0022-538X/85/020596-06$02.00/0
Copyright C) 1985, American Society for Microbiology
Scrapie PrP 27-30 Is
a
Sialoglycoprotein
DAVID C. BOLTON, RUDOLF K. MEYER, ANDSTANLEY B. PRUSINER*
Departments of Neurology and of Biochemistry and Biophysics, University of California, San Francisco, California 94143
Received25 July1984/Accepted 11 October1984
The major scrapie prion protein, designatedPrP27-30, exhibitedboth charge and size heterogeneity after purificationfrom infected hamsterbrains.Eightor morediscretechargeisomers of PrP 27-30 withisoelectric points ranging from approximately pH 4.6 to 7.9 were found by using non-equilibrium pH gradient
electrophoresisinthe first dimensionfollowed by sodium dodecylsulfate-polyacrylamide gel electrophoresisin
the second dimension. Thecharge isomersweredetected by silverstainingaswellasby radioiodination. The
procedures used todisaggregate PrP 27-30 before electrophoresis in the first dimension do notappeartobe
responsible for thecharge heterogeneity. However, heatingPrP27-30to100°C for 15 minin0.1NNaOHor
0.1 N HCI resulted in modification of theprotein and alteration of itselectrophoretic pattern. A PrP27-30 fragment (molecular weight, 17,100 to21,900) obtained bycyanogenbromidecleavage also exhibited charge
and size heterogeneity. Periodic acid-Schiff staining of PrP 27-30 electrophoresed into sodium dodecyl sulfate-polyacrylamide gels demonstrated that carbohydrate residuesareattached to theprotein. Digestion of
PrP 27-30 with neuraminidase and endo-,l-N-acetylglucosaminidase Hresulted in significant changes in the isoelectric pH ofPrP27-30 isomers, whereas digestion with alkaline phosphatase had no effect. Our results
demonstrate that PrP 27-30 isasialoglycoprotein;this is consistent with severalpropertiesof thisproteinand of thescrapie prion.
The scrapie agent appears to differ from viruses in its
apparent small size and resistance to procedures that
inac-tivate nucleic acids(32, 36). Some investigators continueto
classifythescrapieagentas anunconventional virus (42, 43)
despite a lack of definitive data demonstrating that it
con-tains a genomic nucleic acid and thus fulfills commonly
accepted criteria for a virus. The only macromolecule that
has been identified as unique to tissues infected with the
scrapie agent is a protein that purifies with the infectious
particle (4, 37). Several lines ofinvestigation have shown
thisproteintobeastructural component of thescrapieagent
which is required forexpression of biological activity (1, 4,
13, 31, 37, 38, 40; D. C. Bolton, M. P. McKinley, and S. B.
Prusiner, Biochemistry, inpress). Because thescrapieagent
has notbeen demonstrated to be avirus, butits infectivity
dependsat least inpart on aprotein,theterm "prion"was
suggested to denote this class of infectious particles (36).
From this terminology, the protein that is a component of
the scrapie prion has been designated PrP 27-30, forprion
proteinwithanMrof27,000 to30,000 (31,38; Boltonetal.,
in press). Although this nomenclature is subject to change basedon newinformation,we useit in this reportforclarity
andconvenience.
The size heterogeneity of PrP 27-30 and its resistance to
proteasedigestiongreatly facilitated theidentificationof the
protein in fractions enriched for scrapie prions (4, 31, 37;
Boltonetal., in press). Webegan investigatingthe molecular basis for the size heterogeneity of PrP 27-30 by using a
NEPHGE-SDS-PAGE two-dimensional electrophoresis
technique developed by O'Farrell et al. (34). Our initial
studies indicated that PrP 27-30 was heterogenous with respect to both size and charge. One explanation for this result was that the apparent charge isomers actually repre-sented individual unique proteins. Alternatively, the charge heterogeneity we observed could have been caused by the
radioiodination methods required fordetecting low
concen-trations of PrP 27-30 present in partially purified fractions.
* Correspondingauthor.
Improvementsinthemethodfor purifyingand
concentrat-ing scrapie prions led to further characterization of PrP
27-30. Studies with peptide mappingin onedimension pro-vided evidence that PrP 27-30 behaved as a single protein
species (Boltonetal., in press). Further datasupportingthis
hypothesis were obtained by N-terminal amino acid
se-quenceanalysisof PrP27-30purifiedto nearhomogeneity by
high-pressure liquid chromatography (38). The sequence
data demonstrated that PrP 27-30possessed a single amino
terminal sequence,althoughitremainedheterogeneouswith respect tosize. Minor signals obtained duringthe sequence
analysis indicatedsomevariationwithin the Nterminus due
to "ragged ends" (38). In addition, amino acid sequence
studies ofzonal sucrose gradient fractions containing high
titers of scrapie prions and only one major protein, PrP
27-30,exhibitedthesamesingle amino-terminalsequenceas
thatobtainedwith PrP 27-30purified by high-pressure liquid
chromatography. Thus, PrP 27-30 obtained from zonal
su-crose gradient fractions containing infectious prions was
homogeneous with respect to N-terminal amino acid
se-quence, indicating a single protein or a family of related
proteins in the rangeofMr27,000 to 30,000 (38).
