Copyright 0 1973 American Society for Microbiology Printed in U.S.A.
Cell-Free Translation
of
Paramyxovirus
Messenger
RNA
D. W. KINGSBURY
Laboratories of Virology and Immunology, St. Jude Children's Research Hospital, Memphis, Tennessee38101
Received forpublication23July 1973
Polypeptides corresponding in electrophoretic mobilityto virionpolypeptides
1, 3, and5 weremade in areticulocyte cell-freesystem towhich 18S RNA from
Sendai virus-infected cellswas added. Immuneprecipitationwas used toselect
relevant polypeptides from endogenous products. The cell-free product
corre-spondingtovirionpolypeptide 3 (the nucleocapsidstructureunit) wasthe most
abundant; its tryptic peptides comigrated electrophoretically with tryptic
peptides of polypeptide 3 isolated from virions. Other sedimenting classes of
RNAfrominfectedcellsweretested; onlythe28S fraction showed slight activity.
Virion 50S RNA was inactive. These findings support the hypothesis that
complementary RNAtranscriptsofparamyxovirion RNA arethetemplatesfor
viral proteins.
There is much evidence that the
single-stranded
RNA in paramyxoviruses (5) is notmessengerRNA (1, 15). (i) IsolatedvirionRNA
is not infectious (13). (ii) Virions contain an
RNA-directed RNA
polymerase
which makescomplementary
RNAspecies
smaller thanvi-rion RNA in vitro
and
in vivo (11, 26, 27, 31).(iii)
Infected cells
containlarge
amounts ofcomplementary
RNAspecies
(most of whichsediment at about
18S)
late in infection (2, 4,14, 21); these are the
only virus-specific
RNAsknown to be
associated
withpolyribosomes
(3,4);
they
containpolyadenylic
acid
(23),
anindication of messenger
function,
whereasvi-rionRNA does not (8). But a conclusive
demon-stration of messenger function
requires
transla-tion of an
RNA
in a reconstituted cell-freeprotein synthesizing system. This report
de-scribes the
synthesis
ofSendai
virus structuralproteins in a
rabbit
reticulocyte lysate
directedby
18S RNA from infected cells. Aspredicted,
virion RNA was inactive in thesame system.
MATERIALS ANDMETHODS
Virus. Previous reports describecultivation of the
clone ofSendaivirus used in this work(22, 31). Virion RNA. Unlabeled 50S RNA was isolated
from egg-grown virions by sodium dodecyl sulfate
(SDS)-phenol extraction and agarose
chromatogra-phy (13, 22).TheRNAwasprecipitated with ethanol,
washed with ethanol, and storedasdescribed below.
RNA from infected cells. Chicken embryo lung
(CEL) cellmonolayer cultures, 100 mm indiameter,
were inoculatedwith 1 to10plaque-forming units of
Sendai virus percell and wereincubated at 30C in
Eagle minimal essential medium (Earle saline base)
supplemented with 3% heat-inactivated fetal calf
serum, penicillin, streptomycin, and Mycostatin in
5% CO2 in air. At 48 h, the cellswere scraped into phosphate-buffered saline, collected by centrifugation
(500 x g,4C,5min), andsuspendedin 1ml of0.01M
sodium acetate and0.05Msodium chloride (pH 5.2)
perculture. SDS wasaddedtogive0.017M and the
mixturewasshaken withanequal volumeofphenolat
50C (14, 29).Asecondphenolextraction wasdoneat
22C. The aqueous phasewas made0.1M insodium
acetate(pH 5.0);3vol of ethanolwasadded, and the
mixture was placed at -20C for 16 h or longer.
Precipitated RNAwascollected by centrifugationat
12,000 x g,4 C,for 15min, washed threetimeswith
absolute ethanol, dried under vacuum, dissolved in
autoclaved water, and storedat-60C. About100ug
ofRNAwereobtained from each culture.
Cell-freeproteinsynthesis. Rabbitsweretreated
with phenylhydrazine for 6 days as described by
Gilbert and Anderson (7) and bled by cardiac
punc-ture onthe 8thday,when reticulocytosiswasgreater than 90%. Reticulocyte lysate (10) was stored in a
liquid-nitrogen freezer. Reaction mixtures contained
theingredientsspecifiedby Housmanetal.(10),with
the addition of 500
lMCi
of3H-amino
acid mixture(Schwarz/Mann, catalog no. 3130-08) per ml. RNA
solution or water (for endogenous reactions)
repre-sented 18% of the finalreaction volume. Incubation was at 22C for90 min.
