Rochester Institute of Technology
RIT Scholar Works
Theses
Thesis/Dissertation Collections
6-1-1969
The reaction products and the kinetics of the
reactions between hypochlorite ions and thiosulfate
and tetrathionate ions
Thomas Dagon
Follow this and additional works at:
http://scholarworks.rit.edu/theses
This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion
in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact
ritscholarworks@rit.edu
.
Recommended Citation
THE
REACTION PRODUCTS AND rrHE KINE
'
rICS OF
THE
l~EAc'rIONS
BETvvEEN
HYPOCHLORITE IONS
AND
THIOSULF
A
TE
AN.iJ
'
rETRATHIONA
'
rE IONS
r
rHOMAS
J.
DAGON
JUNE,
1969
SPECIAL REPORT
SUBlIUTTED IN PARTIAL
FULFILL:V1ENT OF
THE
RE'~UIRK1ENTS
FOR THE DEGREE OF
MA
STER
OF SCIENCE
John A. White
Project Adviser
R. L. Craven
Department Head
T. E. Strader
Library
.3
ABSTRACT
The
reaction products ofthe
hypochlorite
oxidationof
both
thiosulfate
andtetrathionate
solutions weredetermined.
In
addition,
preliminary
kinetic
studieswere made
dealing
withthe
alkaline chlorination ofthiosulfate
solutions and withthe
hypochlorination
oftetrathionate
solutions.In
the
latter
base,
a pHINTRODUCTION
The
purpose ofthis
researchis
to
investigate
the
mechanism of
the
oxidation ofthiosulfate
andtetrathio
nate with
hypochlorite
andto
study
the
kinetics
ofthese
reactions.
The
thiosulfate
ion
is
an oxy-compound of sulfurin
which
the
sulfuris
presentin
anintermediate
oxidationstate,
positivetwo.
The
most common ofthe
thiosulfates
is
sodiumthiosulfate
(NapSpO^'5HpO),
commonly,
but
in
correctly,
referredto
ashypo.
The
thiosulfate
ion
is
relatively
staolein
a neutral or alkaline media.How
ever,
neitherfree
thiosulfuric
acid norhydrogen
thio
sulfate salts canbe
readily
preparedbecause
of reactions
between
thiosulfate
andhydrogen
ions.
However,
the
free
acidhas
been
isolated
as an etherate at -7&0
from
the
following
reaction:SO^
+H2S
*~H2S205
The
decomposition
reactionsdepend
onthe
hydrogen
ion
concentration.
At
hydrogen
ion
concentrationsbelow
pH4.6,
the
ion
couldbe
formed,
but
would undergoimmediate
decomposition
to
sulfur and sulfurdioxide.
The
lower
the
pH,
the
more rapidis
the
decomposition.
It
has
been
reportedthat
some pentathionateis
slowly
formed
atthe
sametime.
Under
someconditions,
whenhydrogen
sulfide and persulfide are alsoformed
in
smallamounts .
The
thiosulfate
ion
is
a structuralanalog
ofthe
sulfate
ion
in
which a sulfur atomhas
replaced one oxygenatom
in
the
sulfate.This
structureis
compatible withthe
observationthat
if
thiosulfate,
synthesizedusing
ordinary
sulfite and radioactive elementalsulfur,
is
acidified,
almost all ofthe
radioactivity
is
precipitatedwith
the
elemental sulfur whichis
regenerated.'This
shows
that
the
sulfur atomsin
the
thiosulfate
are notequivalent.
This
is
shownbelow:
0
0
0
S
+ :S -0
*-S
:3 -0~
>
3
+ :S -OH
I I l
0
0
OH
The
thiosulfate
ion
is
amoderately
strong
reducing
agent.
The
most common oxidation reactionit
undergoesis
the
conversion ofthe
thiosulfate
to
tetrathionate
by
iodine.
2S203= +I2
*-21" + S406=This
reactionis
widely
usedin
the
volumetricdetermina
tion
ofiodine.
Most
often,
a neutral orslightly
acidic
solution ofiodine
in
potassiumiodide
is
titrated
withthiosulfate.
Then
the
following
reaction occursrapidly
and stoichiometrically. I3" + 2S205= >-31" + S406=
5
-immediately,
it
has
been
shown^that anintermediate
SpO^I
is
formed
by
afast,
reversible reaction.S20^~
+
I,
.< >+
21"
This
intermediate
reacts withiodide.
2S203I%
+I"
This
accountsfor
the
reappearance ofiodine
nearthe
endpoint
in
the
titration
ofvery
dilute
iodine
solutions.The
intermediate
also reacts withthe
thiosulfate,
providing
the
main portion ofthe
overall reaction.+
s2o3=
+ I"
It
has
Deenfound
that
the
ratelaw
for
the
formation
oftetrathionate
involves
the
second power ofthe
SpO,I~
ion
concentration.
Although
this
reactionis
generally
stoichiometric,
several complications can occur.First,
atlow
concentrations ofiodide
(0.003
M)
the
stoichio-metry
is
nolonger
exact,
because
of some sulfateformed
by
the
reaction:S203I" +
3I3~
+
5H20
>"2S04=
+
10H+
+
101"
Also,
tetrathionate
will undergo slow oxidationby
excessiodine
to
form
sulfate ,but
the
reactionis
too
slowto
be
important
underthe
usual analytical conditions.4
long
asthe
pHis
kept
below
5
the
iodine-thio-sulfate reactionis
accurate.However,
in
alkaline solution,
(pH
=8),
iodine
will reactto
form
hypoiodite
ioni
I2
+ 20H" ^ > OT" + I" +H20
thio-sulfate
to
sulfate.This
would affectthe
titration
ofiodine
withthiosulfate.
