CopyrightX 1975 American Society for Microbiology Printed inU.SA.
Evidence for the Existence of Protomers in the Assembly of
Encephalomyocarditis Virus
S. McGREGOR,* L. HALL,I AND R. R. RUECKERT
Biophysics Laboratory and Department of Biochemistry, University of Wisconsin, Madison, Wisconsin 53706 Received forpublication26December1974
Two capsid precursor subunits, which sediment on glycerol gradients at 13S
and 14S, respectively, have been identified in cytoplasmic extracts of
ence-phalomyocarditis virus-infected HeLa cells. The 13S subunit, which was
detected after a 10-minpulselabel with 3H-labeled aminoacids, containedonly
capsidprecursorchainA (mol wt 100,000). Whenthe 10-minpulselabelinsuch
cells was chased for 20 min, the A-containing 13S subunit in the cytoplasmic
extractswasreplacedbya14Ssubunitcontainingequimolarproportions ofthree
chains: a, y,anda.This(e,y,a)-containing14Ssubunitcouldbe dissociatedinto
6Ssubunitswiththesamepolypeptide composition. Thesedimentation
proper-ties and thepolypeptide stoichiometry ofthesethree precursorsubunits, when
compared with those ofthe 13S,
(8,'y,a)s,
and5S,(B,y,a),
subunits derived byaciddissociationofpurified virions, suggest the following structural assignments:
13S, (A) ; 14S, (e,
y,a),;
6S,(w,y,a).
The molecular weights ofthe individuallyisolated capsid chains were determined by gel filtration in 6 M guanidine
hydrochloridetobe: a, 36,000; a, 32,000; ,, 29,500; y, 26,500; and6, 7,800. With
theexceptionoftheb-chain, these values areinreasonable agreement with the
values previously determined
by
electrophoresis on sodium dodecylsulfate-polyacrylamide gels. These data support the hypothesis that picornavirus
capsids are assembled from identical protomers
according
to thefollowing
scheme:
(A)-
(A)5
_(,y,a)s -(b,f,y,a)o n(evy,a)n
where n is the numberof immatureprotomersper virion.
Picomaviruses contain four nonidentical
polypeptidechains here designateda, ,S, y, and
6 (29). All four chains are generated by
post-translationalcleavageofasingle, large(mol wt
100,000) precursor, the A chain(3, 13, 17, 22).
The virions alsofrequently contain traces ofa
fifth polypeptide,
a,
which is an uncleavedprecursorof
#
and 6chains (3, 13).Thecardiovi-ral(encephalomyocarditis [EMC]virus, mouse
Elberfeld [ME ]virus, mengovirus) chainsA,
a,
a, ,B, y, and6correspondtothepolioviral chains
NCVP-la, VP-0, VP-1,VP-2, VP-3, andVP-4,
respectively (13, 32). We have adopted the
cardiovirus nomenclature which lends itself
more readily to the description of oligomeric
structures.
Cytoplasmic extracts of poliovirus-infected
HeLa cells contain two capsid-related
struc-tures, one sedimentingat
148
and the otherat73S (25-27). Both contain the capsid peptides 'Present address: Department ofBiology, 16-717, Mas-sachusetts Institute ofTechnology, Cambridge,Mass. 02139.
a,
'y,
aand are thought to be intermediates incapsidassembly.Inthepresenceof
rough
mem-brane fractions from infected cells, the 14S
subunit can be assembled in vitro into 73S
protein shellswhich are indistinguishablefrom
the RNA-devoid, empty capsids found in
in-fected cells (24, 25, 27). Thekinetics of in vivo
labeling experiments are also consistent with
the postulatedprecursor role ofthese two
sub-units (14, 23).
It has been suggested that the capsid
poly-peptides are organized into two closely related
types of subunits designated "mature proto-mers" (a protomer is defined as the smallest identical subunitofan oligomeric protein [21])
(6,-,',a),
and "immature protomers,"(w,'y,a)
(29, 30). These hypothetical subunits are
pre-sumably derived from the A chain in the
se-quence: A -
(a,y,a)
-p(6,M,y,a).
According to the protomer hypothesis, the
immature empty capsid,
by
analogy with thestructure ofthe virion, is
comprised
of60im-1107
on November 10, 2019 by guest
http://jvi.asm.org/
McGREGOR, HALL, AND RUECKERT mature protomers, (,
'ya!)60,
whereas thesedimentation coefficient of the 14Ssubunit is
aboutthat expected for a pentameric structure,
(E,-y,a). (29). However,
thestoichiometry
re-ported for the polioviral 14S precursor is E
302y5a3
(26).Acapsid-related antigen, which sedimentsat
14S, has also been reported in extracts from
EMC virus-infected KrebsII ascites cells (15).
Inthis communicationweconfirmtheexistence
of such a 14S subunit in EMC virus-infected HeLa cells and show that it has the propertiesof a precursor, not a capsid breakdown product. We also show that this subunit can be dis-sociated intoasmaller subunit withthe
proper-ties expected of the postulated immature pro-tomer. A second subunit, which appears to be
an uncleaved precursor of the 14S subunit, is also described.
MATERIALS ANDMETHODS
Media. Medium A is Eagle minimal medium in Earle saline supplemented with 0.1 mM nonessential aminoacids and2x 10-5 Minositol (20). Medium F is Eagle minimal medium in Earle saline lacking calcium and magnesium andsupplementedwith 0.1 mM glycine and serine and 0.1% Pluronic F68. Me-dium AH is meMe-dium A containing 25 mM N-2-hydroxyethyl-piperazine-N'-2-ethanesulfonic acid buffer,pH 7.4 (3). Medium AL is medium AHlacking amino acids.
Buffers. When Dulbecco phosphate-buffered sa-line (6) is supplemented with 0.1% bovine serum albumin(BSA), it isdesignated PBSA.Buffer IV is 1 Msodium chloridein 0.02 MTris acetate (pH 7.5). TSAis 0.25Msodiumchloride,0.005M Tris acetate (pH 7.4) containing 0.01% BSA. Reticulocyte stan-dardbuffer (RSB) is 0.01 M sodium chloride, 1.5 x 10-3M magnesium chloride and 0.01 M Tris-hydro-chloride(pH 7.4).
Cells.HeLacells,designatedW-HeLa,were origi-nally obtainedthroughthe courtesy ofG.C.Mueller, Department of Oncology, University of Wisconsin. They were propagated in suspension in medium F supplemented with 6% bovine serum by shaking 300-mlcultures insiliconizedFlorenceflasksat37C. The cultures, which did not require trypsinization, grew with a generation time of 24 h. They were continuously maintainedatthisgrowthrateof serial dilution tokeepthecelldensitybetween 0.5 x 106to 6.0 x 101 cells per ml.
Virus. EMC virus was originally obtained from C. Fuerst,University of Toronto. It had beenadaptedby serial passagetogrowthinSarcoma 180 ascitestumor cells (12). The virus was then plaque-purifiedthree times in HeLa cells. Stock virus was prepared by growing the plaque isolate (hereafterdesignated the F2strain)inHeLa cell suspension cultures. Thestock was concentrated 50- to 100-fold by sedimentation. The pelleted virus wasresuspended and purifiedby isopycniccentrifugationincesiumchloride. Thevirus
suspension was thendialyzed against TSA buffer and stored at -70 C. Theinfectivitytiter of thestock virus was determined by plaque assay on Hela cell mono-layersand was 6.5 x 1011 PFU per ml.
Infection of suspension cultures. HeLa cells were grown in suspension culture in medium F supple-mented with 6% bovineserum. The cells were sedi-mented by low-speed centrifugation, washed once, andresuspendedinmedium AL at a concentration of 4 x 107 cells per ml. The cellsuspensionwas inocu-lated with 100 PFU per cell. After an attachment interval of 20 min at room temperature, the suspen-sion was diluted 10-fold with warm medium AL. Actinomycin D was then added to give a final concentration of 5 gg per ml. The infected cell suspension (4 x 101 cells per ml) was incubated at 37 C with gentle agitation.