Antisera. Rabbit serum with antibodies against
Sendai virion polypeptides was prepared asfollows.
Purified egg-grown Sendai virions (31) were
sus-pended in 0.01 M sodium phosphate (pH 7.2) at a
concentration of 2 mg of virion protein per ml and
dialyzed against1MKClin 0.01 Msodiumphosphate
1020
on November 10, 2019 by guest
http://jvi.asm.org/
(pH 7.2). Triton X-100 was added to a final
concen-tration of 2% (28). After 20 min at 23 C, 10 vol of
ethanol was added, and the mixture was placed at
-20 C for 24 h. The precipitate was collected by
centrifugation, suspended in phosphate buffer, and
dialyzed against phosphate buffer containing 0.17 M
NaCl.The antigen was mixed with complete Freund
adjuvant and 2 mg of viral protein was inoculated into each rabbit, the dose divided equally among a foot pad and two leg muscles. A month later the intramus-cular inoculations were repeated, and an intravenous
injection was given. Serum, collected 7 dayslater, had
a hemagglutination-inhibition titer of 500, and
pre-cipitatingantibodies against viral envelope
polypep-tides (28) and nucleocapsids were detected, although
this was notdetermined quantitatively.
Goat serum with antibodies against rabbit gamma globulin was a gift of Luis Borella.
Immune precipitation. Triton X-100 (final
con-centration 0.5%) and 2 uliters of rabbit anti-Sendai
virion serum were added to 70 to 150uliters of reaction
mixture. After incubation at 22 C for 30 min, 40
uliters of goat anti-rabbit serum was added, and
incubation was extended for 2 h more at 22 C.
Immuneprecipitates were collected bycentrifugation
and washed three times with 0.01 M sodium phos-phate, 0.15 M NaCl, 0.01 M EDTA, and 1% Triton
X-100(pH 7.2). Precipitates were dissolved in 0.01 M
sodium phosphate and 0.034 M SDS (pH 7.2) at 100 C for 2 min. Incubation of reaction mixtures with antibodies at 37 C (9) or in the presence of 0.5%
sodiumdeoxycholate (20, 24) were found to increase
nonspecific precipitation, and were therefore avoided.
Polyacrylamide gel electrophoresis. Immune
precipitates containing 50 to 100
Ag
ofprotein wereelectrophoresed in 6-mm diameter10%
polyacrylam-ide gels, and the gels were sliced and processed for
countingasdescribed (30).
Tryptic peptide analysis.
"4C-amino
acid-labeledSendai virion polypeptides were separated in 6-mm
polyacrylamide gels (30). 'H-amino acid-labeled
im-mune-precipitated products ofa 1-ml reaction
pro-grammed by 18S RNA from infected cells were
separated in a 25-mm diameter gel. In both cases,
radioactive polypeptides were eluted by incubating
gel slices in 0.01 M sodium phosphate, 0.003MSDS,
and 0.001 M NaNs (pH 7.2) at 37C for 24 h. Gel
fragments were removed by passing the eluates
through type HA membrane filters(MilliporeCorp.).
Polypeptides werereduced and alkylated (12) inthe
presence of 100
Mug
ofovalbumin (Worthington) andconcentrated by precipitation with 15%
trichloroace-tic acid. Three washes with 5%trichloroacetic acid
and three washes with ethanol were followed by
drying under vacuum. Each samplewassuspendedin
1 mlof 0.1 MNH4 HCO,(pH 8.0)anddigestedwith
10
Mg
ofTPCK-treated trypsin(Worthington)for 3hat 37C.Anadditional5
Mg
oftrypsinwasthenadded,and incubation continued for 12 h. Samples were
driedat60Cin a stream ofdry nitrogen, dissolvedin
pyridine-acetic acid-water (100: 4:896, pH 6.4), and
separated in the same buffer on Whatman 3MM
paperat2,500V, 10C, for 2h. The driedpaperwas
cut into 1-cm segments whichwere placedin liquid
scintillation countingvialscontaining 1 ml of0.1M
NaOH. After 30 min at 22C, 10 ml of PCS
(Amer-sham/Searle) wereadded,the mixtureswereshaken,
and they were counted after 24 h, by which time
chemiluminescence had decayedto an undetectable
level.
