pUC9 (pNRE4) (41). The 120-bp sequence (⫹812 to⫹931) or the 110-bp sequence (⫹822 to⫹931) spanning the pNRE in the C3H MMTV LTR was obtained by PCR and cloned into pCRII (Invitrogen, San Diego, Calif.). Purified plasmid DNAs were digested with EcoRI andHindIII (pNRE4) or EcoRI (p120 or p110) to give 5⬘overhanging ends, and the inserts were isolated on polyacrylamide gels. Fragments were end labeled with Sequenase (version 2.0; Amersham Pharmacia Biotech, Piscataway, N.J.) for EMSA. Oligonucleotide probes were also end labeled with Sequenase after annealing of complementary strands to create 5⬘overhanging ends. One microliter (1:10 dilution) of rabbit anti-CDP serum was used for antibody ablation experiments as described by Liu et al. (41). Competition and SP1-binding experiments were performed as de-scribed previously (41).
DNase I footprinting assays.The binding conditions used for DNase I foot-printing experiments were the same as those described for EMSAs, except that samples contained purified, bacterially expressed CDP representing the C-ter-minal two-thirds of the protein (CR2-Cterm) (39). Full-length CDP is not sol-uble. Approximately 0.5 ng (0.5⫻107cpm/g) of labeled probe and 15 to 30g of purified CDP were combined in a volume of 50l. Following incubation at 4°C, a solution containing 10 mM MgCl2and 5 mM CaCl2(50l) was added, followed by 2l of DNase I (0.02 U/ml freshly diluted from a stock solution) (GIBCO BRL). The reaction mixture was incubated at room temperature for 1 min, and then the reaction was terminated by the addition of 90l of 20 mM EDTA (pH 8.0), 1% sodium dodecyl sulfate (SDS), 0.2 M NaCl, and yeast tRNA at 150g/ml. Samples were then extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) prior to precipitation with ethanol. Precip-itated DNA was resuspended in formamide loading buffer (80% formamide [Sigma Chemical Co.], 10 mM EDTA, 0.1% xylene cyanol [Sigma], 0.1% bro-mophenol blue [Bio-Rad]) and analyzed on 8% polyacrylamide gels containing 8 M urea. Gels were dried and subjected to autoradiography.
Antibodies and Western analysis.Polyclonal rabbit antibodies against gluta-thioneS-transferase (GST)-SATB1 were kindly provided by Paul Gottlieb (Uni-versity of Texas at Austin). Other polyclonal antibodies against GST-SATB1 or GST-CDP (CR2-Cterm) (39) were prepared by immunization of rabbits in ac-cordance with the standard procedures of Cocalico Biologicals, Reamstown, Pa. Antibodies were shown to be specific for CDP or SATB1 as described previously (40). Western blots were prepared by lysis of cells in RIPA buffer (25 mM Tris-HCl [pH 7.8], 150 mM NaCl, 2 mM EDTA, 0.5% NP-40, 0.5% deoxy-cholate, 0.1% SDS) (23). All subsequent steps were performed at room temper-ature. Protein extract (15 to 20g for cell lines and 65g of mammary tissues)
on November 9, 2019 by guest
http://jvi.asm.org/
was subjected to electrophoresis on 8% polyacrylamide gels containing 0.1% SDS and blotted onto nitrocellulose. The membrane was incubated with 5% nonfat dry milk in TBST buffer (20 mM Tris-HCl [pH 7.6], 137 mM NaCl, 0.1% Tween 20) for 1 h and then washed three times for 5 min each with TBST buffer. Anti-CDP (1:500 dilution) or anti-actin (1:150 dilution) (Sigma; A-2066) serum was diluted in TBST buffer containing 1% nonfat dry milk. The antibody was added to the membrane for 1 h, and then blots were washed four times with 50 ml of TBST buffer (for 15 min each time). The membrane then was incubated with horseradish peroxidase-labeled anti-rabbit antibody (1:8,000 dilution) for 40 min and washed three times for 15 min each time with TBST buffer. Binding of the secondary antibody was detected using the ECL Western Blotting Detection System (Amersham).
Preparation of fusion proteins.Recombinant GST-CDP fusion protein was cleaved with thrombin (35) and purified by standard methods (58) as described by Liu et al. (40) before use in immunization or DNA-binding protocols.
RESULTS
CDP DNA-binding activity in the developing mammary
gland. Previous experiments have shown that high levels of
MMTV transcription in the lactating mammary gland result from the action of positive factors, including those that bind to the HRE and the mammary gland-specific enhancer in the LTR (28, 37, 46, 47, 55, 69). However, little is known about negative regulation of MMTV expression in the mammary gland. We have shown previously that at least two homeodo-main transcription factors, CDP and SATB1, bind strongly to NREs in the MMTV LTR (13, 41). These experiments have shown that there is no detectable SATB1 and CDP binding activity for the MMTV NRE in the lactating mammary gland, whereas CDP, but not SATB1, binding activity is detectable in all of the mammary gland cell lines tested (13, 41). Such data suggested that CDP is a suppressor of MMTV expression in relatively undifferentiated cells (e.g., cultured cells) derived from the mammary gland (41).
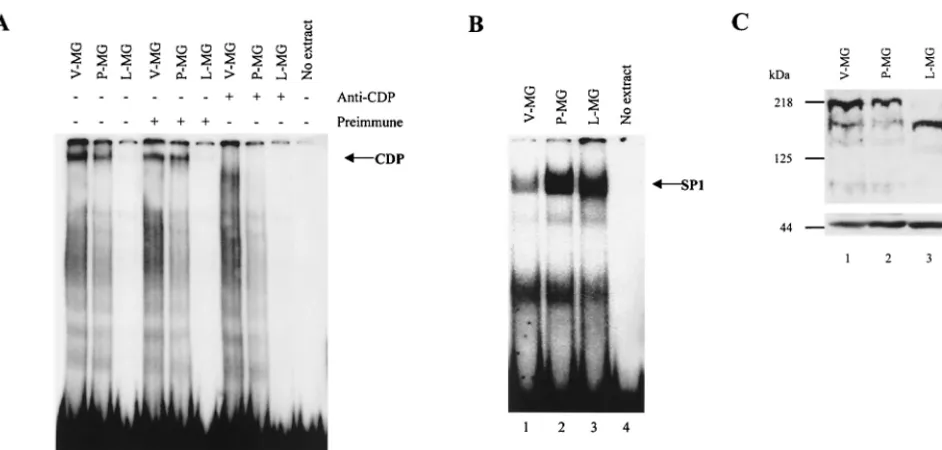
Because MMTV expression increases during mammary gland differentiation (34), it is possible that some of the in-creased viral expression corresponds to a decrease in suppres-sion by CDP. Therefore, we examined CDP binding activity to the MMTV NRE in mammary gland extracts at different de-velopmental stages by EMSA. Nuclear extracts from virgin, first-pregnancy, and lactating mouse mammary glands were incubated with the multimerized 22-bp pNRE probe (probe positions are shown in Fig. 1) prior to electrophoresis on non-denaturing polyacrylamide gels. As anticipated from our pre-vious results (41), lactating mammary gland extracts had un-detectable SATB1 and little or no CDP binding activity for the pNRE (Fig. 2A, lane 3). However, virgin and first-pregnancy mouse mammary glands both had detectable DNA-binding
activities (lanes 1 and 2) that were abolished by preincubation of extracts with anti-CDP serum (lanes 7 and 8), but not with preimmune serum (lanes 4 and 5). SP1 binding activity was detectable in all mammary cell extracts (Fig. 2B). Intriguingly, regressing mammary glands from animals that had finished lactation showed the return of CDP activity (not shown). These results suggested that the level of CDP binding of the MMTV LTR was inversely correlated with the differentiation status of the mammary gland.