Inthis report, dataarepresenteddemonstratingthecharge
heterogeneity of PrP 27-30. Furthermore, we show the
presence of a PAS-staining carbohydrate attached to PrP 27-30 and show that digestion of the denaturedproteinwith neuraminidase or endoglycosidase H affects the charge characteristics ofPrP27-30. Our results indicate that previ-ous attempts todemonstrate PAS-staining carbohydrate on
PrP 27-30probablyfailed dueto aninsufficientamountofthe
protein available for staining. The presence of sialic
acid-containing oligosaccharides on PrP 27-30 helps to explain
severalobservationsabout thebehavior oftheproteinandof the scrapie prion.
MATERIALS ANDMETHODS
Abbreviations. CIAP, calf intestine alkaline phosphatase;
endoglycosidase H, endo-4-N-acetylglucosaminidase H;
HPAP, human placental alkaline phosphatase; lodobeads,
596
on November 10, 2019 by guest
http://jvi.asm.org/
N-chloro-benzenesulfonamide-derivatized
polystyrene
beads; NEPHGE, non-equilibrium pH gradient
electropho-resis; PAGE,
polyacrylamide gel
electrophoresis;
PAS,
pe-riodic acid-Schiff;PrP27-30,
scrapie prion protein
(molecu-larweight, 27,000 to30,000); SDS, sodium dodecyl sulfate.
Materials. Allchemicals were ofthe
highest grades
com-mercially available. Neuraminidase (Clostridium
perfrin-gens) and ovomucoid
protein
werepurchased
fromSigma
Chemical Co.
(St.
Louis, Mo.). Endoglycosidase
H(45)
wasobtained from Health
Research,
Inc.(Albany, N.Y.).
Acrylamide and N,N'methylene bis-acrylamide were
pur-chased from Bio-Rad Laboratories
(Richmond,
Calif.).
Na1251
was purchased from AmershamCorp.
(Arlington
Heights, Ill.).
Source of scrapie prions and purification. A
hamster-adapted isolate ofthe scrapie
prion
was agift
fromRichardMarsh (29). It was
passaged
andprepared
as describedby
Prusiner et al. (37, 39). Theprionswere
purified
asdescribedby Prusiner et al. (37), with modifications
(40).
Beforecleavage with CNBr, PrP 27-30 and a related
protein
(mo-lecular weight, 23,000 to 26,000) were further
purified by
molecular sieve chromatography on tandem 60- and 30-cm
TSK-2000 SW columns (38). PrP 27-30 and the related
protein were
separated
from each otherby
electrophoresis
througha 15%
polyacrylamide gel.
Theproteins
wereelutedfrom appropriate gel
fragments
identifiedby
using
theradioiodinated
proteins
as markers and concentratedby
precipitation (Bolton et
al.,
inpress).
Radioiodination of PrP 27-30. Four
procedures
wereusedtochemically label PrP 27-30 with
125I.
Ingeneral,
proteins
wereconcentrated 10-fold before iodinationby
precipitation
with SDS and quinine hemisulfate orby sedimentationfrom
sucrosegradient fractions diluted with distilledwater
(R.
K.Durbin, personal
communication;
Boltonetal.,
inpress).
Samples in 0.1 Msodiumborate-0.1% SDS
(pH 8.5)
wereiodinated with
methyl-3,5-di-[1251]iodo-p-hydroxybenzimi-date
hydrochloride
by
combining 50-,ul samples
of thepro-teinsuspension with
100-pul samples
ofthe reagentinmeth-anol (47). The reaction mixture was incubated at room
temperature for 24 h with occasional
mixing.
Theradio-labeledproteins wereremoved fromthereaction mixture
by
precipitation with SDS and
quinine
hemisulfate(Durbin,
personal
communication).
Proteins in0.05 Msodium
phosphate-0.1%
SDS(pH
7.5)
were iodinated directly with 1.0 mCi of
Na1251
by thechloramine T procedure of Hunter and Greenwood
(22),
withminor modifications.
Proteins in 0.1 M sodiumborate
(pH 8.5)
withorwithout0.1% SDS were radioiodinated with
N-succinimidyl
3-(4-hydroxy-5-[1251]iodophenyl)
propionate by theprocedure
ofBoltonandHunter, with minor modifications
(3, 4;
Boltonetal., in press).
Sucrose
gradient
fractions withoutprior
concentrationwere radioiodinated with 1 to 2 mCi of
Na125I
withlodo-beads
(Pierce
ChemicalCo.,
Rockford, Ill.).
After theaddi-tionof sodium
phosphate
buffer(pH 6.5)
to a finalconcen-tration of 30 mM, the
proteins
were iodinatedby
theprocedure of Markwell (28). The labeled
proteins
wereremoved from the reaction mixture
by
sedimentation in amicrocentrifuge (Boltonet al., inpress).
NEPHGE-SDS-PAGE two-dimensional gel electrophoresis.
Samples (10or20,ul)in 9.5Murea,5%
3-mercaptoethanol,
2% Nonidet P-40, and 2% Ampholytes were subjected to
NEPHGEin the firstdimensionasdescribed byO'Farrellet
al. (34).Theproteinswere separatedat400 Vfor4 to4.5 h.
The extruded first-dimension
gels
were incubated in 62.5mM
Tris-HCI,
2%SDS,
5%P-mercaptoethanol,
and0.002%bromphenol
blue(pH 6.8)
at room temperature for30to60min before
quick
freezing
in adry
ice-ethanol bath andstorage at
-70°C.
The frozen first-dimensiongels
were thawedby
brief incubation at37°C
andelectrophoresed
immediately
in thesecond dimensionin15%acrylamide gels
asdescribed
previously (34).
Analyses
by
SDS-PAGEinonedimension were
performed
as describedby
Laemmli(26).