RESULTS
Messenger activity in total RNA from
in-fected
cells.Schimke
and co-workers (20, 24) have shown that a messenger RNA need not bepurified
forcell-free translation
ifthere is a way,such as immune precipitation, to isolate the
relevant product. Apparently,
ribosomal RNA
in large amounts
does
not interfere. Thesim-plicity
ofthisapproach
isparticularly
advanta-geous in work with paramyxoviruses, becausethey grow
poorly
in suspensioncultures,
whichis a handicap to producing large amounts of
polyribosomes. In
addition, large
amounts ofcandidate paramyxovirus message are not
poly-ribosome
associated
(4, 15).Accordingly, as infected CEL cell monolayer
cultures
became
available, they
wereextractedas describedinMaterialsandMethods, and the
RNA was
kept
as aprecipitate
in ethanol at-20C until several milligrams were
accumu-lated.
When this material was added to thereticulocyte
protein-synthesizing
system,sev-eral
peaks
ofradioactivity
were seen aftergel
electrophoresis
of the immuneprecipitate
(Fig. 1).These
correspond
inmobility
tovirionpoly-peptides
1, 3, and 5, with asuggestion
ofmaterial
in the region ofpolypeptide
6.Poly-peptide
1 isimplicated
in viral transcriptasefunction (30);
polypeptide
3is thenucleocapsid
structure unit (18); there are several
polypep-tides inregion
number
5, atleastoneofwhichisglycosylated (19); and
polypeptide
6probably
resides on the inside of the viral
envelope
(19).Much less
radioactivity
was present in agel
separation of the immune
precipitate
of anendogenous
reaction. Theonly
discerniblepeak
was in the region of virion
polypeptide
1(Fig.
1); this
probably
represents the"70,000"
molec-ular
weight
reticulocyte polypeptide
describedby
others (17,20).
Messenger activity of virion
RNAand
cell RNAfractions.
To learn whichsedimenting
class
of RNA contained messengeractivity,
total cellRNA was
centrifuged
insucrosegradi-ents, and fractionswere takenasshown in
Fig.
2.The
18S pool
wasactiveinstimulating
aminoacid incorporation into
polypeptides
whichmi-grated
electrophoretically
like virionpolypep-tides1, 3, and 5
(Fig.
3); material likepolypep-tide 6 wasnot
clearly
resolved.Aswith reactions directed
by
total cellRNA,
on November 10, 2019 by guest
http://jvi.asm.org/
N
0X
x i
(-0 20 40 60 80
100
[image:3.501.53.453.58.266.2]DISTANCE MOVED (mm)
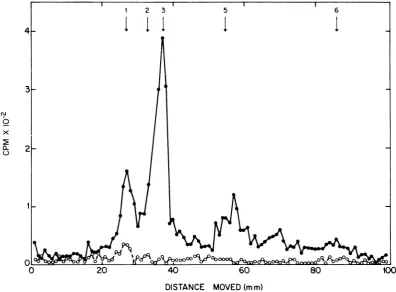
FIG. 1.Polyacrylamide gel electrophoresis of polypeptides in immuneprecipitates ofcell-free reactions. (0)
Endogenous reaction, no added RNA; (0) 1,020
,g
of totalRNA fromSendai virus-infected cellsadded permilliliter. The numbers in this and later figures refer to the positions of virion polypeptides asassigned by
Mountcastleetal.(18). InFig.1,3, and4thesepositionsweredeterminedinstainedgelsrun inparallel.