To determine if the reduction of CDP-specific DNA-binding activity was due to a decrease in CDP levels during differen-tiation, Western blotting was performed on extracts derived from virgin, first-pregnancy, and lactating mouse mammary glands (Fig. 2C). Surprisingly, CDP was detected at all stages in the mammary glands examined, although the level in virgin mouse mammary glands appeared to be sixfold higher than that in pregnant mouse glands as measured by densitometry (compare lanes 1 and 2). In addition, the molecular mass of the CDP detected in lactating mouse mammary glands was approx-imately 50 kDa less than that observed for CDP in virgin or pregnant mouse glands. Although we cannot exclude the pos-sibility that this is an artifact of proteolytic degradation during preparation of extracts, the same extracts did not show degra-dation when anti-actin serum was used (Fig. 2C, lower panel). Thus, CDP binding activity for the pNRE decreases during development of the mammary gland and this result may be due, in part, to the appearance of a novel CDP form. Since the reduction of CDP binding to the pNRE correlates with the elevation of MMTV expression following the onset of lactation in the mammary gland, CDP may participate in the control of MMTV transcription.
Effect of CDP overexpression on transcription from the
MMTV LTR.The tissue-specific activity of CDP during
[image:3.612.124.479.78.203.2]mam-mary gland development suggests a potential role in repression of MMTV transcription. To confirm this hypothesis, we stud-ied the effect of CDP overexpression on MMTV LTR-reporter gene expression using transient-transfection assays of mouse mammary cells. Because all of the cultured cell lines that we have examined contain endogenous CDP that might interfere with the assay (our unpublished observations), the amount of pLC-LUC used in this experiment is critical for our ability to observe effects on CDP overexpression. To determine the op-timal amount of pLC-LUC for these experiments, we trans-fected different amounts of pLC-LUC (20, 40, and 60g) into HC11 cells with 30g of the CDP expression vector and 5g
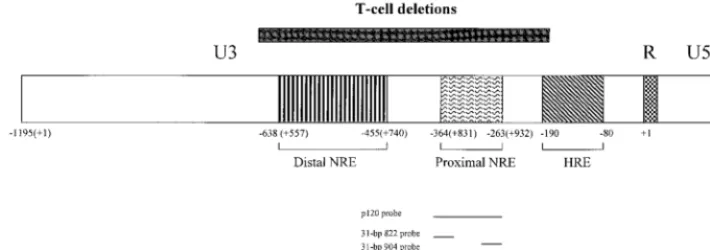
FIG. 1. Diagram of the MMTV LTR. The LTR is divided into U3, R, and U5 regions, and transcription is initiated from the standard MMTV promoter at the first base of the R region. The promoter-proximal and promoter-distal NREs and the HRE are shown by boxes with different types of hatch marks within the U3 region of the LTR. Numbering is shown from the first base of the LTR (⫹1). The region encompassing the largest of the U3 deletions found in thymotropic MMTVs relative to mammotropic MMTV strains is shown by a box over the LTR. The positions of probes used in this study are given below the LTR. The number of bases in each probe also is indicated.
on November 9, 2019 by guest
http://jvi.asm.org/
of pRSVlacZto normalize for the efficiency of DNA uptake. The total amount of DNA in each transfection was kept con-stant by the inclusion of appropriate amounts of vector lacking CDP sequences. The results showed that CDP overexpression suppresses reporter gene expression from the MMTV LTR, and the highest levels of suppression were observed when 40
g of the reporter construct was used (data not shown). To determine the dose dependence of CDP effects on MMTV transcription, we cotransfected increasing amounts of the CDP expression vector into HC11 cells by electroporation using 40g of pLC-LUC and 0.5g of pRL-TK vector as a transfection control. After 48 h of incubation, cells were har-vested and a portion was used for Western blotting to deter-mine CDP levels (Fig. 3A). As expected, densitometry showed that levels of CDP increased approximately 50-fold following transfection with the highest levels of the CDP vector (com-pare lanes 1 and 5) whereas the levels of actin in the same extracts were relatively constant (Fig. 3A, bottom). Four dif-ferent transfections performed in the same manner were also analyzed for reporter gene activity (Fig. 3B). Transfection of 5
g of the CDP vector gave a two- to threefold decrease in luciferase activity compared to endogenous levels of CDP found in HC11 cells transfected with the empty vector, whereas 30g of the CDP-containing vector showed over 20-fold sup-pression of MMTV LTR activity. These levels of CDP did not appear to be toxic to the cells, and the levels of actin (Fig. 3A, bottom) and luciferase expression from the Renilla control vector were relatively constant when different amounts of CDP vector were used for transfection. Similar results were ob-served after CDP overexpression in a second mouse mammary
cell line, NMuMG (Fig. 3C). These experiments showed that transcription from the MMTV LTR is suppressed in a dose-dependent manner by CDP overexpression.
Mapping of CDP binding sites in the pNRE.CDP
overex-pression in transient-transfection experiments indicates that CDP represses expression from the MMTV LTR. This repres-sion may be the result of CDP binding to NRE sequences upstream of the MMTV promoter or, alternatively, the repres-sion may be an indirect effect of other CDP activities. If re-pression of MMTV LTR-reporter gene exre-pression was due to direct CDP binding to the pNRE, mutation of CDP binding sites should alleviate the transcriptional suppression. Our pre-vious experiments showed that both CDP and SATB1 bound to a 22-bp sequence at the 3⬘ end of the pNRE (41). However, mutations of this sequence had little effect on CDP binding in EMSAs with the 120-bp pNRE fragment, suggesting that there is more than one CDP binding site in the pNRE.