Gels were stained with silver
by
the method ofMorrissey
(33).
The
pH
gradient
in the NEPHGEgels
was estimatedby
measuring
thepH
of the elutedAmpholytes
witha Radiom-eterpH
meter.Briefly,
extruded first-dimensiongels
elec-trophoresed
withsample
bufferonly
were cut into 1-cmsegments. The segments were
placed
into aglass
tubecontaining
1 ml of double-distilled water; the tubes werepurged
withnitrogen, sealed,
andincubatedatroom temper-aturefor several hours beforemeasuring
thepH.
PAS
staining.
Samples
ofsucrosegradient-purified prions
containing
approximately
250pug
ofprotein
or 100 ,ug ofovomucoid
protein
wereelectrophoresed
on a 12.5%SDS-polyacrylamide gel
as describedpreviously
(26).
Thegel
was stained with the PAS reagentby
the method ofGlossmanand
Neville,
withminor modifications(9, 12).
ThePAS-stained
gels
werephotographed
and thencounter-stained with Coomassie brilliant blue R-250 and
photo-graphed
again.
Alkaline
phosphatase
treatmentofPrP27-30.Purifiedprion
rods were radioiodinated
by
using
Iodobeads
as describedabove. The
pellet
wassuspended
indigestion
buffer(0.1
MNaCl,
0.015 MTris-HCl,
1 mMdithiothreitol, pH 7.5)
andheated to
100°C
for 15 min todisaggregate
the rods. Thesuspension
was cooledto30°C
anddistributedintoworking
samples.
Thesamples
were incubatedfor 30 minat30°C
inthe
digestion
bufferalone orindigestion
bufferwith 25 UofCIAP
(Boehringer
MannheimBiochemicals,
Indianapolis,
Ind.)
per ml or 25 U ofHPAP(Sigma)
per ml. Inaddition,
samples
wereincubatedwith 25 UofCIAP
orHPAP per mlin the presence of5 mM
p-nitrophenyl
phosphate
(Sigma).
The
digestions
were terminatedby
heating
thesamples
to100°C
for 5min,
and thesamples
wereanalyzed by
NE-PHGE-SDS-PAGE after 10-fold dilution with NEPHGE
sample
buffer.CNBr
cleavage
of PrP 27-30.SDS-PAGE-purified
PrP27-30 was
precipitated
withquinine
hemisulfate(Durbin,
personal
communication)
andsuspended
in70%formicacidat a
protein
concentration ofapproximately
0.2mg/ml.
One-tenth volume of CNBr
(50
mg/ml)
was added to theprotein
suspension
in aglass
vialcontaining
aTeflon-coatedmagnetic stirring
barand sealed withaTeflonseptum(Pierce
Chemical
Co., Rockford,
Ill.).
The vial waspurged
withnitrogen,
and the reaction wasperformed
overnight
in thedark with continuous
stirring (5, 6, 11).
The reaction wasterminated
by
diluting
thesuspension
10-fold with distilled water andquick
freezing
in adry
ice-ethanol bath. Thecleaved
protein
was stored at-70°C,
lyophilized,
andana-lyzed by
SDS-PAGE andNEPHGE-SDS-PAGE.RESULTS
Detection of
charge
isomersby
radioiodination and silverstaining.
In our initial studiesdesigned
toinvestigate
thecharge
of PrP27-30,
sucrosegradient
fractionsprepared
from
scrapie-infected
hamsterbrains werechemically
mod-ified withmethyl-3,4-di-[125I]-iodo-p-hydroxybenzimidate
hydrochloride.
This reagent was used because it has beenreported
to iodinateproteins
whilepreserving
their nativeon November 10, 2019 by guest
http://jvi.asm.org/
NEPHGE so
, ...
A
Lu
0
(A
't
I B
FIG. 1. NEPHGE-SDS-PAGE of scrapie-infected and normal brainsucrosegradient fractions. Samples of scrapie-infected(A)or
normalbrain(B)sucrosegradient fractionswereradioiodinatedwith methyl-3,5-di-['25I]-iodo-p-hydroxybenzimidate hydrochloride at 25°C for 24 h. The fractionswereconcentratedby precipitationwith
SDS and quinine hemisulfate (Durbin, personal communication), suspended in NEPHGE sample buffer, and electrophoresed for
3,000 Vh in the first dimension. The SDS-PAGE in the second dimension was performed in 15% polyacrylamide gels. The gels
werefixed, stained, and driedasdescribedpreviously (4; Boltonet al.,inpress). Theautoradiographwasexposedatroomtemperature
for 40 days. The arrow indicates the most alkaline of the major charge isomers.
charge (47). A series of charge isomers migrating in the size range of PrP 27-30 was found in fractions from scrapie-in-fected brain, but not from normal brain (Fig. 1). Other proteinspresent inthese samples did notappearasa series
ofcharge isomers, although somebroadening oftheprotein
spots was apparent. The low labeling efficiency of this reagent made it impractical to pursue detailed analysis of PrP27-30by NEPHGE-SDS-PAGE.
Improved purification methods provided fractionshaving
aspecific infectivity of between
109-4
and 1010.3 50% infectivedose units per mg ofprotein and containing primarily one
protein, PrP 27-30. Theapparentcharge isomers ofPrP 27-30
werereadily evident in these fractions containing the
exten-sively purified protein (Fig. 2).Silverstainingthe
NEPHGE-SDS-PAGE gels of these fractions conclusively
demon-strated that thepresenceofmultiple charged specieswasnot an artifact due to radiolabeling (Fig. 2). At least eight different charge isomers within the size rangeof PrP 27-30
wereevident in the silver-stainedgel. These charge isomers
haveisoelectricpointsranging from approximately pH4.6to 7.9.