0
10
20
30
EFFLUENT
VOLUME
(ml)
FIG. 2. Separation of RNA from Sendai virus-infected cells in a sucrose gradient. A bout 2 mg ofRNAin2 ml
of0.005 Tris-hydrochloride, 0.001 MEDTA, 0.1 M NaCl, and 0.017 M SDS (pH 7.4) were layeredon a34-ml
linear15 to
30%o
sucrose (wt/vol)gradient in thesame buffer andcentrifugedat18,000rpm,20C, for16 h.Collectionwasfrom the top, with continuous automatic monitoring of ultraviolet absorbance. Theindicated
volumeswere collectedseparately, precipitated with ethanol, and prepared for cell-free proteinsynthesisas
describedinMaterials and Methods.
1022
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.501.120.400.338.579.2]there
was little or noradioactivity
in theposi-tion of virion polypeptide 2, the major virion
glycopolypeptide (19).
Virion 50S RNA was inactive. The
acrylam-ide
gel
pattern of a reactionreceiving
virionRNA
could
not bedistinguished
from theen-1 2 3
4
I
3X
0 x 2
dogenous
pattern(Fig.
3).Also
inactive, givingresults
identical
toen-dogenous
reactions, were the4S and
"50S"
peaks
from infected cells(data
not shown).The28S pool of cell RNA directed the
synthe-sis of small amounts of polypeptides which
DISTANCE MOVED(mm)
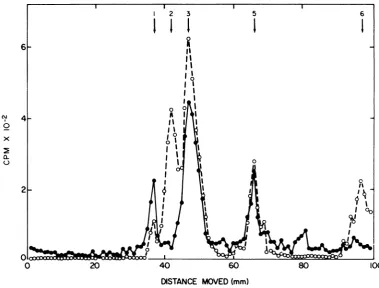
FIG. 3. Electrophoresisofimmuneprecipitates of reactions programmed by 33 ug of 50S RNA from Sendai
virions per milliliter(0);490
,ug
of 18S RNA frominfectedcells per ml(0).x
a-(-)
too
DISTANCE MOVED
(m m)
FIG. 4. Electrophoresis of the immuneprecipitate ofa reactionprogrammed by 480
Mg
of 28S RNA frominfectedcells permilliliter.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.501.48.445.141.433.2] [image:4.501.50.450.469.621.2]KINGSBURY
migrated like virion polypeptides 1 and 3 (Fig.
4), suggesting that some of the more rapidly
sedimenting
viral messenger RNAsspilled overinto this
fraction.
Table
1summarizes the total incorporation in [image:5.501.63.256.151.294.2]the
various reactionsand
the yields in theTABLE 1. Effects ofRNAfractionsonsynthesisof
protein andanti-Sendaivirion-precipitable
radioactivity in thereticulocyte lysatesystem
Totalcounts/ %
Precip-RNAadded jig/mi minx10-/ itated" 0.1m1a
None 8.4 0.70
Total cell 1,020 6.6 2.0
4Scell 560 5.2 1.3
18Scell 490 7.4 2.4
28S cell 480 6.6 1.1
"50S" cell 52 7.7 1.0
50Svirion 33 7.1 0.85
Hot trichloroacetic acid-precipitable
radioactiv-ity.
IPrecipitated by anti-Sendai virion serum as
de-scribed in Materials and Methods.
I I
2 3
6~
_
~
~
~~~~~~~~
IJ
0
c\ 4- II1
0~
x
l l
XU
immune precipitates. It can
be
seenthat all
added RNA
species
depressed
overall
incorpora-tion
moderately and that differences between
immune precipitates of active and inactive
reactions (with respect to virion
polypeptide
synthesis) were not as marked as in the
acryl-amidegel analyses. Presumably,
contaminating
globin, which migrated
outof
the
gels,
accountsfor
the
differences between total
radioactivity
precipitated and
radioactivity
recovered
inthe
gels.
Authenticity
of
the cell-freeproducts.
Co-electrophoresis
of"4C-virion
polypeptides with
the
immune-precipitated
3H-products
of areac-tion
directed
by
18S
RNA
frominfected cells
revealed close
correspondence
ofpeaks
1, 3,and
5
(Fig. 5).
More evidence
wasobtained
by analysis
oftryptic
peptides
ofpolypeptide
3,
the mostabundant
product.
Asshown
inFig.