However,
the
effect onthe
reverse reaction would
be
smallbecause
the
rapidthiosul
fate
reaction would consumethe
iodine
before
it
couldreact with
the
hydroxyl
ion.
In
avery
strong
alkalinesolution,
the
reaction will goquantitatively
to
sulfate.It
was expectedthat
the
chlorine(or hypochlorite)
oxidation of
thiosulfate
wouldbe
similarto
that
ofthe
iodine
oxidation.In
fact,
it
has
been
notedthat
thio-sulfates are oxidized
to
tetrathionates
by
mildoxidizing
+
3
+2agents such as
Fe
^,
Cu
,PbOp,
Na~Op
andSeOp.
One
suchreaction
is
shownby
the
following
equation:2Na2S203
+2FeCl3
>-2NaCl
+2FeCl2
+Na2S406
Since
chlorineis
a strongeroxidant,
it
wouldbe
exoec-1 z
ted
that
the
reaction would yieldmainly
sulfate.Lunge
Jsaid
that
the
chlorination of a sodiumthiosulfate
solution
resultedin
the
following
reaction:Na2S203
+4C12
+5H20
^-2NaHS04
+8HC1
He
also pointed outthat
sometetrathionate
wasprobably
14
produced.
/mother
source statesthat
the
chlorinationof
thiosulfate
resultsin
the
formation
of sulfate andpolythionates.
Later,
it
wasdetermined
that
the
hyoo-chlorination of a
dilute
thiosulfate
solution resultedin
the
following
reaction:3Na2S203
+5NaOCl
~2Na2S04
+Na^Og
+5NaCl
conversion of
tetrathionate
to
sulfate.Glasstone
andHicklina;
-^foundthat
tetrathionate
wasformed
whenthio
sulfate was electrolyzed
in
the
presence ofHpOp.
Finally
in
a1935
articlepertaining
to
an analytical methodfor
determining
available chlorinein
ahypochlorite
solution,
<Villson claimed
that
the
hypochlorination
of athiosulfate
solutionfollowed
one ofthe
three
following
reactions,
depending
onthe
molecular ratios ofthe
"reacting
substances.
2NapS203
+NaCIO
+H20
-Na2S406+
2NaOH
+NaCl
Na2S203
+4NaC10
+H20
*-4HaCl
+Na2SO/|_
+HPS04
3M--i2S20,
+5NaC10
>-Na2S406
+5NaCl
+2Na2S04
In
this
and allthe
othercases,
no mention was madeas
to
the
exact nature of another polythionateformed
asan
intermediate
in
the
oxilation ofthiosulfate.
This
could
hav
been
due
to
the
lack
of rigorous analyticalmethods
for
determining
the
oolythionates atthe
time
the
various experiments were carried out.Under
the
various
possible conditionsinvolved
in
the
chlorination(or
hypochlorination)
ofthiosulfate,
there
is
certainly
apossibility
ofthese
speciesexisting
even,
withthe
exception of
the
tetrathionate,
if
only
in
small amountsand even
if
they
resultfrom
asecondary
reaction ratherthan
from
the
direct
chlorination.The
polythionateshave
the
generalformula
equal
to
from
1
to
4.
These
include
the
trithionate
,Sz0^,
the
tetrathionate,
34l,
~
,
the
pentathionate ,Sc-O^-,
andthe
hexathionate,
3^0,--.
It
has
been
definitely
established
for
allfour
ofthese
speciesthat
there
are sulfur17
chains. '
This
has
disproven
previous proposalscommonly
found
in
older articlesthat
there
mightbe
S
^3
link--j
oages.
Uithionate
is
generally
not considered apolythion-ate.
As
withthiosulfate,
the
free
acids'
are not stable.
They
decompose
rapidly
Into
sulfur,
sulfurdioxide,
andsometimes sulfate
depending
uponthe
conditions.However,
the
salts arefairly
stable under certain other conditions.The
production of polythionatesfrom
thiosulfate,
in
addition
to
tetrathionate,
has
been,
noted.Trithionates
have
been
found
afterthe
treatment
ofthiosulfates
with19
SOp
and alsotreatment
withHpOp.
This
reactionis
givenas
2Na2S203
+H202
^2NaOH
+Na2S406
20
Na2S30&
+Na2S04
+H20
The
formation
ofhexathionate
was noted whenthio
sulfate was
treated
withKNOp
in
cooledhydrochloric
acidAnother
source statesthat
prolongedtreatment
ofthiosul
fate
with sulfurdioxide
resultedin
the
production of21
22
tri,
tetra,
and pentathionates.Another
earlier articlerelates
that
the
treatment
ofthiosulfate
withhydrogen
of some
dithionate,
according
to
the
reaction:4Na2S203
+8H202
-^*-2Na2S04
+Na2S206
+Na^Og
+8H20
Thus,
allthe
polythionates and alsodithionate
can resultfrom
thiosulfate.
Part
ofthe
objectives ofthis
study
is
to
determine
whether or notany
ofthese
substancesare
formed
during
the
chlorination of athiosulfate
solution.
There
are several possible approachesto
the
"chlor
ination"
of a solution.