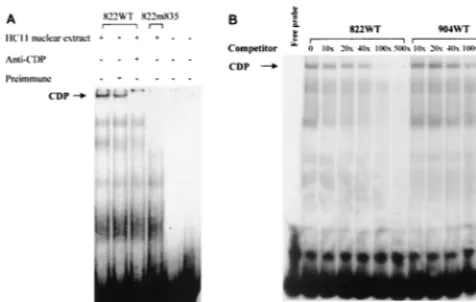
To localize all of the CDP binding sites, we performed a series of DNase I footprinting assays with bacterially expressed purified CDP (40). Because full-length CDP is insoluble, an N-terminal deletion that removed the coiled-coil and CR1 domains of CDP (39) was used for footprinting with an end-labeled 120-bp fragment from the pNRE (Fig. 1). Using both upper- and lower-strand probes, we detected two CDP binding sites (Fig. 4). One site was localized to the 5⬘end of the pNRE (⫹830 to⫹845) using the upper-strand probe and from⫹832 to⫹843 using the lower-strand probe. A second site at the 3⬘
[image:4.612.70.541.72.297.2]end of the pNRE was localized using the upper-strand probe (⫹920 to⫹931). As predicted from our previous experiments, the latter site spanned the 3⬘half of the 7-bp inverted repeat FIG. 2. CDP levels and NRE-binding activity decrease during mammary gland development. (A) CDP binding activity declines during mammary gland differen-tiation. Nuclear extracts were obtained from pooled tissues of virgin (V-MG), first-pregnancy (P-MG), and lactating (L-MG) BALB/c mice as described in Materials and Methods. Extracts (5g) were incubated with no serum (lanes 1 to 3), preimmune rabbit serum (lanes 4 to 6), or rabbit anti-CDP serum (lanes 7 to 9) prior to incubation with a labeled tetramer of a 22-bp oligomer in the pNRE (ca. 2.5 fmol). This oligomer spans the 3⬘CDP binding site of the pNRE shown in Fig. 1 (pNRE4) (41). Monomers and dimers of the 22-bp oligomer do not show detectable CDP binding using nuclear extracts (41). A reaction mixture with the probe in the absence of the extract is shown in lane 10. Samples were analyzed on a 4% nondenaturing polyacrylamide gel. (B) SP1 binding activity in virgin, pregnant, and lactating mouse mammary gland extracts. Approximately 260 fmol of probe (plus strand; 5⬘GGTGACGGGCGGGCCCGCCCCCCTCC 3⬘) was added to 20g of protein from each extract. Binding reaction mixtures were analyzed on gels as described for panel A. (C) Western blots of nuclear extracts from mammary glands at different developmental stages. The mammary gland extracts used were the same as those used for panels A and B. Approximately 65g of each extract was analyzed on an 8% polyacrylamide gel containing SDS prior to transfer to a nitrocellulose membrane. The Western blot shown at the top was incubated with anti-CDP serum, whereas the blot shown at the bottom was developed with anti-actin serum. Both blots were washed and developed as described in Materials and Methods. The positions of molecular size markers are shown to the left.
on November 9, 2019 by guest
http://jvi.asm.org/
and the 5-bp gap and overlapped a SATB1-binding site in the pNRE (41). In agreement with previous results, both of the identified CDP binding regions were AT rich (3, 27) and con-tained the ATTA sequence typical of DNA-binding sites for transcription factors with homeodomains (22, 26).
CDP binding to mutant sequences in the pNRE.To confirm
the additional CDP binding site detected by DNase I footprint-ing, we synthesized 31-bp oligonucleotides containing the wild-type (822WT) CDP binding sites at the 5⬘end of the pNRE starting at position⫹822. A mutant oligomer also was synthe-sized from the same region so that the ATTATA sequence starting at⫹835 was changed to GGTACC, thus generating a KpnI recognition site (Fig. 4). Each oligonucleotide was la-beled and used for EMSAs with nuclear extracts obtained from the mouse mammary gland cell line HC11 (Fig. 5A). As ex-pected, CDP bound to the wild-type oligomer in the presence of preimmune rabbit serum (lane 2) and this binding was abolished in the presence of CDP-specific serum (lane 3). However, introduction of the 4-bp mutation into the wild-type oligomer (m835) eliminated detectable binding by CDP (lane 4). To compare the relative affinity of CDP for the 5⬘and 3⬘
[image:5.612.316.544.72.331.2]binding sites in the pNRE, binding of the labeled 5⬘ oligonu-cleotide (822WT) was competed with increasing amounts of the unlabeled homologous oligomer or an oligomer containing the 3⬘ CDP binding site (904WT) (Fig. 5B). PhosphorImager analysis showed that a 15-fold excess of homologous compet-itor was necessary to obtain a twofold reduction in CDP bind-ing, whereas an approximately 110-fold excess of heterologous FIG. 3. Overexpression of CDP suppresses MMTV LTR-reporter gene
ex-pression in mammary cells. (A) Western blots showing CDP overexex-pression in HC11 mammary cells. HC11 cells were transfected by electroporation with an MMTV LTR-luciferase reporter gene construct (pLC-LUC) (40g) and various ratios of a CDP expression vector or a vector control lacking CDP sequences. After 48 h, cytoplasmic extracts were prepared and divided into two parts. One part of the extract was used for Western blotting (15g for each lane) using antiserum specific for CDP (top) or actin (bottom). Blots were developed as described in the legend to Fig. 2. (B) CDP overexpression suppresses MMTV LTR promoter activity in HC11 cells. The second part of the extract obtained as described for panel A was used for luciferase assays, and the results are ex-pressed relative to MMTV LTR activity in the absence of CDP overexpression. The data shown are averages of three independent experiments, and transfec-tions were performed in triplicate for each experiment. Standard deviatransfec-tions from the mean are indicated by error bars. LUC, luciferase. (C) CDP overexpression suppresses MMTV LTR promoter activity in NMuMG cells. Other parameters are the same as those described for panel B.