Isotopic labeling and purification ofEMC virus. Isotopically labeled amino acids were added to in-fected cell suspensions at 3 h postinfection. Incuba-tion was continuedat37C. At 5 hpostinfection, the cells,containing greaterthan90% ofthe total infectiv-ity, were sedimented and washed once in cold me-dium AL. The virus was released from the cells by freeze-thawing twice. Cell debris was removed by low-speed centrifugation, and the virus waspelleted through a cushionof 30% sucrose in bufferIV. The pellet was resuspended inPBSA by flushingwith a needle and syringe and insoluble material was re-movedbycentrifugation at 12,000 x g for 5 min. The virus in the clarified supernatant fluid was further purified by isopycnic centrifugation in cesium chlo-ride, followed bygel filtration on 6% agarose equili-brated with TSA. The purified virus was stored at -70C.
Glycerol density gradient. Linear gradients of glycerol(wt/wt)containedRSB,0.1% 2-mercaptoeth-anol, and 0.1%BSA. Five-millilitergradients (Spinco SW65rotor) were generatedbythe method of Britten andRoberts (1). Twelve-milliliter gradients (Spinco SW41rotor)werepreparedby layeringfive concentra-tions ofglycerol (30, 25, 20, 15, and 10%)andstoring at6Cfor 6htoallow diffusion. Bothtypesof
gradi-entswerecentrifugedat40,000rpm and had average gravity forces of 114,000 and196,000fortheSW65and SW41rotors,respectively.Allgradientswere fraction-ated by bottom puncture. Radioactivity was deter-mined by scintillation spectrometry in scintillation solventB-10(20).
Dissociationofvirions into 13S and 5Sprotein subunits. Purified EMC virus in TSA buffer was treated with0.1%2-mercaptoethanolat room temper-ature for 10 to 15 min. The reduced virus was dissociated into 13S subunitsby dialysis against0.1 M sodium chloride in 0.05 M sodium citratebuffer, pH 5.7, at 37C for6 h. The 13S subunit was then isolated by sedimentation on a 12-ml, linear, 10 to 30%glycerolgradientat 6C for13hat40,000rpm ina Spinco SW41rotor.
Toprepare 5S subunits, reduced virions were first dissociated at pH 5.7 as described above, and the resulting 13S subunits were then dissociated into 5S subunitsby addingtothedialyzed preparation suffi-cient6Mureatobringthe finalconcentrationto1.5
1108 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
M. After 15minatroomtemperaturethe preparation
wasAppliedtoa12-ml, linear, glycerol gradient and
centrifugedat6Cfor 14 hat40,000rpminaSpinco SW41rotor.
Polyacrylamidegel electrophoresis. Electropho-resiswasby amodification (20) of the procedure of
Maizel (16). The gels contained 9.7% acrylamide, 0.3% ethylene diacrylate (vol/vol), 0.1% N,N,N',N'-tetramethylethylenediamine, 0.1%
so-diumdodecyl sulfate (SDS), 0.5 M ureaand 0.1 M
sodium phosphate at pH 7.2. The electrophoresis bufferwas0.1M sodium phosphate, pH 7.2, contain-ing 0.1% SDS and 0.01 M mercaptopropionic acid. Samples to be electrophoresed on SDS gels were
dissociatedat100C for 5 min in 1% SDS, 0.5 Murea,
and 0.1% 2-mercaptoethanol in 0.01 M sodium phos-phate buffer, pH 7.2. Electrophoresiswascarriedout at9 mApertube.
Soft gels, utilized in the purification of high-molecular-weight subunits, contained 5% polyacryl-amide (acrylpolyacryl-amide-bisacrylpolyacryl-amide ratio of 33), 0.5%
agarose, 0.1%
N,N,N',N'-tetramethylethylenedia-mine, and 0.1 M sodium phosphate buffer (pH 7.2). Thegelswerepolymerizedat37Cand then incubated atroomtemperaturefor 1 h beforeuse.The
electro-phoresis buffer contained 0.1 M sodium phosphate buffer, pH 7.2, and 0.02 M mercaptopropionic acid. Electrophoresiswascarriedout at4mApertube.
Allgelswerefractionated withaGilson automatic
gel fractionator (11). Determination of radioactivity in SDS gel fractions by liquid scintillation counting has been described (20).
Determination of polypeptide molecular weights. Theapparentmolecular weights of individ-ually isolated virus peptidesweredetermined by gel
filtration in 6 M guanidine hydrochloride accordingto the method of Fish et al. (10). The virus peptides, labeled with a 'H-labeled amino acid mixture, were
recovered from peak regions of the fractionated SDS-polyacrylamide gels by extraction with 10 gel volumes ofdistilled watercontaining 0.1% 2-mercaptoethanol and 0.1% BSA for 12 to 48 h. The proteins in this extract were carboxymethylated by the following procedure.
Solutions containing the isolated virion peptides
weremixed with marker proteins (never exceeding 20 mgoftotal) ina1-dram vial containing the
vacuum-dried residue from1ml of 6 Mguanidine hydrochlo-ride in 0.5 M Tris-hydrochloride, pH 8.6. After adjusting the volumeto1.0mlwithdistilled water, 4
plof2-mercaptoethanolwasadded and the solution wasincubatedfor 1 h at 45Ctocompletereduction of disulfide bonds. lodoacetamidewasthen added toa
final concentration of 0.15 M and incubation was
continuedat 45Cfor 10 min inthedark.Alkylation
wasterminatedby adding 30 &l of 2-mercaptoethanol.
Aftercooling, 300 Mgof Blue dextran2000(Pharmacia Fine Chemicals, Piscataway, N. J.) and 78 ;g of sodium 2,4-dinitrophenyl-alanine (Mann Research Laboratories, Inc., New York)wereaddedasvolume markers for the column. Gel filtration of 200-Ml sampleswascarriedouton a6%agarosecolumn (75 by 0.9 cm) equilibrated with 6 M guanidine hydro-chloride. The markerproteinswere
spectrophotomet-rically detected usingamicro-flow cell
(Instrumenta-tion Specialties Co., Lincoln,Neb.) withavolume of
50
Al.
The flowratewasmaintainedat1.5mlperh.Determination of acid-insoluble radioactivity. Samples from glycerol gradients were placed onto Whatman 3MM filter disks and driedat37Cfor4h. The filter disks were washed three times in 5%
trichloroacetic acid at 6C for 10 min. The trichloro-acetic acid was removed by washing twice in 95%
ethanol and thediskswere driedat 100C. The filter diskswerethencounted in scintillation solvent B-10.
Material. Actinomycin D (Dactinomycin) was
ob-tained from Merck, Sharp and Dohme (West Point, Pa.). The 6% agarose (Bio-Gel A-5M) was from
BioRad Laboratories (Richmond, Calif.). The L-[4,5-'H]leucineandL-[U-14C]leucinewerepurchased from
Schwarz/Mann (Orangeburg, N.Y.); the L-[U-14C]-amino acid mixture was from New England
Nu-clear(Boston, Mass.).
RESULTS
Identification oftwocapsid-related protein precursorsubunits.There is evidence that the EMC viral A chain, which is theprecursorof all capsid protein, hasahalf-life of about 7min(4). Accordingly, we sought evidence for the exist-enceofcapsid-relatedstructureswithinthe first
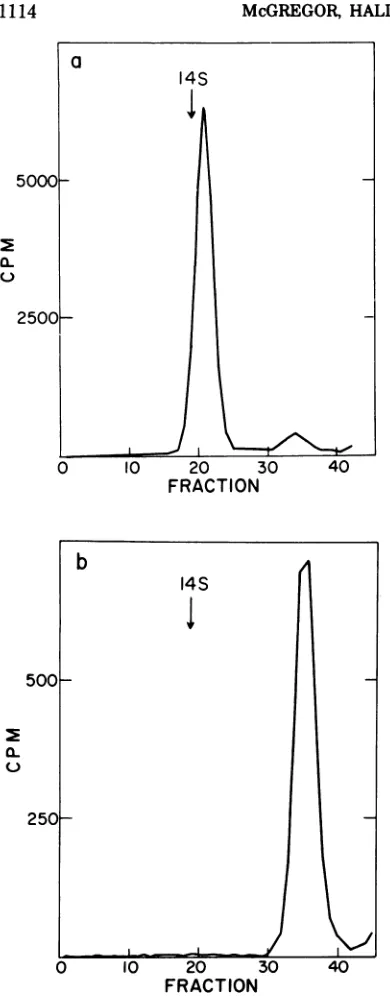
10 min of synthesis. Figure lashows the sedi-mentation profile ofacytoplasmicextractfrom
EMC virus-infected HeLa cells after a 10-min pulse of
[3HRleucine.