Theresistance ofPrP27-30todigestion byproteaseswas
used to determine whether these charge species were iso-mers of PrP 27-30. Sucrose gradient fractions were
radio-iodinated with
Na125I
by the chloramineTmethod. Thehighefficiency oflabeling permitted detection of proteins suchas
PrP 27-30, which were present in low concentration in partially purifiedsucrosegradient fractions (4, 37; Boltonet
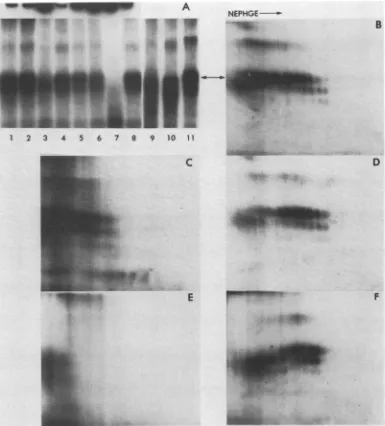
al., in press). Charge isomers of the same size as PrP 27-30 were detected as afaint smear ofradioiodinated proteins in scrapie fractions before protease digestion (Fig. 3A). After digestion with proteinase K, the sample contained signifi-cantly less detectable protein; prolonged exposure of the autoradiograph clearly revealed the presence of a series of spots similar to those described above (Fig. 3B).
Additional samples were radiolabeled with
N-succinim-idyl
3-(4-hydroxy-5-[1251]iodophenyl)
propionate or withNa125I
by using Iodobeads. Analyses of these samplesdemonstrated that the migration of the major charged spe-cies of PrP 27-30 was not influenced by these chemical labeling procedures (Fig. 3C and D). Some minor charge modification of PrP 27-30 may have occurred after these procedures asevidenced by a noticeable broadening of the individualprotein spots. The increasedsensitivity for detect-ing PrP 27-30 obtained with radioiodination prompted us to
use this methoddespite the possibility of introducing minor artifacts.
Disaggregation procedures.During the course of our inves-tigation into the charge heterogeneity in PrP 27-30, we
became concerned thatprocedures used to disaggregate the protein, which exists in rod-shaped or fibrillar structures in sucrose gradient fractions, might generate multiple charge isomers. We have found thatboiling proteins used as molec-ularweight standards in SDS-PAGE sample buffer produced minorchargeand sizevariants detectablebythe NEPHGE-SDS-PAGE two-dimensional technique. To explore this question, we sought additional methods for disaggregating PrP27-30 from its aggregated fibrillar state. Although con-siderable datasuggested that the scrapie prionbehaves like
a hydrophobic particle, several recent observations show
NEPHGE
66. 2 l l
LO
45.0-;
'21.5-}
[image:3.612.84.276.74.348.2]
144-pH 4.1 4.65.3 5.96.67.2 7.98.4 8.9 9.39.59.4 FIG. 2. Unmodified PrP 27-30 shows charge heterogeneity. A
200-plsample ofazonalsucrosegradient fractioncontaining highly
purified scrapie prions was diluted with 500
pul
of0.1 M sodium phosphate (pH6.5)andsedimented for60min inamicrocentrifuge at4°C.Theresulting pelletwas suspendedin 10ptlof0.1 NHCIat25°C byrepeated pipettingwitha micropipettefor 2to3 min. The samplewasthen neutralized with 10pulof0.1 NNaOH,and 20 mg ofurea wasadded followedby20p.1of NEPHGEsamplebuffer.The proteinswereseparated inthe NEPHGE first dimensionfor 1,800 Vh. The second-dimension SDS-PAGE gel contained 15% acryl-amide. Thegelwas stained with silverbythemethod ofMorrissey (33).
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.332.546.439.615.2]
NEPHGE-J
NEPHGE
-c
Au"cn
ar
/
B D
[image:4.612.136.476.77.335.2];m .,.
.w
FIG. 3. Protease digestion andradioiodination do not alter charge heterogeneity. A sample ofascrapie sucrosegradient fractionwas radioiodinated with Na'25I by using chloramine T, and the proteins were removedfrom the remaining free iodine by precipitation with methanol(Boltonetal., in press). The resulting pelletwassuspended in 10mMTris-hydrochloride-0.2%Sarkosyl (pH 7.4) and incubatedat 25°C for 30 min inbuffer alone (A)or 100p.gof proteinase Kper ml (B). After this treatment, theproteins were again precipitated with methanol andseparated by NEPHGE-SDS-PAGE. (A)Scrapiesucrosegradientfraction incubated inTris-hydrochloridealone. Thearrow indicatesfaintlyvisible charge isomers. The autoradiographwasexposed for0.5h.(B)Scrapiesucrosegradient fraction incubated in 100 ,ug ofproteinaseKperml. Theautoradiographwasexposed for5.0 h.(C)Asample ofascrapie zonalsucrosegradient fractioncontaining highly
purified prionswas concentrated by sedimentation to apellet in amicrocentrifuge and radiolabeled with N-succinimidyl
3-(4-hydroxy-5-[1251]iodophenyl)propionate.Theradioiodinatedproteinsweremoved fromtheunreactedreagentby sedimentationto apelletasbefore.The
pelletwassuspendedin 0.1 NHCl,neutralized, and prepared for electrophoresisasdescribedinthelegendtoFig. 2. Theautoradiographwas exposed for15 h at25°C. (D)Asample ofascrapie zonalsucrosegradient fraction containinghighlypurified prionswasradioiodinated with
Na1251 by usinglodobeads.Theradiolabeledproteinswere removedfrom unreacted iodineby sedimentationto apellet inamicrocentrifuge
for60minat4°C. The pelletwassuspended in0.1 NHCI, neutralized, and prepared for electrophoresis asdescribed inthelegendtoFig. 2. Theautoradiograph wasexposed for 2 hat25°C.
that the aggregation ofPrP 27-30 may also be significantly
altered by procedures that modify ionic interactions. Thus,
we explored thedisaggregation ofPrP 27-30 at extremepH
values.