FIG. 4. DNase I footprinting of the pNRE in the MMTV LTR. The 120-bp fragment from the pNRE was end labeled on the lower strand (lanes 2 to 6) or the upper strand (lanes 7 to 11), and footprinting was performed as described in Materials and Methods. Lane 1 shows a Maxam-Gilbert sequencing reaction of the 120-bp fragment. Bacterially produced CDP (CR2-Cterm) (39) was used in the reaction mixtures in lanes 2, 3, 7, and 8 (10g in lanes 3 and 8 and 15g in lanes 2 and 7 with DNase I at 0.8 U/ml). The amount of DNase I was titrated in lanes without CDP (lanes 4 and 9, 0.6 U/ml; lanes 5 and 10, 0.4 U/ml; lanes 6 and 11, 0.2 U/ml). Reaction mixtures were analyzed on an 8% denaturing polyacryl-amide gel. The protected regions are shown to each side of the gel. The numbers in parentheses indicate the locations of the protected regions relative to the first base of the C3H MMTV LTR (44). The locations of mutations used in this study with respect to the DNase I-protected regions are shown below the wild-type (WT) LTR sequence. The cross-hatch marks indicate that the entire region between CDP footprints is not shown. The location of the imperfect inverted repeats at the 3⬘end of the pNRE is also shown by arrows. A 3⬘CDP binding site on the lower strand was not observed.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:5.612.52.294.78.519.2]competitor was required to achieve the same amount of com-petition. This result indicates that the 5⬘CDP binding site in the pNRE has a higher affinity for CDP than does the 3⬘site. Because footprinting assays revealed two CDP binding sites in the pNRE, it was important to examine the effects of binding
11). The small amount of residual binding observed with the m835 probe was due to CDP, since binding was abolished in the presence of CDP-specific serum (lane 12). As expected, combination of mutations at the 5⬘ end of the pNRE (835, 839a, and 839b) with a mutation at the 3⬘end (916) reduced or completely disrupted CDP binding under these conditions (lane 14 and data not shown). Thus, these results confirmed the presence of two CDP binding sites within the pNRE and suggested that mutations in the 5⬘site have a greater effect on CDP binding than mutations in the 3⬘site.
[image:6.612.54.292.74.225.2]To determine the relative affinities of CDP for mutant pNRE binding sites, we performed EMSAs with the labeled wild-type pNRE fragment in the presence of increasing amounts of unlabeled mutant fragments from the same region (locations are shown in Fig. 4). Based on quantitation by Phos-phorImager analysis, mutations in the 3⬘ binding site (m924 and m916) had relatively minor effects on CDP binding to the pNRE, whereas mutations in the 5⬘ binding site (m835 and m839a) had a more dramatic effect, as expected (Fig. 6B to F). However, a fragment containing both 5⬘ and 3⬘ binding site mutations (m835/916) was the poorest competitor for CDP binding to the pNRE (Fig. 6D). Because the double mutant showed some competition for CDP binding to the wild-type FIG. 5. CDP binding site mutations in oligomers containing the 5⬘or 3⬘
binding sites from the pNRE. Nuclear extracts (7g of protein) from HC11 mammary cells were used in EMSAs prior to analysis on 4% nondenaturing polyacrylamide gels. (A) EMSA using oligomer probes with CDP binding site mutations at the 5⬘end of the pNRE. Oligomers spanning the wild-type (822WT) or mutant (822m835) 5⬘CDP binding site in the pNRE were labeled, and ca. 100 fmol of probe was incubated with nuclear extract and anti-CDP or preimmune serum, as indicated, prior to gel analysis. Reaction mixtures without added protein are shown for the 822WT and 822m835 probes in lanes 5 and 6, respec-tively. (B) Competition of unlabeled 5⬘or 3⬘oligomers for CDP binding to the labeled 5⬘oligomer probe. Approximately 105cpm (ca. 100 fmol) of wild-type 822 double-stranded oligomer (822WT) (plus strand; 5⬘GGC AAC AGG TAC ATG ATT ATA TTT ATC TAG GGG 3⬘) was used in an EMSA with a 10- to 500-fold excess of unlabeled homologous (lanes 3 to 7) or heterologous (904WT) (lanes 8 to 12) oligomer (spanning the 5⬘or 3⬘CDP-binding site, respectively, in the pNRE) (plus strand; 5⬘ GGGGCTGGACTAATAGAACATTATTCTG CAA 3⬘).
FIG. 6. Binding of CDP from mammary cells to mutant NREs. Nuclear extracts from HC11 mammary cells (400 ng) were incubated with wild-type or mutant pNRE probes (2⫻104cpm) prior to analysis on nondenaturing polyacrylamide gels. (A) The wild-type 120-bp fragment or mutant fragments (ca. 2.5 fmol) from the same region were end labeled and analyzed on gels without nuclear extract (lanes 1, 5, 9, and 13) or with nuclear extracts (all other lanes). In some cases, reaction mixtures contained preimmune (lanes 3, 7, and 11) or anti-CDP (lanes 4, 8, and 12) serum. (B) Competition of the labeled 110-bp pNRE (ca. 2.5 fmol) with the wild-type (WT) homologous or heterologous (m835) fragments from the same region. Competitor DNA was present in 10- to 200-fold molar excess of labeled DNA. (C) Competition of the labeled 110-bp pNRE with the unlabeled m924 and m916 fragments from the same region. (D) Competition of the labeled 110-bp pNRE with the unlabeled wild-type or double mutant (m835/916) fragments from the same region. (E) Competition of the labeled 110-bp pNRE with the unlabeled mutant (m839a) fragment from the same region. (F) Competition of the labeled 110-bp pNRE with the unlabeled mutant (m839b) or wild-type fragment from the same region.
on November 9, 2019 by guest
http://jvi.asm.org/
NRE, this mutant may retain residual CDP binding activity for the NRE that is not detectable in direct binding assays.
Effects of CDP binding site mutations on MMTV promoter
activity in transfection assays.If CDP suppression of MMTV
expression is due to CDP binding to the MMTV promoter, then CDP binding site mutations should elevate viral gene expression. To test this hypothesis, we inserted the CDP bind-ing site mutations described above into the pNRE of the MMTV C3H LTR-luciferase construct (LC-LUC). The result-ing constructs were tested for reporter gene activity in tran-sient-transfection assays with HC11 cells (Fig. 7).
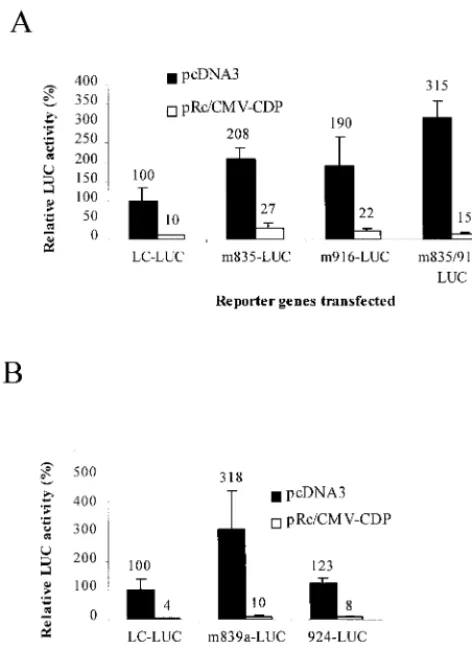
Each construct was coelectroporated with a CDP overex-pression plasmid, and reporter gene exoverex-pression was compared to that of the same construct electroporated with a control vector (Fig. 7A and B). After normalization for DNA uptake, these experiments showed that mutations in the 5⬘or 3⬘CDP binding site of the pNRE elevated luciferase expression two- to fourfold without CDP overexpression, compared to the
wild-type construct. Thus, mutations that affected CDP binding to the pNRE also relieved CDP-mediated LTR suppression, al-though differences between the activity of the 916 mutant and that of the wild-type LTR were marginally significant (P ⫽
0.06). A combination of 5⬘ and 3⬘ binding site mutations (m835/916) showed approximately the same effect on MMTV LTR repression as the single-site mutations alone. However, in most cases, the effect of CDP binding site mutations was di-minished by CDP overexpression (see Discussion).