First,
chlorine gas couldbe
bubbled
into
the
thiosulfate
ortetrathionate
solution.The
efficiency
ofthis
reactionis
poor and unlessthe
system
is
buffered,
the
system willbecome
acidic.A
second approach would
be
to
saturate water withchlorine,
and
then
reactthe
chlorine water withthe
solution.Chlorine
willdissolve.
However,
species otherthan
the
solvated chlorine will
be
present,
since a rapiddispro-portionation occurs.
Two
equilibria result.1.)
Cl2(g)^Cl2(aq)
^
=6.2
x10"2
2.)
Cl2(aq)
+Cl"
+
H0C1
K2
=4.2
x10"4
Thus
at25
C,
the
total
solubility
of chlorinein
wateris
0.091
M.
The
Cl2(aq)
concentration willbe
0.061
M
and
the
H0C1
concentration willbe
0.030 M.
Thus
two
different
oxidizing
agents willactually
be
presentin
23
such a solution.
An
additional complication can occurdue
to
the
disproportionation
oTthe
hypochlorite
ion
ac3C10~
^
2C1"
+
C107
3
Thus
a weakeroxidizing
agent,
the
chlorateion
mightbe
present.
This
disproportionation
is
slow at roomtemper
ature,
but
is
rapid athigh
temperatures,
such as75
C.
A
third
method of "chlorinating"the
thiosulfate
would
be
to
bubble
chlorineinto
the
solution and atthe
same
time
ado. sodiumhydroxide,
keeping
the
ph11.
Hypochlorite
wouldbe
readily
formed
and.would act asthe
oxidizing
agent.The
continuous addition ofhydrox
ide
wouldin
effectbuffer
the
solution.Finally,
a solution ofhypochlorite
couldbe
reactedwith
the
thiosulfate
ortetrathionate
solution.If
allowed
to
proceedin
an unbufferedsystem,
the
pH wouldeventually
drop
wellbelow
7
Due
to
the
nature ofthe
starting material,
andthe
possibleintermediate
products,
the
pH ofthe
system coulddetermine
which materials wouldbe
formed
in
the
reactions.It
wasdecided
that
the
"chlorination" wouldbe
carried out
using
sodiumhypochlorite.
The
"chlorination"would
be
carried out sothat
the
oxidation proceeded abouthalf
way
to
completion.Complete
oxidationin
any
casewould result
in
the
production of sulfateonly
and nopolythionate.
This
workis
descrioed
in
the
experimental
section.
After
the
products ofthe
various reactionshave
been
EXPERIMENTAL
A.
Determination
ofReaction
Products
The
first
part ofthis
work was plannedto
determine
the
productsresulting
from
the
partial oxidation ofthiosulfate
andtetrathionate
by
hypochlorite.
Preliminary
work establishedthe
existance of anintermediate
form
in
the
chlorination ofthiosulfate.
Two
liters
of0.36
M
thiosulfate
solution were chlorinatedfor
75
minutes at aflow
rate of oneliter
of chlorineper minute.
The
apparatus usedis
shownin
Figure
1
onthe
following
page.Ten
M
NaOK
was addedcontinuously
during
the
chlorination sothat
the
chlorine wouldbe
converted
to
hypochlorite.
The
solution was sampled at24
various
time
intervals
and analyzedfor thiosulfate
and25
chemical oxygen
demand.
The
chemical oxygendemand
test
essentially
measures oxidizable material whichis
present.a
known
volume of sampleis
refluxed with an aciddi-chromate mixture and
the
dichromate
reductionis
measured.Whereas
the
thiosulfate
concentration was reducedto
zeroin
thirty
five
minutes,
there
was still an appreciablechemical oxygen
demand.
Since
the
chemical oxygendemand
was measured
using
an automatedTechnicon
methodin
whichchloride
ion
did
not contributeto
the
oxygendemand,
the
only
otherpossibility
wasthat
anintermediate
compound
Figure-
1
In
orderto
verify
that
anintermediate
form
was present,
samples weretaken
after45,
60
and75
minutes ofchlorination.
The
samples were analyzedfor
chloridecontent and sufficient silver nitrate solution was added
to
each solutionto
provide a slight excess afterthe
precipitation of all silver chloride.
If
any
thiosulfate
or polythionate were
present,
a reaction ofthe
type
shown
below
occursresulting
in
adark
precipitate.2AgN0,
+Na23203
+H20
^Ag2S
+H2S04
+2NaN03
If
nothiosulfate
of polythionates werepresent,
only
the
white silver chloride precipitate would result. ./henthe
45
and60
minute samples weretreated,
dark
precipitates
resulted.However,
a white precipitate resultedwhen
the
75
minute sample wastreated
indicating
that
only
sulfate remained.Chemical
oxygendemand
measurements on
the
75
minute solution after chloride removalverified
that
all ofthe
oxidizable material was gone.Chemical
oxygendemand
measurements could notbe
made onsamples
having
low
chemical oxygendemands
andhigh
chloride contents
because
ofthe
formation
of silverchloride precipitate
in
the
analyzerlines.
This
resultsfrom
the
reactionbetween
chlorideion
and silverion
present as a catalyst
in
the
method.Thus
anintermediate
species must result
from
the
chlorination ofthiosulfate
solution.
First,
500
milliliters of a0.20
M
thiosulfate
solution,
solution
A,
was placedin
the
container.Next
37^5
milliliters
of2
M
sodiumhypochlorite
solution was addedto
the
beaker
whilethe
solution wasbeing
constantly
stirred-The
temperature
was monitoredthroughout
the
reaction.pH was also monitored
throughout
the
reaction.The
amountof sodium
hypochlorite
solution was chosento
carry
the
reaction
approximately
one-halfto
completion.The
solution
was stirredfor
one-halfhour.