Disaggregation of rodscontainingPrP27-30wasmeasured
by determining the migrationofthe protein into 15%
SDS-PAGE gels after treatment with NaOH or HCI (Fig. 4A).
Suspending the rods in 0.1 N NaOH or 0.1 N NaOH
containing 1% SDS was clearly effective at disaggregating
PrP 27-30, as was 0.01 N NaOH containing 1% SDS.
Disaggregationwasless when 0.01 N NaOH,0.1 NHCI,or
0.1 N HCI containing 1% SDS was used. Suspending the
rods in 0.01 N HCI resulted in little measurable
disaggrega-tion, but inclusion of 1% SDS in the suspension aided
monomerization ofPrP 27-30.SDS-PAGE sample bufferwas
effectivein disaggregatingtheprion rods at 100°C(Fig. 4A,
lane 9) but had little effect at 25°C (data not shown). Treatment with 25 mM NaCl was equally ineffective (Fig. 4A, lane 10).
We also measured theeffect ofthese disaggregation pro-cedures ontheconformation ofPrP 27-30byexamining the protease resistance of the protein after treatment. In the native state, PrP 27-30 is resistanttodigestion byavarietyof proteases,butbecomes sensitivetodigestionupon denatur-ation (4, 31; Boltonetal., in press). Suspending PrP 27-30 in
0.1 N NaOHor0.1 N NaOHcontaining 1% SDS by repeated
pipettingatroomtemperature denatured theprotein,
result-ing in digestion with proteinase K (Fig. 4B). Similar
treat-ment with 0.01 N NaOH apparently did not denature the
protein,asshownby itsresistancetodigestion. Theaddition
of 1% SDS tothe0.01 N NaOHpromoted denaturation and
digestion.
SuspendingPrP 27-30 in 0.1 N HCI containing 1% SDS,
butnot in0.1 N HCl alone, denatured theprotein, allowing
significant digestion by proteinase K (Fig. 4B). PrP 27-30
also was not denatured after exposure to 0.01 N HCl, but exposure to 0.01 N HCI containing 1% SDS promoted
denaturation anddigestion by proteinase K.
Two-dimensional NEPHGE-SDS-PAGE analysesof
frac-tionsdisaggregated by threeoftheseprocedures areshown
inFig.5.HeatingPrP 27-30to100°C for5mininSDS-PAGE
sample bufferorsuspendingPrP 27-30atroomtemperature
in 0.01 NNaOH with 1% SDSorin 0.1 NHClcontaining1%
SDS produced essentially identical results. Thus, these
procedures resulted in disaggregation of prion rods and at
least partial denaturation of PrP 27-30 and promoted pene-tration of the protein into thefirst-dimension NEPHGE gel
(Fig. SB and C).
Modification byacid and alkali. Our studies ontheeffects of acid and alkalion PrP27-30wereextended by
incubating
i.
#M.-.
...'amoLAIL f
TOP117
on November 10, 2019 by guest
http://jvi.asm.org/
0
1 2 3 4 5 6 7 8 9 10 B_ _m " " ... _
.:i
protein,but lowerconcentrations ofHCl were only
margin-allyeffective. Aslight shift toward more rapid migration was
apparentaftertreatment with 0.1 N HCI. Incubationat60°C
for 30 min in water alone followed by the addition of 2x
concentrated SDS-PAGE buffer to a final concentration of
2% SDS and 5% ,-mercaptoethanol did not result in signif-icantdisaggregation asmeasured by this technique.
Heating PrP 27-30 to 100°C in 0.1 N NaOH resulted in
severemodification of theprotein, evidenced by smearing of
theprotein bandin theSDS-PAGE gel (Fig. 6A).
Modifica-tionofPrP 27-30also resulted fromboiling in0.1 N HCI, but
this alteration was qualitatively different; no change was
seenin the width ofthe protein band,but its migrationwas
significantly increased (Fig. 6A). PrP 27-30 resuspended in
water alone and heated to 100°C for 15 min exhibited no
alteration of its migration in SDS-PAGE gels (Fig. 6A) or
NEPHGE-SDS-PAGE gels (Fig. 6B).
NEPHGE--_ 1!