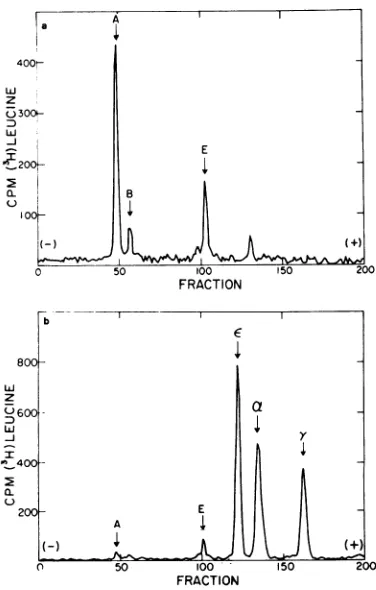
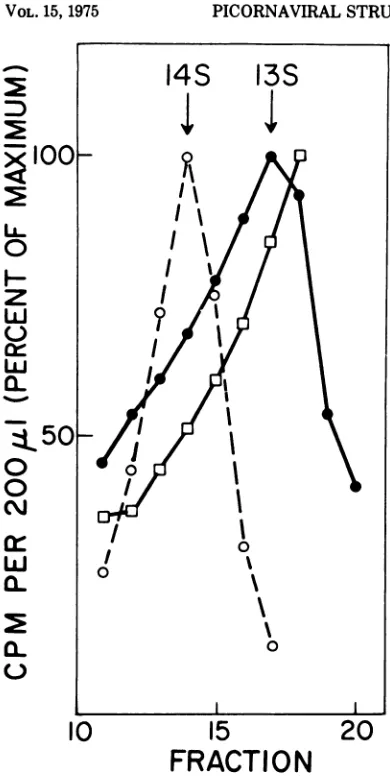
Labeling was carried out at3.5 h postinfection when virtually all protein synthesiswasvirus coded. The bulkofthe acid-insoluble radioactivity sedimented as a broad band in the 2S to lOS region with a small shoulder cosedimenting with a 13S marker. When the label insuchpulsed cellswaschasedfor 20min, the 13S shoulderwasreplaced with a prominent sharp peak which sedimented 8% faster than the 13S marker, or at about 14S (Fig. lb).
Theelectrophoretic profileofthe 13S and 14S peaks on SDS-polyacrylamide gels is shown in
Fig. 2.The 13Speak, observed after the10-min pulse, contained a dominant peak of A chain andatraceofcapsid-related B chain (3). It also
contained about 12% of the recovered label in E
chain, a noncapsid protein (3), and a trace of label in atleast one other minor, unidentified peak. The 14S peak,observed after the20-min chase, consisted predominantly of capsid-related peptides (E,a,y) and only very small
proportions of A and E chains. These results
raise the possibility that the E chain is a
component of the A-containing 13S peak, in
which case the distribution of the A and E
chains in theglycerol gradientshould coincide. To test this possibility, the distribution of
each chain in gradient la was determined by
1109
on November 10, 2019 by guest
http://jvi.asm.org/
MCGREGOR, HALL, AND RUECKERT
j7e
L
\13S
0
U200204
0 20 4w
a.
m
1000-0
In
z
0 20 40 0 20 40
FRACTION
FIG. 1. Sedimentation profile of cytoplasmic ex-tracts from EMC-infected cells. (a) Pulse labeled 10min;(b) pulse labeled 10 min and chased20min. Infected HeLa cells (8 x 107) were pulse labeled
with ['H]leucine (100 M&Ci per ml) at 3.5 h
postin-fection. Labelingwasallowed toproceed for 10min at 37C.Half of the cells werethen Dounce homoge-nized in cold RSB and the cytoplasmic extractwas stored in ice. The other half of the culture was sedimented and resuspended in10mlof mediumAH and the 'H label was chased by incubation for an
additional 20 min at 37C. The cells were then
homogenized. Equivalent amounts of both cytoplas-mic extracts wereplaced onto 12-ml glycerol gradients (10 to30%) and centrifuged at 6 C for 13 h at 40,000 rpm in aSpinco SW41 rotor. A [14C]13Smarker (not shown) prepared by acid dissociation of the EMC virion (seeMaterials and Methods) was included in both gradients. Thegradients werefractionated into 14-drop (270,l)fractions by bottom puncture. Sedi-mentation was fromright to left.
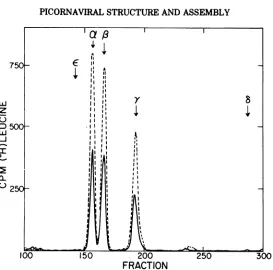
analyzing fractions 10 to 20 on SDS-polyacrylam-ide gels. The total radioactivity in the A and E chains in each gradient fraction was
calcu-latedandplottedagainst fraction number (Fig.
3). The small amount of label in the B chain
precluded analysis by this procedure. Also
shown in Fig. 3 are the results of a similar analysis of the 14S, E-containing peak from
gradient lb. The e, a, and y chains inthe 14S
peak allcoincided, supportingthe idea that all
are elements ofa single structure. In contrast, thelackofcoincidenceinthedistributionofthe Aand E chainsinthe 13S region ofgradientla
argues againstthehypothesis thatthe E chain is a component of the 13S peak. A more
complete analysis (not shown) indicated that
the E chain ispartof aseparate butoverlapping
component whichpeaksatabout 7S.
Thesedatasuggest that theAchain is
assem-bled into an oligomeric structure before being
cleavedintothe smallers, a, andychains. After
cleavage, the sedimentation velocity of the precursor increased from 13S to 14S. In addi-tion, it should be pointed out that while the e-containing peak was very symmetrical the A-containingpeak was not, but tended to skew toward the 14S peak (Fig. 3).
Polypeptides in the 2S to lOS region. The monomeric form of the A chain, with a mass of 100,000daltons, would be expected to sediment
atabout6S(29).. A search for such a monomeric
structure inthe 2S to10S region of the gradient (Fig. la) revealed less than 2% of the total radioactivity in this region in A chains. How-ever, after the 20-min chase (Fig. lb), approxi-mately 10% of the total label in the 2S to 10S region was found in e chains. A trace of label was also found in capsid-related peptides in a narrow band at the top of the gradient. The
bulk of the label in the 2S to 10S region was
a 400r
z u 3001-0
LU
Zj E
5o2002
0 50 100 150 200
FRACTION
[image:4.505.62.256.60.261.2]FRACTION
FIG. 2. Electrophoretic profile of peak fractions from densitygradients inFig. 1. Samples of 200MI were electrophoresed on 20-cm, SDS-polyacrylamide
gelsat 9 mAper tube for approximately18 h.(a)13S subunit(fraction 17) from gradienta inFig.1.(b)14S subunit(fraction 14) from gradient b inFig.1.
1110 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.505.267.455.309.604.2]14S
13S
I
I
1'\
I I'
F
-I
I
0
0
15
FRACTION
FIG. 3. Distributionof virus-coded peptides inthe 13S to 14S region of sedimentation gradients of cytoplasmic extracts. Gradientfractions from Fig. 1
(a and b) were analyzed by SDS-polyacrylamide gel
electrophoresis. Theamountof each virus peptideper
fraction was calculated and their distributions were
plotted. The values plotted for the A chain and E chainwerefrom (a); the value for theechainwasfrom
(b) (Fig. 1). The distribution ofaand-ychains (not
shown) coincided with that of the e chain. The
maximum values for each peptide were: A, 1,163
counts/min; E, 544counts/min; and e,2,576counts/
min. A chain, *--*; e chain, 0--- 0; and E chain, O--O.
associated with the noncapsid polypeptides E
(molwt56,000),H (12,000),and I(11,000).The Echain sedimentedatabout7S and theHand
Ichainsatabout5S.Theamountof these three
noncapsid proteins inthe2Sto10Sregionwas
increased at least fivefold when the extracts
were treated with 0.5% Nonidet P-40 (NP-40)
beforecentrifugation.