The
solution wasthen
analyzedfor
thiosulfate,
sulfite,
trithionate,
tetrathionate,
pentathionate , andhexathionate
using
the
24
method of
Kurtenacher
andGoldbactf.
This
methodis
des
cribed
in Appendix
I.
Three
other experiments were carried outinvolving
variation of
the
pH.Experimental
conditions wereiden
tical
withthe
exception ofthe
pH andthe
volume ofhypo
chlorite added.
In
the
first
case,
Solution
B,
the
reaction
was allowedto
proceed andthe
pH was adjustedto
7.1
with3
milliliters of7
M
sulfuric acid.In
the
second
case,
Solution
C,
40
milliliters of2
M
sodiumhypo
chlorite and
5
milliliters of10
M
sodiumhydroxide
wasadded
to
the
solution.The
causticin
effectbuffered
the
solution at pH12.
This
is
the
pH at which alkalinechlorination would
be
carried out.Next
50
millilitersof
2
M
sodiumhypochlorite
solution was addedto
the
soladjust
the
pHto
4.6.
This
solutionis
labeled
asSol
ution
D.
A
0.40
M
thiosulfate
solution was prepared.500
milliliters of
this
solution was placedin
a oneliter
beaker.
100
milliliters of2
M
sodiumhypochlorite
wasthen
addedto
the
thiosulfate
solution.This
mixture wassampled and analyzed after
thirty
minutes,
(Solution
E),
and again after
four
hours,
(Solution
F).*This
was
done
to
evaluatehow
wellthe
reactant and products were equilibrated
among
themselves.
Several
milliliters of sulfuricacid was added
to
adjustthe
pHto
71
Two
tests
were carried outto
determine
the
productsresulting
from
the
hypochlorination
of atetrathionate
solution.
First,
250
milliliters of a0.44
M
tetrathionate
solution was mixed with
7
milliliters of2
M
sodiumhypo
chlorite
solution,
(Solution
G).
Finally,
250
millilitersof
the
sametetrathionate
solution was reacted with10
milliliters of
2
M
sodiumhypochlorite
andthe
pH was adjusted
to
7
with several milliliters of sulfuric acid.This
solutionis
labeled
asSolution
H.
In
allthe
abovecases,
the
solutions were analyzedas mentioned previously.
All
results are givenin
the
next section.
B.
The
Kinetics
ofthe
Oxidation
ofthe
Thiosulfate
Ion
and
the
Tetrathionate
Ion
by
the
Hypochlorite
Ion.
the
kinetics
ofthe
thiosulfate
oxidation.This
work wascarried out
using
the
equipment shownpreviously
in
Fig
ure
I.
Chlorine
wasbubbled
into
the
solution at a constant rate and
the
pH washeld
constant at pH11.5
by
the
addition of10 M
sodiumhydroxide.
Thus
the
hydrox
ide
ion
concentration wasin
excess and was not expectedto
affectthe
rate ofthe
following
reaction:S23=
+
4C12
+10
0H~
*~
2S04=
+
5H2
+8G1"
Three
reactions were carried out withthiosulfate
concentrations
of0.181
M
(Solution
I
),
0.363
M
(Solution
S),
and
0.725
M
(Solution N).
The
chlorineflow
ratefor
these
experiments wereheld
constant at oneliter
ofchlorine per minute.
Two
more experiments were carriedout
using
0.363
M
thiosulfate
solution and chlorineflow
rates of
0.5
(Solution
L)
and1.5
(Solution
h)
liters
perminute.
The
results ofthese
experiments are givenin
the
following
section.The
limitations
ofthis
kinetic
study
were recognizedand a more
thorough
study
was undertaken.It
wasdecided
to
study
the
kinetics
ofthe
reactionbetween
tetrathionate
and
hypochlorite.
The
expected reactionis:
7C10" + S406= + 60H" *~ 4304= + 7C1" +
3H20
The
study
wasdone
spectrophotometrically.It
wasknown
that
the
hypochlorite
ion
has
a peak absorption near300
26
millimicrons.
If
none ofthe
other species which mightreaction rate could
be
monitoredby
mixing
the
tetra
thionate
andhypochlorite
andfollowing
the
absorptionwith
time.
Absorption
curves were run onthe
solutionsshown
below between
250
and350
millimicrons.NaCIO
solutionNa2S20^
solutionNa2S04
solutionNaCl
solutionNa2S40^
solutionNapSO^
solutionThe
curvesfor
the
tetrathionate
andhypochlorite
solutions are shown
in
Figures
2
and3.