A
0a
FIG. 4. Disaggr gationanddenaturation ofPrP27-30in acidand alkali. Asample ofascrapie sucrose gradient fraction was radio-iodinated withNaI251by using lodobeads.Theradiolabeled material was distributed into 10 samples, and each was sedimented to a pellet. The pellets were suspended in acid or alkali by repeated pipetting and then neutralized and analyzed for disaggregation of prion rodsordenaturation ofPrP27-30.(A)Disaggregation of prion rodswasmeasuredasafunction ofthemigration ofPrP 27-30into a 15% SDS-PAGE gel after addition ofan equal volume of 2x SDS-PAGE sample bufferat25°C. The autoradiographwasexposed for3hat 25°C. (B)Denaturation ofPrP 27-30was measuredas a function of thesusceptibility of the proteintodigestion by protein-ase K.Tris-hydrochloride (pH 7.4)wasaddedto afinal concentra-tion of10mM,andproteinaseK wasaddedtoaconcentration of100 ,ug/ml. The samples were incubated at 25C for 30 min. The digestionwasterminated by adding phenylmethylsulfonyl fluorideto 1mM, anequal volume of2xSDS-PAGE sample bufferwasadded, andimmediately the sampleswere boiledfor 5min. The autoradi-ograph was exposed at 25°C for 16.3 h. The arrows show the position of PrP 27-30. The acid and alkali treatments were as
follows.Lanes: 1, 0.1 NNaOH; 2,0.1 NNaOH-1%SDS;3, 0.01 N NaOH; 4,0.01NNaOH-1% SDS;5, 0.1 N HCI;6, 0.1 N HCl-1% SDS;7, 0.01 NHCI; 8,0.01 NHCl-1%SDS; 9, SDS-PAGE sample buffer, 100°C for5min; 10,25 mM NaCl.
the protein in HCI orNaOH at highertemperatures. Disag-gregation of PrP 27-30was measuredby increased
penetra-tion of the protein in polyacrylamide gels as described
above. Radioiodinated PrP 27-30wasconcentrated by
sedi-mentation inamicrocentrifuge. The pellets weresuspended
in NaOH or HCl solutions by repeated pipetting and
incu-batedat 60°C for30 min or 100°C for 15 min (Fig. 6A). At
60°C, either 0.1 or 0.01 N NaOH was as effective at disaggregatingPrP27-30asboiling thesample in SDS-PAGE
samplebuffer. Heatingwith0.001 N NaOHwasineffective.
Somemodification of PrP 27-30wasapparentaftertreatment
with0.1 NNaOHas seenby theincreased smearing ofPrP
27-30 in thegel duringelectrophoresis. Heating PrP 27-30to 60°C in 0.1 N HCl was effective in disaggregating the
FIG. 5. Disaggregation procedures do notaffectcharge
hetero-geneity. Samples ofascrapie sucrosegradientfractionwere
radio-iodinatedwithNal'25 by using lodobeads,and theproteinsin these
sampleswereremovedfrom the unreacted iodinebysedimentation
toapellet. The pellets were suspendedin (A) SDS-PAGE sample
buffer followedby heatingto100°Cfor5min,(B)0.01 N NaOH-1%
SDSat25°Cfor1to2min,or(C)0.1 N HCl-1% SDSat25°Cfor 1
to2min. SamplesBandCwereneutralizedwith theconjugateacid and base, and then 20-,ul samples of each were prepared for
NEPHGE-SDS-PAGE by adding an equal volume of NEPHGE
sample bufferand 20mgofurea.Theautoradiographswereexposed
for 17 hat25°C.
B
C
',Rwv
-l",w w "W9
Z.-fIrlt
Po 00
17' .:f:Azf f'f" :.::.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.105.249.79.360.2] [image:5.612.339.532.259.619.2]A
* ws
....,
NEPHGE---
-"qh'#_I
1 2
_
_
5 6 - m m01 2 3 4 5 6 7 8 9 10 l1
:,
AN'.k :r. w
C
A
B
D
'41
F
FIG. 6. Modification of PrP 27-30 by heating in acid and alkali. (A) Proteins in samples of ascrapie sucrose gradient fraction were
radiolabeledwithNa1251 by using lodobeadsandconcentratedby sedimentationtoapellet.Thepelletsweresuspendedinacidoralkali and heatedto60°C for 30min(lanes 1 through 7)orto 100°C for15 min (lanes9through 11). Disaggregationormodification of PrP 27-30was
assessed from itsmigration in a15%SDS-PAGEgel. Inlanes 1 through 8, the wellsofthestackinggel areshowntoindicate radiolabeled material thatfailedto enterthegel.Thedoublearrowindicates thepositionof PrP27-30. Lanes: 1,0.1 N NaOH; 2,0.01NNaOH; 3,0.001 NNaOH; 4,0.1 N HCl; 5,0.01 NHCl; 6,0.001 N HCI; 7,wateronly; 8,heatedto100°Cfor 5 mininSDS-PAGEsample buffer; 9,0.1 N
NaOH;10, 0.1 NHCI;11,wateronly. (B through F) NEPHGE-SDS-PAGE separationofproteinstreated under selected conditionsasin A.
(B) Proteins heatedto100°Cfor15mininwater.Theautoradiograph was.exposedfor34 h. (C)Proteinsheated to60°Cfor30minin0.1 N
NaOH. Theautoradiographwasexposed for51 h.(D) Proteinsheatedto60°Cfor 30minin 0.1 N HCI.Theautoradiographwasexposedfor 39 h.(E) Proteins heatedto100°C for15minin 0.1 NNaOH. Theautoradiographwasexposedfor37 h.(F)Proteinsheatedto100°Cfor 15
minin0.1 N HCl. Theautoradiographwasexposed for 34 h.
Modificationof PrP 27-30during heatingto60°Cfor 30min
in 0.1 N NaOH was confirmed by NEPHGE-SDS-PAGE
electrophoresis (Fig. 6C). In the firstdimension, thecharge isomers were shifted to a more acidic pH, and increased
smearing was observed in the first and second dimensions.