Distribution of A chains in the pellet. In the course of these experiments, 40 to 60% of the total A-chain label in the cytoplasmic extracts
was found in the membrane-rich pellet at the bottom of the glycerol gradients. When cyto-plasmic extracts of pulse-labeled, infected cells were centrifuged on discontinuous sucrose gra-dients (5), 30 to 35% of the A chain was found in the rough endoplasmic reticulum band; an
additional 5% was found in the smooth endo-plasmic reticulum band. In an attempt to detectamonomeric (6S) form of the A chainon
membranes, pellets from glycerol gradients were solubilized with 0.5% NP-40 and sedi-mented on glycerol gradients. A chains were found in the 148 but not in the 6S region. This result suggests that the hypothetical A chain, monomericsubunits are rapidly assembled into the 14S form. Another possibility is that A chains werepresent, but, because of a tendency to precipitate or attach to surfaces, were not solubilizedby NP-40 treatment.
Electrophoretic purification ofcapsid pre-cursors on nondissociating polyacrylamide
gels. When material from the 14S region of a
density gradient (Fig. lb) waselectrophoresed
on nondissociating (no SDS) polyacrylamide
gelsatpH7.2,twocomponentswereobserved, a
largepeak I andaslowerpeakII(Fig. 4). Peak II
[image:5.505.56.251.60.448.2]wastypically lessabundant than that shown in Fig. 4. To examine the possibility that these peaks might correspond to the A-containing,
13S precursor and the cleaved, 14S precursor
subunits, each component was eluted from the
gel and its polypeptide composition was deter-mined by electrophoresis on
SDS-polyacryla-mide gels.
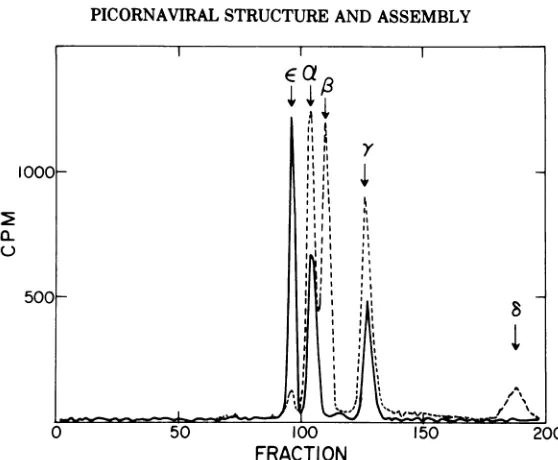
The electrophoretic profile of peak I (Fig. 5)
revealed three major polypeptides; these comi-grated with the c, a, and y chains of the EMC virion. Sedimentation analysis of peak I on glycerol gradients further showed a single peak
sedimenting at 14S (not shown); hence the
majority ofthe applied 14S material survived
electrophoresis and elution without
degrada-tion. The total lack of E chains in this profile further supports our earlier conclusion that the E chain is not an integral element of the 14S
subunit. In addition, the absence of f chains indicatesthat the 14Ssubunit is a precursor of the virion and not a degradative product.
A similar electrophoretic analysis of peak II revealed93% of the recovered radioactivity in E, a, and ychains (not shown); these chainswere
present in the same relative proportions as in peakI.Tracesoflabel were also foundincapsid precursorchains A (0.7%) and B (0.7%) and in a
100-%
i
lIo
0
LA-z
LJ
0Lii
a--L50
0
0
0
10
1111
on November 10, 2019 by guest
http://jvi.asm.org/
McGREGOR, HALL, AND RUECKERT
1500_
0
1000_
cc I Io
w~~~~~~~~~~~~~
50010
30
50
[image:6.505.57.252.62.362.2]FRACTION
FIG. 4. Electrophoretic profile of the intact 14S
precursor subunit on a 5% polyacrylamide gel (no SDS). The 14S subunitwasisolatedfrom cytoplasmic extractsof[3H]leucine-labeledEMC-infected cellsas
described inFig. lb. Afterdialysis against RSB, the subunit was further purified by sedimentation in a
secondglycerol density gradient. The 14Speak frac-tions werepooled andelectrophoresedona5% poly-acrylamidegel containing 0.1 M sodiumphosphate, pH 7.2, and 0.02Mmercaptopropionate but lacking SDS (see Materials and Methods). Electrophoresis
was carried out at 4mA per tubefor 32 h. The gel
wasfractionated and the protein subunitwaseluted into distilled water containing 0.1% 2-mercapto-ethanol. Fractionswereincubatedforatleast 12 hat 6Cbefore sampleswereremovedfordeterminationof radioactivity.
noncapsidchain G (4%).Hence, peakIIcannot contain more than a trace amountofsubunits
composedof Achain. It consists insteadmainly
ofpeakI-related material, possiblyinthe form ofanaggregate, aconformeroreven a
dissocia-tion product. Because of its smaller size, the
mobility ofsuch a dissociation product would
normally be expected toexceed that ofpeakI.
However, similar electrophoretic studies of the
virion-derived 13S, (,y,a)5, and 5S,
(0,,y,a),
subunits(see below) yieldedtheopposite result;
i.e., the 5S subunit migrated slower than the
13S. Such a result could be explained by a
decrease in the isoelectric point of the protein accompanying the dissociation of the 13S sub-unit.
Attempts to electrophoretically purify the A-containing 13S peak from pulse-labeled, cy-toplasmic extracts were frustrated by thesmall amount ofradioactivity available and by seri-ous losses encountered during storage and ma-nipulation of this material.
Dissociation of the 14S capsid precursor
with urea. Figure 6 shows the sedimentation profile of the 14S subunit after a30-min treat-ment with 1.5 M urea at 35 C. Approximately 55% ofthe 14S subunitswasdissociated by this treatment into material which sedimented just ahead of a 5Smarker; this smaller component wasassigned a nominal sedimentation value of 6S. An additional 30% of the radioactivity sedimen,ted as undissociated 14S subunits. The remaining 15% of the material was adsorbed to the centrifuge tube. To minimize the loss of material due to adsorption, the 6S region was collected into siliconized glass vials. When ex-aminedby electrophoresis on SDS-polyacrylam-ide gels, the 6S material exhibited a polypep-tide composition very similar to that of the parent, 14S subunit (Fig. 7; see also Table 4,
below).
13S and 5S subunits from pH-dissociated
EMC virions. ME virus (7) and mengovirus
(18), which are serologically related to EMC
virus, can be dissociated through a 13S-14S intermediate, (,y,a)8, intoprotomeric 5S sub-units,
(B,y,a).
In this section we describe the properties of the analogous subunits derived from pH-dissociated EMC virions (Fig. 8). Thesedimentation profile of the dissociation
prod-uct of
[ICJleucine-labeled
EMC virions (Fig.8a) was indistinguishable from that of the
(#,,y,a),
subunits from ME virus (data notshown). Weassumetherefore that the 13S-14S
subunitsderived from EMC viruscorrespondin
structure tothose from ME virus.
The
(#,-y,a),
subunit of ME virus hasprevi-ously been assigned a sedimentation value of 14S (7); on this basis, the (e,y,a)-containing
precursorsubunitofEMC virus wasassigned a
valueof15S(29).The sedimentation coefficient
ofthemengovirus
(#,,y,a)5
subunit, determinedby analytical ultracentrifugation, has been reported to be 13.4S (19). In this paper, we
have attempted to simplify the nomenclature
by adopting a nominal value of 13S for the
(#,,y,a),
subunit ofMEvirus, EMC virus, andmengovirus. This leadsto acalculatedvalueof 14S for the EMC virus capsid precursor (Fig. lb), avaluewhich has beenextensivelyusedin
1112 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
500
0 50 100 150 200
FRACTION
FIG. 5. SDS-polyacrylamide gelpattern ofthepurified 14Sprecursorsubunit. The subunit elutedfrom fraction 14 to 15ofFig. 4 was coelectrophoresed with ["4CJEMC virus on a20-cmpolyacrylamide gelat 9 mA pertube forapproximately15h. Arrowsindicate thepositions oftheEMCvirionpolypeptides.