Above
300
millimicrons,
only
these
absorb andconsequently
only
these
two
absorbtion curves are shown.The
tetrathionate
absorption
is
fairly
significant at300
millimicrons,
how
ever at
320
millimicrons,
the
absorptionis
minimal atthe
planned experimental concentration whilethe
hypo
chlorite still
has
a significant absorption.At
this
wavelength
the
ratio ofthe
absorbance ofhypochlorite
to
the
absorbance oftetrathionate
wasthe
maximum.Con
sequently,
it
wasdetermined
that
the
kinetics
couldbe
studied
by
following
the
absorption at320
millimicrons.The
experiments were carried out sothat
the
follow
ing
concentrations of chemicals were reacted.a)
2
mm/1NaCIO
+2
mm/1Na2S406
b)
2
mm/1NaCIO
+1
mm/1Na2S406
c)
2
mm/1NaCIO
+0.5
mm/1Na2S406
d)
4
mm/1NaCIO
+1
mm/1Na2S406
e)
1
mm/1NaCIO
+1
mm/1o ro
W
m
CO CD +-> CO5
CM/
a o
tT
. H O O co x:^
4-> CO U W CDW
11
w
=3 'S> o
f
)
2
mm/1NaCIO
+1.5
mm/1NapS406
g)
2
mm/1NaCIO
+1
mm/1Na2S406
+ acid.h)
2
mm/1NaCIO
+1
mm/1Na2S406
+base
In
each casethe
two
solutions were /prepared separately
immediately
priorto
analysis.The
two
solutionswere
then
mixedtogether
in
a1-cm
silica cell.The
absorption curves were plotted
beginning
at30
secondsafter mixing.
This
wasthe
time
required#to
mixthe
solutions and
to
placethe
cellin
the
instrument.
In
eachcase,
pH andtemperature
were monitoredin
a separatebut
identical
setup.The
reactions were all run at25
C
Because
ofthe
high
dilutions,
the
solutionsdid
not needto
be
thermostatically
insulated.
Finally,
two
additionalreactions were run
varying
the
pHto
the
showthe
effectof
the
hydrogen
ion
concentration onthe
reaction rate.All
results are shownin
the
following
section.The
equipment usedin
the
various experimentsis
des
cribed
below.
The
gasdispersion
tube
usedin
the
chlorination
was an18
mm polyethylenefilter
candle purchasedfrom
the
Bel-Art
Product
Company.
The
pH ofthe
solutionswas measured
using
aCorning
Model
12
pHMeter.
A
Cary
Model
14M
Spectrophotometer
was usedto
follow
the
reactionrates.
The
chlorinefeed
rate was measuredusing
aDwyer
Model
500
flowmeter.
All
chemicals used wereBaker
andAdamson Reagent
hypochlorite
solution was a15%
solution of"Sunny
Sol."The
sodiumtetrathionate
was preparedby
K&K
Laboratories,
RESULTS
A.
Determination
ofReaction
Products
The
results ofthe
hypochlorination
ofthe
different
thiosulfate
andtetrathionate
solutions are shownin
Table
I.
It
is
evidentthat
tetrathionate
is
indeed
formed
in
the
reactionbetween
thiosulfate
andhypoahlorite.
How
ever,
it
seemsthat
the
trithionate
ion
is
alsopresent,
possibly
either as anintermediate
or asthe
result of asecondary
reaction.In
fact
the
trithionate
wasthe
predominant
speciesin
five
ofthe
six experiments.In
two
of
the
experiments,
analysis showedthe
presence ofpentathionate.
The
small amounts couldhave
been
withinthe
range ofthe
experimental error ofthe
method.How
ever,
whenthe
tetrathionate
washypochlorinated,
pentathionate
wasdefinitely
produced.Material
balances
indicated
that
sulfate was alsoformed.
No
hexathionate
was
found
in
any
ofthe
solutions.It
is
probablethat
the
thiosulfate
was oxidizedto~
tetrathionate.
The
tetrathionate
then
couldhave
partially
decomposed
to
form
free
sulfur,
which wasfound
in
severalof
the
solutions,
andtrithionate.
Pentathionate
couldresult
from
the
partial oxidation ofthiosulfate,
tri
thionate,
ortetrathionate
orfrom
the
reactionbetween
[image:23.559.62.494.228.701.2]Table
I
RESULTS
OF
THE
HYPOCHLORINATION
OF
THIOSULFATE
AND
TETRATHIONATE SOLUTIONS
So In.
*
Orig.
S2p
S23
S02
3
S56
S46
s56
Temp.
pH
*
Time
A
0.20
0.12
0
.037 .0120
+7
C
8.9
30
B
0.20
0.101
0
.025 .020 .003
7.1
30
C
0.20
0.126
0
.024 .0040
+11C
12.2
30
d y
0.20
0.074
0
.023 .025 .005 +10
c
4.6
30
E
0.33
0.198
0
.017 .015 .010 +16
C
7.1
30
F
0.33
0.208
0
.019 .016.003 +16
C
7.0
240
&y
* *
0.44
"o.on
0
.094 .144 .112 +40
2.7
30
H
* *
0.44
0.037
0
.056 .153 .088 +5
C
7.0
30
*
All
concentrationsin
moles perliter
**
All
time
values
in
minutes.* * *
Values
are moles per
liter
ofS^O^-.
y
Some
colloidal sulfur wasformed
during
reaction. [image:24.559.62.489.135.472.2]28
Another
possibility
involves
the
following
2S406=
^
S506= +
S506=
In
the
hypochlorination
ofthe
tetrathionate,
asmall amount of
thiosulfate
wasfound
along
withtri
thionate,
tetrathionate,
and pentathionate.A
materialbalance
showedthat
some ofthe
tetrathionate
musthave
been
oxidizedto
sulfate.The
thiosulfate,
trithionate,
and pentathionate
probably
resultedfrom
reactions otherthan
the
hypochlorite
oxidationinvolving
the
tetrathion
ate.
In
basic
solution,
tetrathionate
is
known
to
de
compose
to
form
trithionate.
The
results obtainedin
Solutions
E
andF
showthat
little
reaction occurred afterthe
first
one-halfhour.