Because CDP is a matrix-associated region-binding protein (7, 15, 41, 64), complete chromatin structure may be important for maximum effects on repressor function. Therefore, analysis of CDP binding site mutants in stable-transfection assays al-lowed us to analyze integrated templates and to ensure that the majority of cells contained the reporter construct. Each of the constructs was cotransfected with a selectable marker plasmid into six independent cultures of HC11 cells using a lipid-me-diated method (DMRIE-C). The six cultures containing the same plasmid were harvested, pooled, and assayed for lucif-erase activity; the luciflucif-erase activity then was normalized for DNA content using a quantitative PCR method. The results showed that mutation of the 5⬘CDP binding site in the pNRE (m835) gave approximately sixfold (600%) elevation of lucif-erase activity from the MMTV LTR compared to transfectants containing wild-type LTR-luciferase constructs (Fig. 8). Two or four base pair changes at position ⫹839 also increased luciferase activity four- or sixfold, respectively, compared to the wild-type construct, whereas a mutation at ⫹924 that changed 3 bp of the 3⬘binding site in the pNRE (and had little effect on CDP DNA binding) had little effect on luciferase expression. Mutation of both the 5⬘and 3⬘CDP binding sites (m835/916) in LTR-reporter gene constructs gave a similar (5-fold) elevation of luciferase activity, compared to wild-type constructs, as constructs with mutations in the 5⬘ site alone. Therefore, results from transient- and stable-transfection as-says suggested that a single mutation in the pNRE is sufficient to alter transcriptional suppression by CDP.
DISCUSSION
A specific role for CDP in the developing mammary gland.
[image:7.612.311.549.72.219.2]Studies of CDP levels in the differentiating mammary gland showed that CDP levels declined during development of the
FIG. 7. Transient-transfection analysis of MMTV LTR-reporter gene con-structs with pNRE mutations. HC11 cells were transfected by electroporation with the C3H MMTV LTR-luciferase (LUC) plasmid (pLC-LUC) or mutant plasmids (40g), the pcDNA3 vector with or without CDP sequences (20g), and 0.7g of pRL-TK. Transfections were performed in triplicate with HC11 cells. Because of the large number of samples tested, mutants were tested in separate experiments (shown as panels A and B). After normalization for DNA uptake, results are reported relative to the average of three transfections of pLC-LUC with the pcDNA3 control vector (assigned a value of 100). The error bars show standard deviations from the mean. Results were analyzed by the one-tailed Studentttest. Analysis showed that values from m835, 835/916, and 839a were significantly different than those from wild-type pLC-LUC (P ⫽ ⬍0.05), whereas m916 values were marginally different (P⫽0.06). As expected, m924 results were not significantly different than those from the wild-type plas-mid (P⫽0.18).
FIG. 8. Stable transfections of MMTV LTR-reporter gene constructs with pNRE mutations. Transfections were performed as described in Materials and Methods using DMRIE-C reagent. Pooled colonies obtained for each plasmid construct were assayed for luciferase (LUC) activity, and the results were nor-malized for the amount of transfected DNA. Results for mutants are reported relative to those obtained with wild-type C3H MMTV LTR constructs in the same assay (assigned a value of 100).
on November 9, 2019 by guest
http://jvi.asm.org/
[image:7.612.58.294.74.398.2]regulator of mammary gland-specific transcription.
Although CDP binding site mutations were able to relieve suppression of the MMTV LTR-reporter gene up to sixfold in stable-transfection assays (Fig. 8), these same binding site mu-tations had a more modest effect in transient-transfection as-says either with or without CDP overexpression (Fig. 7). There are several possible explanations for this. (i) We have mapped at least six CDP binding sites within the MMTV NRE, and there is at least one other potential site in the 5⬘portion of the LTR (TFSEARCH, version 1.3, Y. Akiyama, Kyoto Universi-ty). Therefore, we believe that mutation of one or two sites is unlikely to completely relieve suppression by CDP. (ii) We have shown that most of the mutations do not completely eliminate CDP binding to the target site. Even in mutants that had two binding site alterations, residual competition was ob-served for CDP binding to wild-type sites. In addition, overex-pression of CDP will maximize CDP binding to low-affinity mutant sites, thus minimizing the effect of the mutation in reporter assays. (iii) CDP has been reported to repress tran-scription of genes by blocking the binding of activator proteins (43). Thus, our CDP binding site mutations also may have affected the binding of transcriptional activator proteins, so that the overall reporter gene expression in the absence of CDP binding will not achieve levels observed without CDP overexpression. Any or all of these factors may contribute to the modest effect of two CDP binding site mutations in the transient transfections.
MMTV is known to replicate in B- and T-lymphoid cells during the transmission of virus from mother’s milk in the newborn murine gut to target epithelial cells in the mammary gland (9, 24, 29). We have shown previously that MMTV expression is repressed by the homeodomain protein SATB1, and we have proposed that SATB1 restricts MMTV expression in many tissues other than the mammary gland, particularly T cells (40, 41). Our studies also have shown that SATB1 binding activity for the MMTV NREs is absent in mammary gland tissue, as well as in cultured mammary cell lines (13, 41). Thus, it appears that negative regulation of MMTV expression is different in differentiated mammary gland tissue than in T cells. Our experiments suggest that a decrease in CDP molec-ular mass may cause loss of CDP DNA-binding activity in the lactating mammary gland. This may represent a novel post-transcriptional mechanism, such as alternative splicing or pro-tein processing, for control of CDP-mediated repression. Re-cent experiments with other viruses, such as human papillomavirus (HPV), suggest that loss of CDP binding to HPV early promoters correlates with cellular differentiation and high virus expression (1). Thus, loss of negative regulatory proteins during mammary gland differentiation combined with the action of positive factors, such as hormone-activated ste-roid receptors and Stat factors (53, 54), leads to high MMTV expression in the lactating mammary gland.