BoilingPrP27-30 in 0.1 N NaOH causedseveremodification of theprotein. Electrophoresisin theNEPHGE-SDS-PAGE systemconfirmed the smeared, indistinct pattern observed withSDS-PAGE. In thetwo-dimensional system, PrP 27-30
appeared to be shifted to more acidic forms (Fig. 6E), but poorresolution made interpretation of this observation
un-certain.
Incubation in 0.1 N HCI at 60 or 100°C had a different
effect. The number of charge isomers was reduced, but
distinct charged species were still observed,
giving
theimpression that the acidic isomers ofPrP 27-30 were
con-vertedtospecieswith amorealkaline isoelectricpoint (Fig.
6D andF).Theapparentdecreasein themolecular
weight
of PrP 27-30 thatwas observed in SDS-PAGE afterheating
to60°C in HCI (Fig. 6A) was accompanied by a shift toward
morealkaline isoelectricpoints intheNEPHGE-SDS-PAGE
system. Thesechangeswere seen moreclearlyafter
incuba-tionat 100°C; fourmajorcharged species havingisoelectric
pointsbetween pH 6.9and 7.9are shownin
Fig.
6F. TheseP"W
mm
,11, i.u
W.iI :"Vr.: 11,W
w
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.114.499.72.498.2]NEPHGE
--A cn
-v
0 m
B |
21.5-
31-
21.5-
31-
21.5-C
D
31-
21.5-I l
3.9 4.3 5.0 5.6 6.2 6.8 7.5 81 8.6 9.1 9.3 9.3
FIG. 7. Digestion withglycosidases alters charge characteristics ofPrP27-30. Proteins from zonalrotor sucrosegradient fractions wereradioiodinated by the chloramine T procedure and then boiled in0.1 Msodium acetate (pH 5.5)for15min. Theproteinswerethen incubatedat 37°C for 12 h at a protein concentration of approxi-mately 0.7 mg/ml in the presence of neuraminidase (62 U/ml), endoglycosidaseH(3U/ml),orboth enzymes. Thedigestionswere terminated by heating to 100°C for 5 min, and the samples were prepared for NEPHGE-SDS-PAGE. (A)Digestion buffer alone. (B) Neuraminidase. (C) Endoglycosidase H. (D) Neuriminidase and endoglycosidase H.Theautoradiographswereexposed for16h.
be similar to those produced by digestion with
neuramin-idase,except that an additional species wasfound having an
isoelectric point of approximately 7.9. Digestion with both enzymes resulted in the apparent conversion ofthe eight
normalchargeisomersto four majorspecies, similarto what
was seen afterdigestion with neuraminidase alone (Fig. 7D). However, three of these species appeared to coincide with the three mostalkaline isomers produced by treatment with
neuraminidase (Fig. 7B), whereas thefourth speciesaligned
more closely with the most alkaline isomer produced by
digestion withendoglycosidase H(Fig. 7C). Wenoted that,
although significant alterations in the chargecharacteristics of PrP 27-30 were produced by digestion with these en-zymes, no significant change in the molecular size was detectable by SDS-PAGE (Fig. 7).
Detection by PASstaining.Electrophoresis ofPrP 27-30by SDS-PAGE and subsequent PAS staining clearly
demon-strated that the protein contained carbohydrate residues
(Fig.8A). PrP 27-30 andovomucoidexhibited the pink color
characteristic ofglycoproteins stained by the PAS method.
The proteins used as molecular weight standards did not
stain with PAS (Fig. 8A), except for slight staining of
ovalbumin, but could be easily seen when this gel was
counterstained with Coomassie brilliant blue(Fig. 8B). We
found that PrP 27-30 and ovomucoid protein retained the
characteristic pink colorofthe PAS reagentandapparently
did notbindCoomassieblue when counterstained withthat
dye.
An attempt was made to measure the effect ofdigestion with neuraminidase and endoglycosidase H on the PAS
staining of PrP 27-30. Samples of zonal sucrose gradient
fractions containing approximately 250 ,ug of protein and
109
650% infective dose unitswere concentratedbyprecip-itationwithtrichloroacetic acid and deoxycholate. The
pel-lets wereresuspended in25 ,u of0.1Msodium acetate(pH
5.5) and boiled for 15 min. A5-,ul sample ofan analogous
fractionradioiodinated with125I at aprotein concentration of
1 mg/ml was added to each ofthe samples, and they were
heated to 100°C for 15 min. The proteins at afinal
concen-tration ofapproximately 6.4 mg/ml were incubated for12h
at 37°C in thepresence of neuraminidase (54 U/ml),
endo-glycosidase H (2.5 U/ml), or bothenzymes. The digestions
isomers coincidewith the most alkaline forms ofPrP 27-30
seenpreviously (Fig.2and6B).Thesedata areinagreement
with thegeneral observationthat the alkaline species ofPrP
27-30 migrated at smaller apparent molecularweights than
didtheacidic species (Fig. 1, 2, 3, 5, and6B).
Digestion with glycosidases alters charge characteristics.
Digestion ofPrP27-30by neuraminidaseorendoglycosidase
Hresulted in asignificant alteration ofthecharge
character-isticsasseenbyNEPHGE-SDS-PAGE.PrP 27-30incubated
in thepresence ofbuffer aloneproduced the characteristic
two-dimensionalpattern ofmultiplecharge isomers. At least
eight discrete charge isomers were observed, ranging in
isoelectricpHfromapproximately pH 4.6 to 7.5 (Fig. 7A).