5S E
14S
20
1500X
13S 1200z
4
~~~~~~
~9006-L1000
3000 30
w
O.. 20 50 100 150 200
2 ~~~~~~~~~~~~~~~~~~~FRACTION
a. FIG. 7. SDS-polyacrylamide gelpattern of the6S
~500-
peak fr-om Fig. 6. Electrophoresed on a22-cm poly.acrylamide gel at 9 mA per tubeforapproximately18 h.
describing the poliovirus capsid precursor,
whichpresumably hasa similarstructure.
The 13S subunit was
completely
dissociated) 10 20 30 40 into smaller 5Ssubunits by treatment with 1.5
FRACTION M urea at room temperature (Fig. 8b). Its
FIG. 6. Sedimentation profile of the 14S subunit stability differed significantly in this respect after treatment withurea. Thepurified14Ssubunit from that of the 14S precursor subunit which
from Fig. 4was heated at 35Cfor30 minafterthe was only partially dissociated even when ex-additionof 6 M ureatogiveafinalconcentrationof posed to anequivalenturea concentrationfor a 1.5M urea. It wassedimentedon a12-mllinear15 to
35%glycerol gradient at 6 C for14 h at40,000rpmin a longer
time
at a higher temperature (35 C Spinco SW41 rotor. A[14CJ5S
subunit was included rather than 21 C).as a sedimentation marker (not shown). A parallel SDS-polyacrylamide gel analysis of the 13S gradient contained a ["C ]13Smarkerpreparedfrom and 5S subunits demonstrated that both sub-EMC virus(notshown). units were
composed
ofa, ,B,and ychains(Fig.
1113on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.505.111.390.53.283.2] [image:7.505.58.450.323.572.2]McGREGOR, HALL, AND RUECKERT
a 9). The distribution of
[H
H]leucine
in thepro-14S
files of the twosubunits was very similar(see
Table 3).
Stoichiometry of polypeptides in the EMC virion. The number of polypeptide chains of species i (nf),
and
molecular weight (mi), in a5000_
capsid
of known mass (M) can be calculatedusing equation (1) if the relativedistribution of radioactivity
(r,)
and the relative specificactiv-ity
(a1)
are known:X ni
=
(ai)(ri)
(M)/(m)
(1)
2500 Purified EMC virions, labeled with amixture of
2500 15radioactive amino acids, weredisrupted with
SDS and electrophoresed on SDS-polyacrylam-ide gels. The distribution of radioactivity in each polypeptide peak was determined (Table 1, column 2). Assumingthat all five chains were
labeled to a constant specific activity by the radioactive amino acid mixture, the average
0
10
20 30 40 chain stoichiometry was calculated usingpep-FRACTION tide molecular weights determined by two
dif-ferent methods: (i) electrophoresis on
SDS-polyacrylamide gels, and (ii) gelfiltration chro-matography in 6 M guanidine hydrochloride.
b4S
Thestoichiometry
obtained usingtheelectro-14S
l phoretically determined molecular weights wasf2a60#57769b
45
(Table 1), comparedtoaformula of,f2a0:5'ys06,s
predicted for a60-protomer
capsid containing two immature protomers.500_
Agreement
with the theoreticalstoichiometry
fell well within the 6 to 10% reliability limit attributed to theelectrophoretic method(8, 33), except for the two smallest chains, y(15% high) and6(25% low). Theapparentmolecularweight
0 of the y chain of ME virus, which is
electro-phoretically indistinguishable from that of
250 EMC virus, is known to be sensitive to small
changes in electrophoretic conditions, varying
from 23,000 to 26,500 (8, 29). Anapparently low delta chain content of the virion has also been observed with ME virus and was traced to an
anomalous molecular weight value (30, 31).
Such anomalies are now known to occur
com-0)
10
20 30 40 monly inSDS-gelelectrophoresis withpolypep-0FRACTION
tides smaller than 15,000 daltons (8, 28). To examine this issue with EMC virus, the FIG. 8. Sedimentation profile of the 13S (a) and 5S molecular weight ofeachpolypeptide
was deter-(b) subunits derivedfrom
the EMC virion by acid minedby gel'filtration chromatography in 6 M dissociation. The 13S subunitwasprepared bydialyz-ing purified,thiol-reduced,
['4C]leucine-labeled
EMC guanidinehydrochloride
usingisolated,
virusagainst 0.1M sodium chloride, 0.05 Msodcbm [H
Jleucine-labeled
chainsextractedfrom SDS-citrate at pH 5.7 (see Materials and Methods). The polyacrylamide gels (Table 1). The stoichiome-dissociated virus was sedimented on a 12-mi, linear,10 to 30% glycerol gradient at 6 C for 12 h at 40,000 temperature. The dissociated virus was then sedi-rpm in a Spinco SW41 rotor. The 5S subunit was also mented on a12-ml, linear, 15to35%glycerolgradient
prepared
from thepurified
virion. After acid dissocia- at 6 C for 14 h at 40,000 rpm in an SW41 rotor.Ation of the virus at pH 5.7 in0.1M sodium chloride, it ['H114S marker was included inboth gradients (not was further treated with 1.5 M urea for 15min at room shown). Sedimentation isfromrightto left.
1114 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.505.66.261.57.555.2]Lu z
I
U"
0)
(-FRCTvv
FRACTION
FiG. 9. SDS-polyacrylamide gelpattern of the 13S and 5S subunits derivedfrom the virion. The peak fractions from Fig. 8 (a and b) were electrophoresed on separate 30-cm polyacrylamidegelsat 9 mA per tube for approximately20h.
[("C
EMCviruswascoelectrophoresedwith eachsubunit(notshown).Arrowsindicate the positions of the EMC virionpolypeptides.[H'H]13S
subunit(---);['H]5S
subunit (--).TAmLE 1. Molarproportionsofpolypeptide chains in EMC virions
SDS-gelelectrophoresis 6MGUHCLgelfiltrationb Peptide radioactivity(%)a Apparent No. of chains Apparent No. of chains
molwtC perviriond molwt perviriond
1.1±0.1 40,000 1.6 36,500 200 1.7
a 35.0 ± 0.1 34,000 59.7 32,000±100 63.4
0 29.3 ± 0.2 30,000 56.6 29,500±200 57.6
ly 27.5 ± 0.3 23,000 69.3 26,500± 150 60.2
a 7.0 0.1 9,000 45.1 7,800 ± 150 52.1
aThe averagevalues (4 averageerror)of three determinationsutilizing purifiedEMCviruslabeledwitha mixtureof 15"'C-labeledaminoacids.Thestandarderror ofcountingwas3% for echain.
bTheapparentmolecularweightsweredeterminedbygelfiltrationchromatographyofisolatedpeptidesin 6 Mguanidinehydrochloride(GUHCL).Theaverageerrorsgiven indicatetheprecisionof two orthree separate determinations.
cFromButterworth et al. (3).
dThe number of chains per virion was calculated using equation(1) (see text).
trycalculated with thesemolecularweight
val-ues was
(*63058n7062.
The molecular weightvalue required to obtain a value of 58 delta chains per virion from the radioactivity
distri-bution data is 7,000 rather than the value of
7,800actually found. These values can be
com-pared with similarly determined values for the
delta chain: EMC virus, 6,800 (2); ME virus and mengovirus, 7,200; and poliovirus, 7,600 (31). Using the average molecular weight value
of all these determinations, 7,300, yielded a
calculated delta chaincontentof56per virion.
There is good agreement between the chain
stoichiometry calculated bythesemethods and
that expected for a 60-protomer virion. This is
possibly fortuitous considering the 7 to 10%
reliability limit attributed to the gel filtration
method ofdetermining molecularweights, and theuncertainty ofthe errorinvolved in
assum-ing the polypeptides are labeled to a constant
1115
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.505.116.389.44.314.2]McGREGOR, HALL, AND RUECKERT
specificactivity by the amino acid mixture (10). Inaddition, there is also goodagreementonthe mass of the capsid protomer calculated by different methods: 100,000 daltons for the A
chain (3); 97,000 and 96,000 daltons for the immature (e + 'y +a) andmature(6 +,B+ y+
a) protomersusing themassvaluesdetermined by SDS-gel electrophoresis; and 95,000 and 96,000 daltons for the immature and mature protomersusing themassvalues determined by gel filtration chromatography.