Mice lacking expression of the full-length CDP have been
transcriptional suppressor CDP (41). One of the CDP-binding sites was localized to a 22-bp oligonucleotide in the pNRE that overlapped an imperfect inverted repeat containing a binding site for homeodomain protein SATB1 (41). Using DNase I protection assays, we confirmed that there was a CDP binding site localized between ⫹920 and ⫹931, and we identified a second site at⫹830 to⫹845 (Fig. 4). As expected, mutations in either of these sites reduced CDP binding to the 120-bp pNRE in gel shift assays (Fig. 5). Mutations in both binding sites abolished or further reduced binding, as measured in gel shift assays, suggesting that CDP interactions with the MMTV NRE are additive.
Previous experiments using the gene for the cytochromeb heavy-chain component (gp91) of the phagocyte NADPH-ox-idase (phox) have shown that CDP has at least four different binding sites upstream of the gp91-phoxpromoter (42). More-over, there appear to be multiple CDP binding sites in the HPV long control region (1), as well as in the matrix-associated region of the immunoglobulin heavy-chain intronic enhancer (64, 70). Results obtained with the gp91-phoxpromoter sug-gested that the promoter-proximal CDP binding site has the highest affinity for CDP and that the affinity for CDP decreases with the distance from the promoter (42). However, this may not be generally true for CDP-mediated repression since the most promoter-proximal CDP binding site in the MMTV pNRE appeared to have a lower affinity for CDP than the 5⬘
site (Fig. 5). EMSAs using oligonucleotides spanning the 5⬘or 3⬘CDP binding sites in the pNRE and extracts from mammary cells showed CDP binding to the 5⬘ site but no detectable binding to the 3⬘site (data not shown). Nevertheless, purified CDP fragments containing CR2-Cterm showed very similar binding to both the 5⬘and 3⬘sites in the pNRE, suggesting that the full-length CDP found in nuclear extracts can discriminate between the two sites.
CDP has four different DNA-binding domains, and each of these domains has a different specificity for DNA, although the CR2 and CR3 domains appear to have the most similar DNA-binding properties (3, 27). Thus, the presence of CR1 in the full-length protein may decrease its affinity for the 3⬘ site or increase its affinity for the 5⬘site in the pNRE. Alternatively, differences in the abilities of the native and bacterial proteins to bind to the 3⬘site in the pNRE may result from the ability of native CDP to dimerize with certain proteins due to the coil domain within the N-terminal region; the coiled-coil is missing in the bacterially expressed proteins. CDP has been shown to interact with a number of proteins in vivo, including SATB1, histone deacetylase 1 (HDAC1), and Rb (38, 40, 63), and SATB1 has been shown to interact with three of the four CDP DNA-binding domains (CR1, CR2, and the homeodomain) (40). Together, these observations argue that cooperative interactions between different CDP DNA-binding domains and between different CDP molecules bound to ad-jacent DNA-binding sites may be important for promoter
on November 9, 2019 by guest
http://jvi.asm.org/
ognition in vivo. The ability of CDP to bind to the promoters and enhancers of a large number of genes, including those for c-mos,␥-globin, Ncam, histones, gp91-phox, HPV E1, E6, and E7, c-myc, the immunoglobulin heavy and light chains, TCR, and CD8␣, MMTV, and the cystic fibrosis transmembrane conductance regulatory gene (1, 7, 8, 15, 16, 21, 31, 38, 41, 57, 61, 64), may depend upon the presence of multiple DNA-binding domains with different DNA-DNA-binding and protein in-teraction specificities.
Mechanism of CDP transcriptional suppression.
Experi-ments by Mailly et al. (43) have indicated that CDP represses transcription by competition for DNA binding by positive reg-ulators, as well as by active repression, perhaps mediated by chromatin reorganization (38). It is likely that transient-trans-fection assays measure competition for transcription factor binding to DNA but not chromatin reorganization. Both tran-sient- and stable-transfection assays for MMTV promoter function were affected by CDP binding site mutations, proba-bly because CDP binding to DNA is a requirement for both types of repression.
CDP is known to have multiple binding sites in the promot-ers or enhancpromot-ers of other genes, such as the immunoglobulin heavy-chain intronic enhancer or the gp91-phoxpromoter (42, 70), where CDP binding sites are thought to overlap binding sites for transcriptional activator proteins (57, 64). Because CDP has a stronger affinity for these sites than the activator protein, binding of CDP prevents binding of the activator, thus leading to repression of gene expression. Although most of these activators are not known, recent results suggest that immunoglobulin heavy-chain transcription may be, in part, re-stricted to B cells through the binding of B-cell-specific acti-vators, such as Bright (30), that accompany the decreasing levels of CDP repressor present during B-cell differentiation (56). The identities of activators that bind to MMTV LTR sites occupied by CDP during mammary gland differentiation are unknown. However, it is an attractive possibility that CDP binding sites could be used to isolate proteins that activate transcription following the loss of CDP binding activity that accompanies differentiation of the mammary gland. Because the loss of CDP occurs in a number of differentiating cell types (1, 56, 57), this strategy also may be applicable to the isolation of transcriptional activators in other tissues.
ACKNOWLEDGMENTS
We acknowledge Susan Ross, Paul Gottlieb, and members of the Dudley laboratory for careful review of the manuscript.
This work was supported by grant R01CA34780 from the National Institutes of Health.
REFERENCES
1.Ai, W., E. Toussaint, and A. Roman.1999. CCAAT displacement protein binds to and negatively regulates human papillomavirus type 6 E6, E7, and E1 promoters. J. Virol.73:4220–4229.
2.Andres, V., B. Nadal-Ginard, and V. Mahdavi.1992.Clox, a mammalian homeobox gene related toDrosophila cut, encodes DNA-binding regulatory proteins differentially expressed during development. Development116:321– 334.
3.Aufiero, B., E. J. Neufeld, and S. H. Orkin.1994. Sequence-specific DNA binding of individual cut repeats of the human CCAAT displacement/cut homeodomain protein. Proc. Natl. Acad. Sci. USA91:7757–7761. 4.Ball, J. K., L. O. Arthur, and G. A. Dekaban.1985. The involvement of a
type-B retrovirus in the induction of thymic lymphomas. Virology140:159– 172.
5.Ball, J. K., H. Diggelmann, G. A. Dekaban, G. F. Grossi, R. Semmler, P. A. Waight, and R. F. Fletcher.1988. Alterations in the U3 region of the long terminal repeat of an infectious thymotropic type B retrovirus. J. Virol.
62:2985–2993.
6.Ball, R. K., R. R. Friis, C. A. Schoenenberger, W. Doppler, and B. Groner.
1988. Prolactin regulation of-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J.
7:2089–2095.
7.Banan, M., I. C. Rojas, W. H. Lee, H. L. King, J. V. Harriss, R. Kobayashi, C. F. Webb, and P. D. Gottlieb.1997. Interaction of the nuclear matrix-associated region (MAR)-binding proteins, SATB1 and CDP/Cux, with a MAR element (L2a) in an upstream regulatory region of the mouse CD8␣ gene. J. Biol. Chem.272:18440–18452.