Experimental
work also showedthat
the
pHhas
agreat
deal
of effect onthe
species present.In
one casea
0.64 M
thiosulfate
solution was mixed with0.20 M
sodiumhypochlorite
solution.At
bhe
end ofthe
reaction,
the
thiosulfate
contenthad
been
reducedto
about5
g/1 asNaoSo0-,5H~0.
The
pH wasapproximately
2.8.
The
pH of d. d z><-the
solution wasthen
adjustedto
11.5
with10 M
sodiumhydroxide.
The
solution was again analyzed andthe
thio
sulfate concentration was
found
to
be
55
g/1.The
tetra
thionate,
orany
other polythionatepresent,
couldhave
been
decomposed
in
the
basic
solutionto
reformthiosul
fate
as shownby
the
following
reaction.When
another100
milliliters ofhypochlorite
solution wasadded
to
the
solution,
the
pHdropped
againto
about3
and analysis showed
less
than
5
g/1 ofthiosulfate
remaining.
Chemical
oxygendemand
data
verifiedthis
explanation.
B.
The
Kinetics
ofthe
Oxidation
ofthe
Thiosulfate
Ion
by
Chlorine.
The
following
results were obtainedin
the
experiments
dealing
withthe
kinetics
ofthe
chlorination ofthiosulfate
solutions.The
thiosulfate
concentrationsand
the
chemical oxygendemands
were monitored at various
time
intervals.
From
the
graphs ofthe
thiosulfate
and chemical oxygen
demand
concentrations versustime,
shown
in
Figures
4a, 4b,
5a,
and5b,
the
initial
rateswere
determined.
It
wasfound
that
halfing
or quartering
the
thiosulfate
concentration(original
concentrationof
180
g/1Na2S20:,5H20)
had
no effect onthe
rate.This
could
have
been
due
to
the
thiosulfate
being
presentin
such a
large
excessthat
concentration changes wouldhave
no effect on
the
rate.When
the
chemical oxygendemand
concentration was plotted versus
time
on semi-logarithmicpaper,
Figure
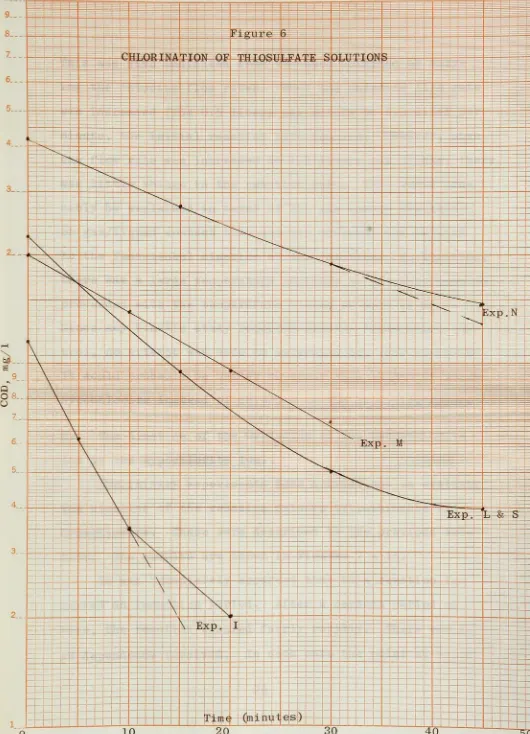
6,
a straightline
resulted.This
is
in
dicative
of afirst-order
reaction.Since
the
hydroxide
was present
in
excess,
asapparently
the
thiosulfate
was,
[image:26.559.57.480.89.694.2]o o o o
10._ 9.. 7__ 5... &d_ Q O O 5_ 2..
-5 -1 "J !
-f =
Y""1 ^
zt
Jrp^
m
~ __= -E _ . ~ _^=
Z
=i
,=L- ?3s;
31
= -_-_: ~ --;.::. - =s
1
=!~ _|
_ ;-;h
:%
;Figuite
= -3 -4 -. -i - !-~
: ~?r_
= :z^
-OfEL(
P
n N A T K
m
0F
rt
v\11
-
-E
irIT
s 0 LIJ1:i 0N
z
~
1
1
__ =E E E = ~ :_ r_ = = if 5; _ ^ ^v. -- -1 . ;^-\>
. _.,...Exp.
h-Ar
f
i'^TTTl:
--N
N
^^
ij
H
j-~~ .___ _^ ---=
|
^_ = ==1
1 v == = '" :"\
r^= g-V
E EE TE=
~ -= = X
z
r^
Z
= EE = == =P
J
= 1= ~ -1 I-\
\
-- -VH]
>. 1T
>/ >
-3
i V \\
L \ --<Fix
P- +
-T in
iti(
mii1Lite s)
[image:31.559.6.536.14.748.2]-This
was alsoindicated
from
the
data
obtainedby
vary
ing
the
chlorineflow
rates.When
the
chlorineflow
ratewas
increased
from
0.5
liters
per minuteto
1.0
liter
perminute,
the
initial
reaction ratedoubled.
However,
whenthe
flow
rate wasincreased
to
1.5
liters
perminute,
there
was
little
changein
the
reaction rate.This
would probably
be
explainedin
terms
ofthe
gasbubble
frequency
or gas/liquid contact
time
and wasprobably
not relatedto
the
fundamental
kinetics
ofthe
reaction.In
addition,
there
was alarge
temperature
increase
asthe
chlorinationproceeded with
the
temperature
rising
as much as75
C.