6, the
polypeptide made
invitroclosely resembled the
nucleocapsid
structureunitfrom
virions.Differ-encesin
relative
peak
heights
canbe ascribed
todifferences
inspecific
activities ofindividual
5 6
60 80 10
60 80 100
DISTANCE MOVED (mm)
FIG. 5. 8H-polypeptides synthesized in a cell-free reaction containing 18S RNA from
electrophoresedin thesamegelas
"4C-polypeptides
fromSendai virions(0). infected cells(0)
1024
J. VIROL.on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.501.67.447.331.619.2]amino
acids in the
twolabeled
amino acid
mixtures
used and
toeffects of cell
pools
on4C-amino acid
labeling
ofvirions.
DISCUSSION
50S Sendai
virionRNA
isprobably
amixtureoftwo kinds of
molecules
ofoppositepolarity,
the
major speciesbeing complementary
to thevirus-specified 18S RNA
ofinfected
cells (21,25). It seems
clear that
neitherkind of50S RNA
is operative as
messenger
forthe virionpolypep-tides
precipitated
by the
antiserumused
inthis
study. This does
notrule
outthe
possibility that
other viral
polypeptides might be templated by
50S RNA.
Although
50S RNA
from virions or infectedcells
wasused
atabout
3 to 6%of theconcentra-tion of
the other
RNA preparations,
thisshould
have
been enough
for afair
test, at least ofvirion
RNA, which
isexclusively virus-specific,
whereas
18S
virus-specific RNA certainly
repre-sents
less than
3%of the totalRNA
ininfected
cells.
I havedetermined
the amount ofRNA
from
infected
cells which binds
tocellulose
(22;D.
W.Kingsbury, unpublished data),
presum-ably because
of
itspoly A
content (16, 23). Only2
-N
x
a-
(-about 0.5%
of the total cell RNA bound tocellulose,
and thisprobably includes cellular
adenylate-rich
RNA as well as themajority of
the viral
18S
RNA(22).
This material has notyetbeen tested in the
reticulocyte
system.The
18S
RNA fraction frominfected
cellscontained the mostactive
template.
Thisshowsthat viral messenger RNA is smaller than virion
RNA. There is
already ample evidence
that thevirus-specific
RNAwhich
sediments
at18S
isexclusively complementary
tothe major species
ofvirion RNA
(2, 4).
Double-stranded
and partiallydouble-stranded
RNA
species,representing
template-product complexes involved
inreplication and
transcription
ofviralRNA, sediment
wellahead
of
18S RNA;
they shouldbe
present mainlyin
the
28S pool (22).
This pool wasrelatively
inactive, either
because
there is littlemessenger
RNA that
sediments that
rapidly
orbecause
ofinhibitory
effects ofdouble-stranded
RNAon
protein
synthesis
(6). The latterexplanation
seems less
likely, because overall incorporation
was not
preferentially depressed
by the28S
pool.
What was not
identified among the cell-free
products
is asinteresting
asthe things that
FRACTION NUMBER
FIG. 6. High-voltage electrophoresis of 3H-tryptic peptides
(0)
from
polypeptide
3 made in vitro and"4C-tryptic
peptides(0) from polypeptide3isolatedfrom
virions.Theorigin
isfraction
25,andtheanode isattheleft.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.501.49.452.356.609.2]were. Nonstructural viral proteins unrelated antigenically to virion polypeptides would not have
been recognized
by the antiserum,and willhave to be looked for by other means.
Polypep-tide
6 was notclearly
seen; it will be necessaryto learn if the antiserum containsenough
anti-body to it. Polypeptide 2, the major
glycopoly-peptide
of virions, appeared tobe absent.
Itmay have
been
made, but notglycosylated,
andtherefore
maylack the requisite antigenic sites.
Or it may have been
precipitated by antibody,
but migrated
anomalously
underanother
com-ponent; more
detailed
peptide analysis
mayresolve this possibility. The most interesting
possibility
is that it was notmade,
suggestingthat
glycosylation
andtranslation
arecoupled.Support
forthis
idea comes from recent work onthe cell-free translation of
vesicular
stomatitisviruscomplementary
RNA,
wherelittle,
ifany,ofthe
glycosylated
virionpolypeptide
waspro-duced,
despite
efficientsynthesis
ofall the
othermajor viral
polypeptides (T.
Morrison,
etal.,
1973, in
press).