8.Barberis, A., G. Superti-Furga, and M. Busslinger.1987. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell50:347–359. 9.Beutner, U., E. Kraus, D. Kitamura, K. Rajewsky, and B. T. Huber.1994. B
cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J. Exp. Med.179:1457–1466. 10. Blochlinger, K., R. Bodmer, J. Jack, L. Y. Jan, and Y. N. Jan.1988. Primary
structure and expression of a product fromcut, a locus involved in specifying sensory organ identity inDrosophila. Nature333:629–635.
11. Blochlinger, K., L. Y. Jan, and Y. N. Jan.1991. Transformation of sensory organ identity by ectopic expression of Cut in Drosophila. Genes Dev.
5:1124–1135.
12. Bodmer, R., S. Barbel, S. Sheperd, J. W. Jack, L. Y. Jan, and Y. N. Jan.1987. Transformation of sensory organs by mutations of thecutlocus ofD. mela-nogaster. Cell51:293–307.
13. Bramblett, D., C. L. Hsu, M. Lozano, K. Earnest, C. Fabritius, and J. Dudley.1995. A redundant nuclear protein binding site contributes to neg-ative regulation of the mouse mammary tumor virus long terminal repeat. J. Virol.69:7868–7876.
14. Bruggemeier, U., M. Kalff, S. Franke, C. Scheidereit, and Beato.1991. Ubiquitous transcription factor OTF-1 mediates induction of the MMTV promoter through synergistic interaction with hormone receptors. Cell64:
565–572.
15. Chattopadhyay, S., C. E. Whitehurst, and J. Chen.1998. A nuclear matrix attachment region upstream of the T cell receptorgene enhancer binds Cux/CDP and SATB1 and modulates enhancer-dependent reporter gene expression but not endogenous gene expression. J. Biol. Chem.273:29838– 29846.
16. Cunningham, J. M., M. E. Purucker, S. M. Jane, B. Safer, E. F. Vanin, P. A. Ney, C. H. Lowrey, and A. W. Nienhuis.1994. The regulatory element 3⬘to theA␥-globin gene binds to the nuclear matrix and interacts with special A-T-rich binding protein 1 (SATB1), an SAR/MAR-associating region DNA binding protein. Blood84:1298–1308.
17. Dekaban, G. A., and J. K. Ball.1984. Integration of type B retroviral DNA in virus-induced primary murine thymic lymphomas. J. Virol.52:784–792. 18. Dickson, C., R. Smith, S. Brookes, and G. Peters.1990. Proviral insertions
within theint-2gene can generate multiple anomalous transcripts but leave the protein-coding domain intact. J. Virol.64:784–793.
19. Dignam, J. D., P. L. Martin, B. S. Shastry, and R. G. Roeder.1983. Eukary-otic gene transcription with purified components. Methods Enzymol.101:
582–598.
20. Dudley, J. P.1999. Mouse mammary tumor virus p. 965–972. InR. G. Webster and A. Granoff (ed.), Encyclopedia of virology. Academic Press Ltd., London, United Kingdom.
21. Dufort, D., and A. Nepveu.1994. The human cut homeodomain protein represses transcription from thec-mycpromoter. Mol. Cell. Biol.14:4251– 4257.
22. Friedman-Einat, M., P. Einat, M. Snyder, and F. Ruddle.1996. Target gene identification: target specific transcriptional activation by three murine ho-meodomain/VP16 hybrid proteins inSaccharomyces cerevisiae. J. Exp. Zool.
274:145–156.
23. Gilead, Z., Y. H. Jeng, W. S. Wold, K. Sugawara, H. M. Rho, M. L. Harter, and M. Green.1976. Immunological identification of two adenovirus 2-in-duced early proteins possibly involved in cell transformation. Nature264:
263–266.
24. Golovkina, T. V., A. Chervonsky, J. P. Dudley, and S. R. Ross.1992. Trans-genic mouse mammary tumor virus superantigen expression prevents viral infection. Cell69:637–645.
25. Golovkina, T. V., A. B. Jaffe, and S. R. Ross.1994. Coexpression of exoge-nous and endogeexoge-nous mouse mammary tumor virus RNA in vivo results in viral recombination and broadens the virus host range. J. Virol.68:5019– 5026.
26. Guazzi, S., M. L. Pintonello, A. Vigano, and E. Boncinelli.1998. Regulatory interactions between the human HOXB1, HOXB2, and HOXB3 proteins and the upstream sequence of theOtx2gene in embryonal carcinoma cells. J. Biol. Chem.273:11092–11099.
27. Harada, R., G. Berube, O. J. Tamplin, C. Denis-Larose, and A. Nepveu.
1995. DNA-binding specificity of the cut repeats from the human cut-like protein. Mol. Cell. Biol.15:129–140.
28. Haraguchi, S., R. A. Good, R. W. Engelman, S. Greene, and N. K. Day.1997. Prolactin, epidermal growth factor or transforming growth factor-␣activate a mammary cell-specific enhancer in mouse mammary tumor virus-long terminal repeat. Mol. Cell. Endocrinol.129:145–155.
29. Held, W., G. A. Waanders, A. N. Shakhov, L. Scarpellino, H. Acha-Orbea, and H. R. MacDonald.1993. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmis-sion. Cell74:529–540.
on November 9, 2019 by guest
http://jvi.asm.org/
BALB/c mammary gland. J. Virol.59:518–521.
35. Lavallie, E. R., J. M. McCoy, D. B. Smith, and P. Riggs.1994. Enzymatic and chemical cleavage of fusion proteins, p. 16.4.5–16.4.17.InF. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
36. Lee, W. T., O. Prakash, D. Klein, and N. H. Sarkar.1987. Structural alter-ations in the long terminal repeat of an acquired mouse mammary tumor virus provirus in a T-cell leukemia of DBA/2 mice. Virology159:39–48. 37. Lefebvre, P., D. S. Berard, M. G. Cordingley, and G. L. Hager.1991. Two
regions of the mouse mammary tumor virus long terminal repeat regulate the activity of its promoter in mammary cell lines. Mol. Cell. Biol.11:2529– 2537.
38. Li, S., L. Moy, N. Pittman, G. Shue, B. Aufiero, E. J. Neufeld, N. S. LeLeiko, and M. J. Walsh.1999. Transcriptional repression of the cystic fibrosis transmembrane conductance regulator gene, mediated by CCAAT displace-ment protein/cut homolog, is associated with histone deacetylation. J. Biol. Chem.274:7803–7815.