The nonintegral amounts ofe chain (1.6 and
1.7)observedintheseexperimentsdonot
neces-sarily reflect a 15 to 20% error in a true
stoichiometry value of 2. A nonintegral value could be attributedtoaheterogeneous
popula-tion of virions containing 0, 1, 2, 3 or more
immature protomers (7). For example, a value of 1.6 might represent a mixture of virions,
40% containing one immature protomer and
60% containing two. In this case, the average stoichiometrywouldappeartobe
E,.,a60#68.d860-Distribution of leucine residues in capsid polypeptides. [3
H]leucine
is incorporated intoEMC viral protein without significant conver-sion to other amino acids (D. Omilianowski, personalcommunication). Therefore,the
distri-bution of label intheelectrophoretic profilesof
eachof thecapsid-precursorandcapsid-product subunits could be used to measure their chain
stoichiometry if the leucine content of each
capsid chainwere known. These valuescan be
computedfrom theradioactivity profileof puri-fied 3H-labeled leucine virions by assuming thepolypeptide stoichiometry predicted bythe
60-protomer model.
The capsid protein of EMC virus is reported
to contain 8.3 mol% leucine (9). Assuming a
mass of 111 daltons per average amino acid
residue (29), the leucine content ofEMC virus protein iscalculated to be (0.083/111 =) 7.5 x
10-4mol/g ofprotein.Hence, aprotomerwitha massof96,000daltons would contain about (7.5
x 10-4x 96,000 =) 72leucine residues anda
60-protomercapsidabout (60x 72=)4,300leucine
residues.
The leucine contentof each chaincannowbe
calculated fora capsid of knownstoichiometry (Table 2). For example, the number of leucine residues in a virion complement ofa chains is
(0.330 x 4320 =) 1,430, or (1,430/60 =) 24
leucine residues per a chain. The results of
similarcalculations for each chain in the virion are summarized in Table 2 for a 60-protomer
capsid containing (n) immature protomers,
wheren = 2 andn = 3. The calculatedleucine
contentofa, 13, y, and6chains isnotaffectedby
the variation in (n).However, in thecaseofthe
echain,the number ofleucineresiduesis45ifn
= 2, but30ifn = 3.The latter valueagreeswith
theleucinecontentof plus6chains, whichare
the cleavage products of the e chain. This
suggests that n = 3, i.e., the average virion in
thispreparation contained three echains.
The alternative interpretation, that n = 2,
would require the loss of 15 leucine residues during the cleavage of theechain.This hypoth-esisrequiresthat themassoftheechain exceed thatofitscleavage products (13 + 6)by atleast 1,700daltons (the mass of15leucineresidues).
Adiscrepancyofthismagnitudemight conceiv-ably go undetected since the estimated uncer-taintyofthe molecularweight determinationis
on the orderof 10%, or 43,600daltons, for the
epsilon chain.However, such asequencewould
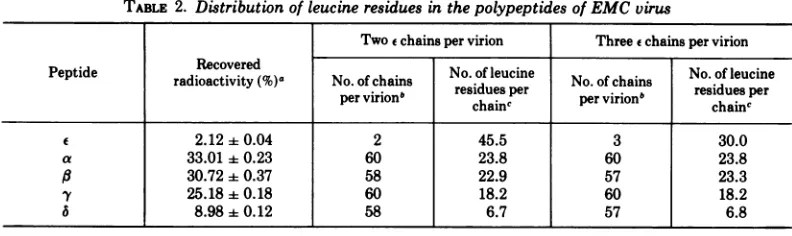
TABLE 2. Distributionofleucineresidues in thepolypeptides ofEMCvirus
Two e chains per virion Threeechains per virion
Peptide Recovered No. ofleucine No. ofleucine
radioactivity (%)a No.of chains residuesper No. of chains residuesper pervirionb chainc pervirion" chainc
2.12+0.04 2 45.5 3 30.0
a 33.01+0.23 60 23.8 60 23.8
ft 30.7240.37 58 22.9 57 23.3
y 25.18 0.18 60 18.2 60 18.2
a6 I 8.98+0.12 58 6.7 57 6.8
aThe average values(4+ averageerror) oftwodeterminations utilizing
[(H
lleucine-labeled
EMC virus. Thechainswereseparatedon SDS-polyacrylamide gels.
bAssuming60protomerspervirion,including(n)immatureprotomers (e,-y,a)and(60-n)matureprotomers Ml,$,y,a).
cThe number of leucine residues per chain was calculated by dividing the product of the total leucine residuesinthe virion and the fraction of['H
#leucine
in aparticularpeptidebythenumber of chains per virion. Itwasassumed thatthe virion contained 4,320 leucine residues.1116 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:10.505.64.460.478.596.2]havetobeveryleucine rich(>50%) in orderto
have such a small mass. Further evidence against this hypothesis isdiscussed below.
Peptide stoichiometry in capsid-related subunits. Electrophoretic profiles of [3H Jeu-cine-labeled 13S,
(f,,y,a),
, and 5S,(M,y,a),
subunits(Fig. 9), derived by acid dissociation of EMCvirions, providedveryreproducible distri-butions of radioactivity. These aresummarized in Table 3 (columns 3 and 5, respectively).
These data were used to calculate the molar
stoichiometry of the polypeptide chains assum-ing that the a chain contained 24 leucine
residues; the ,B chain, 23; and the y chain, 18
(Table 2, column 6). The chainstoichiometry of the 13S and 5S subunits fell within a few
percent of the theoretical equimolar ratios (Table 3, columns 4 and 6). These results strongly reinforcedourconfidence in the values determined for the leucine content of each
virion chainandin theability of this methodto determine chain stoichiometry in capsid sub-units withconsiderable precision.
It should be noted here that the calculated chainstoichiometry dependsonthedistribution
ofleucine in the subunit and inthe virion, but
not onthe gross leucine contentor massofthe
average amino acid residue in the capsid
pro-tein. Thus an error inthe value of0.083 mol% used for the leucinecontentofthevirioncapsid
orinthe value of111forthemassoftheaverage
amino acid residue would change the apparent
number of leucine residuesperchainbutnotthe
relative chain stoichiometry.
Thechain stoichiometry of the14Sprecursor subunit and the 6S subunit was determined assuming the e chain contained 30 leucine residues (Table 2, column 6). The ratio ofall threechainsinthe14Ssubunit fell within 2% of
theequimolar values predicted by theprotomer TABLE3 3. Distributionof['H]leucineinthe13S and 5S subunits derivedfromthe virion
13Ssubunit 5S subunit
Peptide Fraction of leucine Recovered Recovered
residues' radioactivity Molarratioc radioactivity Molarratioc
(%)b (%)d
a 24/65 36.3 L0.7 0.98+ 0.02 36.3 + 0.8 0.98 + 0.02
0 23/65 36.1+0.5 1.02+ 0.02 35.9+ 0.8 1.01 + 0.02
ly 18/65 27.6+1.0 1.00+0.04 27.8+0.3 1.00+0.01
aFrom Table 2,
last
column, assuminga(#,-y,a)protomercontains 65 leucine residues.bThe average values (+ average
error)
of four determinations utilizingthepurifiedsubunit labeled with['H leucine.The 13S subunitwaspreparedby dissociating thepurifiedvirionasdescribedinFig.8.Thechains wereseparatedonSDS-polyacrylamide gels (seeFig.9).
cThe relative molaramountofeach chain (n) wascalculatedfrom the relationn = r/m, where(r) isthe fractionofradioactivity and (m) isthe fraction ofleucineresidues in thechain (column 2).Forexample, the numberof achains wascalculatedtobe0.363 . 24/65 = 0.98.
dThe average values (+ averageerror) of five determinationsutilizing thepurified subunit labeled with
['H leucine.The5Ssubunitwasprepared by dissociatingthe13S subunitasdescribedinFig.8.Thepeptides
[image:11.505.52.448.504.604.2]wereseparatedonSDS-polyacrylamide gels (see Fig. 9).