Since
any kinetic
study
requires carefultemperature
control,
no attempt was madeto
determine
any
rate constants.It
wouldprobably
be
better
to
study
this
oxidationusing
hypochlorite
instead
of chlorine asthe
oxidizing
agent.C.
The
Kinetics
ofthe
Oxidation
ofthe
Tetrathionate
Ion
by
the
Hypochlorite
Ion.
Preliminary
experiments were carried outto
evaluatethe
kinetics
ofthe
reactionbetween
tetrathionate
andhypochlorite.
These
weredescribed
in
the
previous section.
The
results are shownin
Figures
7-15.
It
wasimmediately
apparentthat
this
reactionin
volved an
induction
period.After
a certain period oftime,
the
reaction occuredfairly
rapidly.
There
was aCD s +-> ro
U -H C
H
CO
c o
o
CO
it
became
apparantthat
a reaction wasoccurring"corres-ponded
closely
withthe
time
at whichthe
pH reaction mixture
became
acidic.In
Figures
7,
8,
9
and12,
it
canbe
seen
that
the
induction
periodincreases
withdecreasing
tetrathionate
concentration.However,
the
initial
reactionrate seemed
to
be
the
same whenthe
tetrathionate
concentration
was variedfrom
2
mm/1to
1
mm/1.(Figure
7>
8,
and
12).
When
the
tetrathionate
concentration waslower
ed
to
0.5
mm/1,
the
reaction rate was much slower andthe
induction
time
wasgreatly
increased.
Decreasing
the
sodiumhypochlorite
concentrationin
creased
the
induction
periodbut
did
not resultin
adef
inite
decrease
in
the
reaction rate until alevel
of1
mm/1of
tetrathionate
was reached.Then
adefinite
decrease
occured.
However,
adiscrepancy
arose(Figure
11),
withthe
shorterinduction
period.This
shouldbe
investigated.
Several
experiments were runto
determine
the
effectof
adding
acid orbase
to
the
reacting
mixture.When
0.05
milliliters of concentratedhydrochloric
acid wasadded
to
the
reacting
mixture of2
milliliters of2
mm/1NaCIO
and1
mm/1Na~S406,
nohypochlorite
remained at30
seconds.
A
similar result occured when0.05
millilitersof
0.6
M
hydrochloric
acid was added.In
each casethe
pH went
below
3
almostimmediately.
When
0.05
millilitersof
0.05
M
hydrochloric acid wasadded,
the
induction
perwas quite
fast.
This
reactionis
shownin
Figure
13*
The
pH wasinitially
8.4
aftermixing but
quickly
wentbelow
3
In
Figure
14,
the
result ofmixing
0.05
millilitersof
0.0125
M
hydrochloric
acid withthe
reaction mixtureis
shown.Here
the
induction
periodincreased
to
75
seconds.
Initial
pH aftermixing
was8.7
andbecame
acidic after a period of about one minute.*
Finally,
the
result ofadding
0.05
milliliters of2.5
M
sodiumhydroxide
to
the
reaction mixtureis
shownin
Figure
12.
In
this
casethe
initial
pH ofthe
reactDISCUSSION
It
has
been
shownthat
the
hypochlorination
of athiosulfate
solution resultsin
the
production oftetra
thionate,
sulfate,
trithionate
andprobably
pentathionate.The
latter
two
probably
resultedfrom
secondary
reactionsinvolving
tetrathionate.
It
was also shownthat
the
hypochlorination
oftetrathionate
resulted*in
the
production
oftrithionate,
pentathionate,
and sulfate.Again
it
may be
that,
withthe
exception ofsulfate,
the
other species result
from
secondary
reactions.The
analytical
techniques
(Appendix
I)
are suchthat
it
is
im
possible
to
detect
which species areformed
directly.
Possibly
polarography
couldbe
usedto
establishthis.
The
study
ofthe
kinetics
ofthe
chlorination ofthiosulfate
indicated
that
this
reaction wasfirst
orderin
chlorine andprobably
pseudo zero orderin
thiosulfate.
This
study
was notvery
rigorous.The
study
ofthe
kinetics
ofthe
hypochlorination
of
tetrathionate
resultedin
establishing
the
existanceof a
definite
induction
period whichis
highly
pHde
pendent.
The
elucidation ofthe
exactkinetic
ratelaw
will require additional work.
The
ratelaw
will probably
involve
ahydrogen
ion
concentrationterm.
In
addition,
kinetic
studies shouldbe
done
atdifferent
temperatures
ACKNOWLEDGMENTS
In
conclusionI
wouldlike
to
expressmy
appreciation
to
Dr.
John
A.
.Vhitefor
his
suggestions andcriticisms
for
the
improvement
ofthis
paper.Finally,
I
would alsolike
to
acknowledgemy
gratitudeto
the
Eastman
Kodak
Company
for
the
use oftheir
instruments
BIBLIOGRAPHY
A.
SPECIFIC
REFERENCES
1.
Cotton
andWilkinson,
"Advanced
Inorganic
Chemistry,"2ed
Edition.
Interscience
Publishers,
pg.550,
1966.
2.
Foerster
andHornig,
Zeitschrift
fur
anorganischeChemie, 86,
125,
1922.
3.
Voge
andLibby,
J.
Am.
Chem.
Soc
. ,59,
2474,
1937.
4.
Voge
andLibby,
J.
Am.
Chem.
Soc,
61,
1032,
1939-5.