39. Lievens, P. M., J. J. Donady, C. Tufarelli, and E. J. Neufeld.1995. Repressor activity of CCAAT displacement protein in HL-60 myeloid leukemia cells. J. Biol. Chem.270:12745–12750.
40. Liu, J., A. Barnett, E. J. Neufeld, and J. P. Dudley.1999. Homeoproteins CDP and SATB1 interact: potential for tissue-specific regulation. Mol. Cell. Biol.19:4918–4926.
41. Liu, J., D. Bramblett, Q. Zhu, M. Lozano, R. Kobayashi, S. R. Ross, and J. P. Dudley.1997. The matrix attachment region-binding protein SATB1 partic-ipates in negative regulation of tissue-specific gene expression. Mol. Cell. Biol.17:5275–5287.
42. Luo, W., and D. G. Skalnik.1996. CCAAT displacement protein competes with multiple transcriptional activators for binding to four sites in the prox-imal gp91phoxpromoter. J. Biol. Chem.271:18203–18210.
43. Mailly, F., G. Berube, R. Harada, P. L. Mao, S. Phillips, and A. Nepveu.
1996. The human cut homeodomain protein can repress gene expression by two distinct mechanisms: active repression and competition for binding site occupancy. Mol. Cell. Biol.16:5346–5357.
44. Majors, J. E., and H. E. Varmus.1983. Nucleotide sequencing of an appar-ent proviral copy ofenvmRNA defines determinants of expression of the mouse mammary tumor virusenvgene. J. Virol.47:495–504.
45. Michalides, R., and E. Wagenaar.1986. Site-specific rearrangements in the long terminal repeat of extra mouse mammary tumor proviruses in murine T-cell leukemias. Virology154:76–84.
46. Mink, S., E. Hartig, P. Jennewein, W. Doppler, and A. C. Cato.1992. A mammary cell-specific enhancer in mouse mammary tumor virus DNA is composed of multiple regulatory elements including binding sites for CTF/ NFI and a novel transcription factor, mammary cell-activating factor. Mol. Cell. Biol.12:4906–4918.
47. Mok, E., T. V. Golovkina, and S. R. Ross.1992. A mouse mammary tumor virus mammary gland enhancer confers tissue-specific but not lactation-dependent expression in transgenic mice. J. Virol.66:7529–7532. 48. Morley, K. L., M. G. Toohey, and D. O. Peterson.1987. Transcriptional
repression of a hormone-responsive promoter. Nucleic Acids Res.15:6973– 6989.
49. Neufeld, E. J., D. G. Skalnik, P. M. Lievens, and S. H. Orkin.1992. Human CCAAT displacement protein is homologous to theDrosophila homeopro-tein, cut. Nat. Genet.1:50–55.
50. Nusse, R.1990. The intgenes in mouse mammary tumorigenesis and in
55. Ross, S. R., C. L. Hsu, Y. Choi, E. Mok, and J. P. Dudley.1990. Negative regulation in correct tissue-specific expression of mouse mammary tumor virus in transgenic mice. Mol. Cell. Biol.10:5822–5829.
56. Scheuermann, R. H., and U. Chen.1989. A developmental-specific factor binds to suppressor sites flanking the immunoglobulin heavy-chain enhancer. Genes Dev.3:1255–1266.
57. Skalnik, D. G., E. C. Strauss, and S. H. Orkin.1991. CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phoxgene pro-moter. J. Biol. Chem.266:16736–16744.
58. Smith, D. B.1993. Purification of glutathione-S-transferase fusion proteins. Methods Mol. Cell. Biol.4:220–229.
59. Toohey, M. G., J. W. Lee, M. Huang, and D. O. Peterson.1990. Functional elements of the steroid hormone-responsive promoter of mouse mammary tumor virus. J. Virol.64:4477–4488.
60. Tufarelli, C., Y. Fujiwara, D. C. Zappulla, and E. J. Neufeld.1998. Hair defects and pup loss in mice with targeted deletion of the first cut repeat domain of the Cux/CDP homeoprotein gene. Dev. Biol.200:69–81. 61. Valarche, I., J. P. Tissier-Seta, M. R. Hirsch, S. Martinez, C. Goridis, and
J. F. Brunet.1993. The mouse homeodomain protein Phox2 regulates Ncam promoter activity in concert with Cux/CDP and is a putative determinant of neurotransmitter phenotype. Development119:881–896.
62. van Leeuwen, F., and R. Nusse.1995. Oncogene activation and oncogene cooperation in MMTV-induced mouse mammary cancer. Semin. Cancer Biol.6:127–133.
63. van Wijnen, A. J., C. Cooper, P. Odgren, F. Aziz, A. De Luca, R. A. Shakoori, A. Giordano, P. J. Quesenberry, J. B. Lian, G. S. Stein, and J. L. Stein.1997. Cell cycle-dependent modifications in activities of pRb-related tumor sup-pressors and proliferation-specific CDP/cut homeodomain factors in murine hematopoietic progenitor cells. J. Cell. Biochem.66:512–523.
64. Wang, Z., A. Goldstein, R.-T. Zong, D. Lin, E. J. Neufeld, R. H. Scheuer-mann, and P. W. Tucker.1999. Cux/CDP homeoprotein is a component of NF-NR and represses the immunoglobulin heavy chain intronic enhancer by antagonizing the Bright transcription activator. Mol. Cell. Biol.19:284– 295.
65. Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner.1990. Direct gene transfer into mouse musclein vivo. Science
247:1465–1468.
66. Xu, L., T. J. Wrona, and J. P. Dudley.1996. Exogenous mouse mammary tumor virus (MMTV) infection induces endogenous MMTVsagexpression. Virology215:113–123.
67. Xu, L., T. J. Wrona, and J. P. Dudley.1997. Strain-specific expression of spliced MMTV RNAs containing the superantigen gene. Virology236:54– 65.
68. Yanagawa, S.-I., K. Kakimi, H. Tanaka, A. Murakami, Y. Nakagawa, Y. Kubo, Y. Yamada, H. Hiai, K. Kuribayashi, T. Masuda, and A. Ishimoto.
1993. Mouse mammary tumor virus with rearranged long terminal repeats causes murine lymphomas. J. Virol.67:112–118.
69. Yanagawa, S.-I., H. Tanaka, and A. Ishimoto.1991. Identification of a novel mammary cell line-specific enhancer element in the long terminal repeat of mouse mammary tumor virus, which interacts with its hormone-responsive element. J. Virol.65:526–531.
70. Zong, R. T., and R. H. Scheuermann.1995. Mutually exclusive interaction of a novel matrix attachment region binding protein and the NF-NR enhancer repressor. Implications for regulation of immunoglobulin heavy chain ex-pression. J. Biol. Chem.270:24010–24018.