TABLE 4. Distribution of['H]leucineinthe14Sprecursorand its 6S subunit
14Ssubunit 6S subunit
Peptide Fraction of leucine Recovered Recovered
residues' radioactivity Molarratioc radioactivity Molarratioc
(%)b (%)d
30/72 42.4 0.6 1.02+ 0.02 44.1+1.0 1.06 0.02
a 24/72 32.6+ 0.6 0.98+ 0.02 33.1 + 0.5 0.99+ 0.01
y 18/72 25.0+ 0.3 1.00+0.01 22.8 ± 0.6 0.91±0.03
aFromTable 2.
bThe average values (i average error) of three determinationsutilizingthepurifiedsubunit labeled with
['Hleucine.
ThechainswereseparatedonSDS-polyacrylamide gels (see Fig. 5).cDescribed in footnote c, Table 3. For example, the number of e-chains was calculated to be 0.424 30/72 =
1.02.
dThe average value (i average error) of four determinations utilizing the purifiedsubunit labeled with ['H eucine. Thechains wereseparated on SDS-polyacrylamide gels (see Fig. 7).
on November 10, 2019 by guest
http://jvi.asm.org/
McGREGOR, HALL, AND RUECKERT
hypothesis (Table 4, column 4).
However, thepeptideratio of the 6Ssubunit,
which had been generated by dissociating the 14S subunit with urea, deviated significantly from the equimolar values expected for an
immature protomer (Table 4, column 6). Nor-malizing to the e chain yielded a ratio of 1.00:0.93:0.86 for the e, a,and ychains, respec-tively. Thisdiscrepancycould be accounted for by partial degradation of about 15% of the immature protomers. Such degradation would notbe surprisinginview oftherelativelyharsh urea treatment required to dissociate the 14S precursor intothe 6Ssubunit (see above).
DISCUSSION
The results reported here reveal a satisfying internal consistency between the chain ratios predictedbythe protomerhypothesisand those actuallyfound for the virion and its four related subunits. Moreover, the finding of exact (i.e., within a few percent) equimolar stoichiometry
inthe 14S precursorsubunit, using the leucine compositions calculated for each chain in the virion, justifies a previously unstated, but fun-damental, assumption.Thatis, the 14S
precur-sor subunit loses little, if any, mass (leucine
13S
14S
(A)~~~~~~~~~~o (A5 -*
Or')5
((,CAPSID ASSEMBLY
VIRION
CAPSID DISSOCIATION
S9
r.
a)S
': r.
a)
<
5S
13S
residues) during or after the maturation cleav-age to form the virion.
In fact, leucine might have been lost during maturation in at least two different ways. One, suggested by the result in Table 2, is by elimination of a small, undetected, leucine-rich fragment from the e chain when it is cleaved to formthef, and 6 chains. Another way is by loss of chains, e.g., delta chains, from the capsid during maturation or purification of the virions.
In either case, the apparentleucine content of
theechain,calculatedbysumming thenumber
ofleucine residuesinthe , and 6 chains, would have been low. This would have resulted in a high estimate for the relative number ofechains in the immature protomer. The fact that this did not occur allows us to make two conclusions:
(i)the leucine content of the , and 6 chains is a good measure of the leucine content of the e chain; and (ii) the purified EMC virion prepara-tion did contain the assumed full complement ofeach chain. The latter is reinforced by a sim-ilarconclusion drawn previously in studies with ME virus (31).
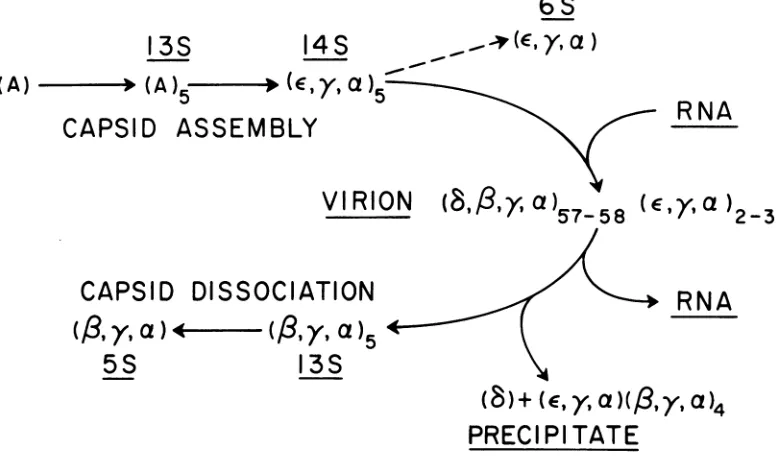
Our current view of the early stages of capsid assembly, and the structural relationship of the subunits involved, are summarized in Fig. 10.
6S
_-- r
a)
RNA
,y,
a
)2
-3RNA
's+ '
.a)(:
a)4
PRECI
PI
TATE
FIG. 10. Model for theassembly and dissociation of the EMC virion. The capsid precursor protein, A, is assembled into 13Ssubunits, whicharethoughttobepentameric oligomers. Cleavageofeach A chain at two specific processingsites then follows. The8%increase insedimentationvelocity attending this processing step suggests aconformational change preceding assembly of14Ssubunits into the 60-protomer capsid. The 14S precursorcould be dissociated with 1.5 urea into a 6S subunitcontaining approximatelyequimolar proportions ofe, y,andachains andfitsthedescriptionofanimmature protomer. EMCvirionscanbe dissociated into 13S and 5S subunitsindistinguishablein sedimentationvelocityandchainstoichiometryfromthose of ME virus. Hence, the dissociation pathway appearstofollow that proposed previously for the serologically related ME virus(7).
1118 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:12.505.71.459.362.590.2]Also shown are thesubunits which are derived
by dissociation ofthevirion.The assignmentof
a pentameric structure to the 13S and 14S
precursorsubunitsisstilltentativeand isbased
on measurements of sedimentation position. Theseindicate that the14Sprecursorsediments
8% faster (Fig. lb) than the
(B,y,a),
subunit,which has a known mass of about 425,000 daltons (7). The molecular weightsofparticles ofsimilarshapeanddensityarerelated to their sedimentation velocities by the relationship
S1/S
2 =(MJ/M2)
213or, inrearrangedform,M2 =Mi(Si/S2)- /2. Assuming thegradient is
approxi-mately isokinetic for the two particles, the mass ofthe14S precursor subunit is calculated to be 480,000 daltons. This is exactly the mass ex-pected for a pentamer of 96,000-dalton proto-mers.
However, a similar calculationyields amass ofonly 425,000 daltons for the 13S,
A-contain-ing precursor. Since cleavage processing is
un-likelytoincreasethemassordensityofthe13S
subunit, the 8% increase in the sedimentation
velocity accompanying its maturationevidently
reflects adecrease in the hydrodynamic radius. This in tum implies a substantial conforma-tional change. In view of the surface changes
implied by marked differences between the antigenic determinants of the 14S, 73S, and virion ofpoliovirus (reviewed in reference 29),
the occurrence of such conformational changes
is not unprecedented. Indeed, it now seems likely that substantial rearrangements of sur-face structure may accompany each step in picornavirus assembly.
A monomeric form of the capsid precursor,
polypeptide A, which is knowntobeaprimary
gene product in infected cells, would be
ex-pected to sedimentat5Sto6S(30).However,a
search for such a form, or for its cleaved
derivative,
(,,y,a),
was negative. It is possiblethat the monomeric A chain, which is thought
to be synthesized on the rough endoplasmic
reticulum, is assembled on the membrane
be-fore its release in thesoluble 13S form orhasa
rate of assembly which is rapid enough to
precludeappreciableaccumulation of the
mono-meric form. Absence of the
(e,-y,a)
monomer suggests that polymerization of the A chains may be prerequisite for chaincleavage.Ithas been suggestedthateachpicornavirus
protomer requires two different types of inter-subunit bonding regions to accomplish capsid
assembly (30). Ordering of atwo-step bonding
sequence might be controlled by adelay
infor-mation of the stereospecific, complementary surface configuration required for the second
bindingstep until the productofthefirststep is
complete. We suggest that the conformational
shift accompanying the cleavage of the 13S subunit may representjust such an unmasking of a second bonding site, and that the double cleavage of the A chain may berequiredtoallow sufficientconfigurational change to accomplish
thisunmasking.