Laitinen,
H.A.,
"Chemical
Analysis,"*McGraw
Hill
Publishers,
pg.400,
I960.
6.
Awtrey
andConnick,
J.
Am.
Chem.
Soc,
73,
1341,
1951
7.
Allen
andKeefer,
J.
Am.
Chem.
Soc,
77,
2957,
1955.
8.
Bradbury
andHanbly,
Australian
J.
Sci.
Research,
9.
Abel,
Z.
anorg. u allgemChem.,
74,
395,
1912.
10.
Ephraim,
F.
,"Inorganic
Chemistry,"
6th
Edition.
Interscience
Publishers,
pg.582,
1954.
11.
Bushwell
andGallaher,
Ind.
Eng.
Chem,
15,
1187,
1923-12.
Hewett
andMann,
J.
Chem.
Soc,
103,
325,
1913.
13.
Lunge,
Ber.,
12, 404,
1879.
14.
Dienert
and uVandenbulche ,Compt.
rend.,
167,
29,
1919.
15.
Glasstone
andHickling,
J.
Chem.
Soc,
2345
and2800,
1932.
16.
Willson,
Anal.
Ed.,
7,
44,
1935.
17.
Cotton
andV/ilkinson,
ibid.,
pg.553.
18.
Zachariasen,
J.
Chem.
Physics,
2,
109,
1934.
19.
Raschig,
"Schwefel
undStuckstof
f
studies , " pg.284.
20.
Weitz
andAchterberg,
Ber.,
61,
399,
1928.
21.
Friend,
J.N.,
editor,
"A
Textbook
ofInorganic
Chemistry,
Vol.
VII,
Part
II,"Charles
Griffin
and
Company,
London,
pg.198,
1931.
22.
Luchow,
Zeitschrift
fur
analytischeChemie,
32,
53,
1893.
23.
Cotton
and vVilkinson,ibid.,
pg.568.
24.
Kurtenacher
andGoldbach,
A.
Anorg.
Allgen.
Chemie.,
166,
177-89,
1927-25.
"Standard
Methods
for
the
Examination
ofWater
andWastewater,"
APHA,
11th
Edition,
I960.
26.
King,
E.L.,
"How
Chemical
Reactions
Occur,"W.A.
Benjamin,
Inc.,
1964,
pg.20.
27.
Barrett
andDurrant,
J.
Chem.
Soc,
1416,
1927
28
Kurtenacher andKaufman,
Z.
Anorg
allgemChem.,
148,
43,
1925.
B.
GENERAL REFERENCES.
1.
Durrant,
P.J.
andDurrant,
B.
,"Introduction
to
Advanced
Inorganic
Chemistry,"Longmans,
Green
and
Co.,
Ltd.
,1962.
2.
Eisenhauer,
H.R.,
"The
Ozonation
ofPhenolic
Wastes,"JZi/.P.C.F., Vol.40,
No.
11,
Part
1,
Nov.
1968,
pg.
1887-1899.
3.
Gutmann,
V-,
"Halogen
Chemistry,
Vol.2,"
Academic
Press,
New
York,
1967.
4.
Heslop,
R.B.
andRobinson,
P.L.,
"Inorganic
Chemistry,"Elsevier
Publishing Company,
London,
I960.
5.
Kirk,
R.E.
andOthner,
D.F.,
Editors,
"Encyclopedia
of
Chemical
Technology,"Vol.
14.
The
Inter
science
Encyclopedia,
Inc.,
New
York,
1955.
6.
Laidler, K.J.,
"Chemical
Kinetics,"2ed
Edition,
McGraw-Hill
Book
Company,
New
York,
1965.
7.
ivlellor,
J.W.,
"A
Comprehensive
Treatise
On
Inorganic
and
Theoretical
Chemistry,"Longmans,
Green
andCo.,
Ltd.,
Volume
X,
London,
1930.
8.
"Supplement
to
Mellor's
Comprehensive
Treatise
On
Inorganic
andTheoretical
Chemistry,
Supplement
II,
Part
1,"Longmans,
Green
andCo.,
Ltd.,
London,
L956.
9.
Schmidt,
M.
, andSand,
T.,
J.
Inorg.
Nucl.
Chem.,
26,
1165-1171,
1964.
APP1WDI2
?4
Outline
ofthe
Method
for
the
Determination
ofSulfite,
Thiosulfate,
andPolythionates
in
the
Presence
ofOne
Another.
A.
S2^3~
~
Tie
u"sul^i':e with
formaldehyde
andtitrate
with standard
iodine
solution.b.
SO,--
Use
the
difference
between
the
iodine
titra
tion
with and withoutformaldehyde.
C.
Other
Polythionates.
*1.
sn5=
(n>3)
+ excess*-S36=
+
S23"*
Tie
up
excess
SO,-with
formaldehyde
andtitrate
the
thiosulfate
withiodine.
2.
3
Oa
(n>3)
+ +Ho0
*-So0z=
+
S0
=+ +
HON
n
6
2
d.3
4
Titrate
withiodine
first
to
remove sulfite andthio
sulfate.
Then
react with cyanide andtitrate
the
resulting
thiosulfate
withiodine.
3.
S
06= + excess S=-2
S20,=+
(n-3)
S.
First
titrate
withiodine
to
remove sulfite andthio
sulfate.
Then
remove excess
S-with zinc carbonate.
Finally
titrate
with standardiodine.
4.
The
trithionate,
tetrathionate
and pentathionateconcentrations can
then
be
calculated.