In theSendai virus
system,the
synthesis
ofpolypeptide migrating
like theminor virion
glycopolypeptide
number 5 arguesagainst this
idea,
but all of the virionpolypep-tides which
appear inthis
region
of agel
are notnecessarily
glycosylated
(19).B.
S. Collins and
M.A. Bratt
(Proc.
Nat.Acad.
Sci.,
inpress) have
recently separated
Newcastle disease
virus(NDV)
complementary
RNA into several
species which differed
inabundance,
and there
werecorrelations
be-tween
the size
andabundance
ofeach RNA
species and the size and abundance
ofNDV
polypeptides. This
suggeststhat
paramyxo-virus messenger RNAs are monocistronic.
The
cell-free
systemprovides
awayto testthis and
to
identify
the message foreach
polypeptide.
It isnoteworthy
that nopolypeptides
larger than
known
virion components wereseeninthe
Sen-dai virus cell-free system,
arguing against
acleavage
mechanism in theproduction
ofpoly-peptides
1, 3, and5.Assuming
that eachproduct
ofthe cell-freesystem is identical to the virion polypeptide
with the same electrophoretic
mobility,
itap-pears that the proportions of the cell-free
prod-ucts are not the same as the proportions of
polypeptides in virions
(Fig.
5). This is mostmarked with respect to polypeptide 1, which
was moreabundant in the cell-freeproduct. But
infected cells contain relatively more
polypep-tide 1 than virions do (30). Thus, the
reconsti-tuted
protein-synthesizing
system may indeedreflect messenger abundance or other
determi-nants of messenger
efficiency
that prevail inintact cells.
ACKNOWLEDGMENTS
I am grateful for help provided by Luis Borella, Edna Duck,Pankaj Ganguly, Preston Marx, Andrew Moseley, Paul Mui, Robert Naegele, Allen Portner, Ruth Ann Scroggs, WilliamWalker, RobertWebster, and Diane Woods.
This study was supported by Public Health Service researchgrantAI-05343 from the NationalInstitute of Allergy and Infectious Diseases, by Childhood Cancer Research Centergrant CA-08480 from the National Cancer Institute, and by ALSAC. I received Public Health Service Career Development Award HD-14,491 from the National Institute ofChild Health and Human Development.
LITERATURE CITED
1. Baltimore, D. 1971. Expressionofanimalvirus genomes. Bacteriol. Rev. 35:235-241.
2. Blair, C. D., and W. S. Robinson. 1968. Replication of Sendai virus. I. Comparison of the viral RNA and virus-specific RNA synthesis with Newcastle disease virus.Virology 35:537-549.
3. Blair, C. D., and W. S. Robinson. 1970. Replicationof. Sendai virus. II. Steps in virus assembly. J. Virol. 5:639-650.
4.Bratt, M. A., and W. S. Robinson.1967.Ribonucleicacid synthesisincells infected with Newcastle disease virus. J. Mol. Biol. 23:1-21.
5.Duesberg, P. H. 1968. Physical propertiesofRous sar-coma virus RNA. Proc. Nat. Acad. Sci. U.S.A. 60:1511-1518.
6. Ehrenfeld, E., and T. Hunt. 1971. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc. Nat. Acad.Sci. U.S.A. 68:1075-1078.
7. Gilbert,J.M.,andW.F. Anderson.1971.
tRNA-depend-ent cell-freehemoglobin synthesis, p. 542-549.In K. Moldave and L. Grossman(ed.), Methodsin enzymol-ogy, vol. 20, partC. Academic PressInc.,New York. 8. Gillespie,D., S.Marshall,and R.C. Gallo.1972.RNAof
RNAtumourviruses containspolyA.NatureN.Biol. 236:227-231.
9. Horwitz, M. S., and M. D. Scharff. 1969. Immunological precipitationofradioactively labeledviralproteins, p. 297-315. In K. Habel and N. P. Salzman (ed.), Fundamental techniquesin virology. Academic Press Inc., New York.
10. Housman, D., M. Jacobs-Lorena, U. L. Rajbhandary, and H. F. Lodish. 1970. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature (London) 227:913-918.
11. Huang, A. S., D. Baltimore, and M. A. Bratt. 1971. Ribonucleic acid polymerase in virions ofNewcastle diseasevirus:comparisonwith thevesicular stomatitis viruspolymerase. J. Virol. 7:389-394.