ACKNOWLEDGMENTS
This research wassupported bygrantVC 26C fromthe American CancerSociety.R.R.R. holds aFacultyResearch award(PRa-106)from the American CancerSociety.
LITERATURECITED
1. Britten,R.J., andR. B. Roberts. 1960.Highresolution density gradient sedimentation analysis. Science 131:32-33.
2. Burness, A.T.H., S. M.Fox, and I. V. Pardoe. 1974. The polypeptidecomposition oftheencephalomyocarditis virusparticle. J. Gen. Virol. 23:225-236.
3. Butterworth,B.E.,L.Hall,C. M. Stoltzfus, and R. R. Rueckert. 1971.Virus-specific proteins synthesizedin encephalomyocarditisvirus infected HeLa cells.Proc. Natl. Acad. Sci. U.S.A. 68:3083-3087.
4. Butterworth,B.E.,andR. R. Rueckert. 1972.Kineticsof synthesisandcleavageofencephalomyocarditisvirus specific proteins. Virology50:535-549.
5. Caliguiri,L.A., andI. Tamm. 1970. The role of cytoplas-mic membranes in poliovirus biosynthesis. Virology 42:100-111.
6.Dulbecco, R., and M.Vogt. 1954.Plaque formation and isolation of pure lines with poliomyelitis viruses. J. Exp. Med. 99:167-182.
7. Dunker, A. K., and R. R. Rueckert. 1971. Fragments generated by pH dissociation ofME virus andtheir relation to the structure of thevirion. J. Mol. Biol. 58:217-235.
8. Dunker,A.K., and R. R. Rueckert. 1969.Observations on molecular weight determinations on polyacrylamide gel.J. Biol.Chem. 244:5074-5080.
9. Faulkner, P., E. M.Martin, S.Sved,R.C.Valentine, and T.S. Work. 1961.Studiesonprotein and nucleicacid metabolisminvirus-infectedmammaliancells.2.The isolation, crystallization and chemicalcharacterization of mouse encephalomyocarditis virus. Biochem. J. 80:597-605.
10. Fish, W. W., K. G. Mann, and C.Tanford. 1969. The estimation ofpolypeptide chain molecular weightsby gelfiltration in 6 Mguanidinehydrochloride.J. Biol. Chem.244:4989-4994.
11. Gilson, W., R. Gilson, and R. R. Rueckert. 1972. An automatic high-precision acrylamide gel fractionator. Anal. Biochem. 47:321-328.
12. Hall, L., and R. R.Rueckert. 1971.Infectionofmouse fibroblastsby cardioviruses: premature uncoating and itspreventionbyelevatedpH and magnesium chloride. Virology 43:152-165.
13. Jacobson, M. F., J. Asso,and D.Baltimore. 1970. Further evidenceon the formation ofpoliovirus proteins. J. Mol. Biol.49:657-669.
14. Jacobson,M.F.,and D.Baltimore.1968.Morphogenesis ofpoliovirus.I. Association of the viral RNA with coat proteinJ.Mol. Biol.33:369-378.
15. Kerr,I. M.,E.M.Martin,M.G. Hamilton, and T.S. Work. 1965. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. The formation of avirus-specific antigeninKrebsII ascites-tumourcellsinfectedwithencephalomyocarditisvirus. Biochem.J.94:337-344.
16. Maizel,J.V.,Jr.1966.Acrylamide-gelelectrophorograms 1119
on November 10, 2019 by guest
http://jvi.asm.org/
McGREGOR, HALL, AND RUECKERT by mechanical fractionation: radioactive adenovirus
proteins.Science151:988-990.
17. Lucas-Lenard, J. 1974. Cleavage of mengovirus poly-proteins in vivo. J. Virol. 14:261-269.
18. Mak, T. W., J. S. Colter, and D. G. Scraba. 1974.
Structure of themengovirion.II. Physicochemical and
electron microscopic analysis of degraded virus. Vi-rology57:543-553.
19. Mak, T. W., D. J.O'Callaghan, C. M.Kay, andJ.Si Colter. 1971. Studies of the protein subunit of pH inactivated Mengovirus variants. I. Physicochemical properties. Virology43:579-587.
20. Medappa,K.C., C. McLean, and R. R. Rueckert.1971.
On thestructureofrhinovirus 1A.Virology 44:259-270. 21. Monod, J., J. Wyman, and J.Changeux. 1965. On the
nature ofallosteric transitions: aplausible model. J. Mol. Biol. 12:88-118.
22.Paucha, E.,J.Seehafer,and J. S. Colter. 1974.Synthesis ofviral-specificpolypeptidesinmengovirusinfectedL
cells: evidence forasymmetric translation of theviral
genome.Virology61:315-326.
23. Penman, S., Y. Becker, and J. E. Darnell. 1964. A
cytoplasmic structure involved in the synthesis and
assembly of poliovirus components. J. Mol. Biol. 8:541-555.
24. Perlin,M., and B. A.Phillips.1973.Invitroassemblyof polioviruses.Ill.Assemblyof14Sparticlesintoempty capsids bypoliovirus-infected HeLacellmembranes.
Virology53:107-114.
25. Phillips,B. A. 1969. Invitro assemblyofpolioviruses.I.
Kinetics of theassembly ofemptycapsids and the role ofextractsfrominfected cells.Virology39:811-821. 26. Phillips,B. A., and R. Fennell. 1973. Polypeptide
compo-sition of poliovirions, naturally occurring empty
cap-sids,and 14Sprecursorparticles. J. Virol.12:291-299.
27. Phillips, B. A., D. F. Summers, and J. V. Maizel, Jr. 1968.Invitro assemblyofpoliovirus-related particles. Virology 35:216-226.
28. Reynolds, J.A., andC.Tanford. 1970. Thegross confor-mation ofprotein dodecyl sulfatecomplexes. J. Biol. Chem.245:5161-5165.
29. Rueckert, R. R. 1971. Picornaviral architecture, p.
255-306. In K. Maramorosch and E. Kurstak (ed.),
Comparativevirology. Academic Press Inc., NewYork. 30. Rueckert, R. R.,A. K.Dunker,andC.M.Stoltzfus.1969. Thestructureof mouse-Elberfeldvirus:amodel. Proc.
Natl. Acad.Sci.U.S.A.62:912-919.
31. Stoltzfus, C. M., and R. R. Rueckert. 1972. Capsid polypeptidesofmouse Elberfeldvirus.I. Aminoacid compositions andmolarratios in the virion.J. Virol. 10:347-355.
32. Summers,D.F.,J. V.Maizel, Jr., andJ. E. Darnell, Jr. 1965.Evidencefor virus-specific noncapsid proteins in poliovirusinfected HeLa cells. Proc. Natl. Acad. Sci. U.S.A.54:505-513.
33. Weber, K., and M. Osborne. 1969. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 215:4406-4412.
1120 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
![FIG. 4.precursorpHwasSDS).subunitsecondSDSextractsdescribedacrylamide6tionswasethanol.into C Electrophoretic profile of the intact 14S subunit on a 5% polyacrylamide gel (no The 14S subunit was isolated from cytoplasmic of [3H]leucine-labeled EMC-infected](https://thumb-us.123doks.com/thumbv2/123dok_us/1572809.109882/6.505.57.252.62.362/precursorphwassds-subunitsecondsdsextractsdescribedacrylamide-tionswasethanol-electrophoretic-polyacrylamide-isolated-cytoplasmic-infected.webp)
![TABLE 4. Distribution of ['H]leucine in the 14S precursor and its 6S subunit](https://thumb-us.123doks.com/thumbv2/123dok_us/1572809.109882/11.505.52.448.504.604/table-distribution-h-leucine-s-precursor-s-subunit.webp)