12. Jacobson, M. F., J. Asso, and D. Baltimore.1970.Further evidence on the formation of poliovirus proteins. J. Mol.Biol.49:657-669.
13. Kingsbury,D. W.1966. Newcastle disease virusRNA.I. Isolation and preliminary characterization of RNA fromvirusparticles. J. Mol. Biol. 18:195-203. 14. Kingsbury,D. W.1966.Newcastle diseasevirus RNA.II.
Preferential synthesisofRNAcomplementary to pa-rental RNA by chick embryo cells. J. Mol. Biol. 18:204-214.
15. Kingsbury,D. W. 1972.Paramyxovirus replication.Curr. Top.Microbiol. Immunol. 59:1-33.
16. Kitos,P.A., G.Saxon, andH. Amos.1972. Theisolation ofpolyadenylate with unreacted cellulose. Biochem. Biophys.Res.Commun. 47:1426-1436.
17. McDowell, M. J.,W. K.Joklik, L.Villa-Komaroff, and H. F. Lodish. 1972. Translation of reovirusmessenger
on November 10, 2019 by guest
http://jvi.asm.org/
RNAs synthesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc. Nat. Acad. Sci.U.S.A. 69:2649-2653.
18. Mountcastle, W. E., R. W. Compans, L. A. Caliguiri, and P.W.Choppin.1970.Nucleocapsidproteinsubunitsof simian virus 5, Newcastle disease virus, and Sendai virus.J.Virol. 6:677-684.
19. Mountcastle, W. E., R. W. Compans, and P.W.Choppin. 1971.Proteinsand glycoproteinsofparamyxoviruses:a
comparison ofsimian virus5,Newcastle diseasevirus, andSendai virus. J. Virol. 7:47-52.
20. Palmiter, R. D. 1973.Ovalbumin messengerribonucleic acid translation. Comparableratesofpolypeptide initi-ationandelongationonovalbuminandglobin messen-gerribonucleic acid inarabbit reticulocyte lysate. J.
Biol.Chem. 248:2095-2106.
21. Portner, A., and D. W. Kingsbury. 1970.Complementary RNAsinparamyxovirionsand paramyxovirus-infected cells. Nature(London) 228:1196-1197.
22. Portner, A., and D. W. Kingsbury. 1972.Identification of transcriptive andreplicative intermediates in Sendai virus-infected cells. Virology 47:711-725.
23. Pridgen, C., and D. W. Kingsbury.1972.Adenylate-rich
sequences in Sendai virus transcripts from infected
cells. J. Virol.10:314-317.
24.Rhoads, R. E., G. S. McKnight, and R. T. Schimke.1971.
Synthesis of ovalbumin inarabbitreticulocyte cell-free
systemprogrammed with hen oviduct ribonucleic acid. J. Biol.Chem. 246:7407-7410.
25. Robinson, W. S. 1970. Self-annealing of subgroup 2 myxovirusRNAs.Nature(London) 225:944-945. 26. Robinson, W. S. 1971.SendaivirusRNAsynthesis and
nucleocapsid formation in thepresence of
cyclohexi-mide.Virology44:494-502.
27. Robinson, W. S. 1971. Ribonucleic acid polymerase activity in Sendai virionsandnucleocapsid. J. Virol. 8:81-86.
28. Scheid,A.S., L.A.Caliguiri, R. W.Compans, and P.W. Choppin. 1972. Isolation of paramyxovirus glyco-proteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycopro-tein.Virology 50:640-652.
29. Scherrer, K., and J. E. Darnell. 1962. Sedimentation characteristics ofrapidly labeled RNA fromHeLa cells. Biochem.Biophys. Res. Commun. 7:486-490. 30. Stone, H.O., D. W.Kingsbury, and R.W.Darlington.
1972.Sendai virus-induced transcriptasefrominfected cells: polypeptides in the transcriptive complex. J. Virol. 10:1037-1043.
31. Stone, H. O., A.Portner, and D. W. Kingsbury. 1971. Ribonucleic acidtranscriptase in Sendai virions and infected cells. J. Virol. 8:174-180.