Incubating the protein in the presence of neuraminidase
caused an apparent shift in the isoelectric points of the
isomers and reducedthenumberof isomersseenfromeight
tofourmajorspecies having isoelectricpoints between pH 6.5and 7.5 (Fig.7B). The effectproduced bydigestion with
endoglycosidase H wasqualitatively similar to that caused
by neuraminidase, except that six major isomers were
ob-served(Fig. 7C). These species exhibited isoelectric points
ranging from approximately pH 6.5 to 7.9. The isomers
produced bydigestion withendoglycosidase H appeared to
A 1 2 3 B 1 2 3
4-'
.I
it
_
*
m
-00-~~~..
FIG. 8. PAS staining of PrP 27-30. Samples of zonal rotor
sucrosegradient-purified scrapie prionswere electrophoresed into anSDS-polyacrylamide gel. (A) Stained with PAS. (B) Same gel
counterstained with Coomassie blue. Lanes: 1, molecular weight proteinstandards; 2, zonal rotorsucrose gradient scrapie fraction
containingprimarilyPrP27-30; 3, ovomucoidprotein.
31- ::;,i..ANL. A..
0.
-tt:.f:: ..g
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.612.81.277.71.387.2] [image:7.612.337.532.514.667.2]A1
S_
2 3 4
._I'M,..
NEPHGE
-B
U'
C
?, .s
FIG. 9. CNBrcleavage ofPrP27-30 andarelatedprotein. Purified fractionswerepreparedandcleaved with CNBrasdescribed in thetext.
(A) Lanes: 1, gel-purified PrP27-30; 2, CNBr cleavage fragments ofPrP27-30; 3, PrP-related protein, Mrof24,000to 27,000; 4, CNBr cleavagefragments of PrP-relatedprotein. The separationwasperformedon a20% SDS-PAGEgel. The autoradiographwasexposed for45.5 h at25°C. (B)NEPHGE-SDS-PAGEseparation ofPrP 27-30CNBrcleavagefragments.(C) NEPHGE-SDS-PAGEseparationof PrP-related protein CNBr cleavage fragments. Theautoradiographsin B andC wereexposedat 25°C for13days.
were stopped by heating the samples to 100°C for 30 min after adding an equal volume ofSDS-PAGE sample buffer
containing 2% SDS and 5%,-mercaptoethanol. Analysis of
these samplesbySDS-PAGE revealed nodifferencesin the
PAS staining characteristics or theelectrophoretic mobility
of PrP 27-30 during SDS-PAGE (datanot shown).
Charge heterogeneity was not altered by digestion with
alkaline phosphatase. Radioiodinated PrP 27-30 was heated
in digestion buffer to 100°C for 15 min to disaggregate the
prion rods anddenature the protein. DigestionwithCIAPor
HPAP(25 U/ml) for30 min did notalter the charge
hetero-geneity of PrP 27-30 (data not shown). Each phosphatase
was shown to be active in the presence of PrP 27-30 by
cleavage of phophate fromp-nitrophenyl phosphate in
reac-tion mixtures to whichthis substrate wasadded.
CNBr cleavage. Cleavage of PrP 27-30 with CNBr pro-ducedonemajor fragment (Mr, 17,100to21,900),whichalso
was heterogeneous with respect to charge (Fig. 9). The
chargeisomers of thisfragment migratedcoincidentally with
the isomers from the largeroftwo fragments generated by
CNBr cleavage ofa related protein that migrates ahead of
PrP 27-30 in SDS-PAGE systems (Bolton et al., in press). Other studieshave suggested thatthis smallerprotein (PrP
23-26) represents a proteinase K digestion product ofPrP
27-30produced during purification oftheprion (Boltonetal., in press). It is of interest that the differences inisoelectric
pHfound between each of the charge isomersoftheCNBr
fragments were greater than those demonstrated by the
chargeisomersof PrP23-26,PrP27-30, oracid-modifiedPrP
27-30.
DISCUSSION
The datapresented in this report clearly demonstrate that
oligosaccharides areattachedtoPrP27-30 andthatterminal
sialic acids are responsible forat least some ofthe charge
heterogeneityexhibited bythisprotein.However, it may be
significant that extensive digestion ofthis protein by
neur-aminidase alone, or inconjunctionwithendoglycosidase H,
failed to convert all of the charge isomers into a single
species. Although this study does not provide direct
evi-dence forphosphorylation as a source ofcharge
heteroge-neity, some of our observations raise the possibility that
phosphorylation
oftyrosine residues may contribute tothechargeheterogeneity ofPrP27-30. Itis
possible
that severaldifferent modifications may be responsible for the charge
isomerization observed(14-16, 23).
Size microheterogeneity exhibited during SDS-PAGE is
characteristic ofmany
glycoproteins
(10, 18, 19). Thisprop-erty was an important factor for the identification ofPrP
27-30 because it distinguished this protein from other pro-teins ofsimilar apparent Mr which were found in fractions
prepared from both scrapie-infected and normal brain (4,
37). At the time of its discovery, the characteristic size
heterogeneity of PrP 27-30prompted speculationthat itwas
aglycoprotein, but attemptstodemonstratethe presence of
oligosaccharides
by PAS staining were unsuccessful untilrecently.
Sialoglycoproteins often show charge heterogeneity due
to
variation
in the numberofneuraminic acid residues added(2, 41). In this report,we haveestablishedthatthepresence
ofsialic acidonPrP 27-30is responsible,atleastin part, for the charge heterogeneity of this protein. Digestion with neuraminidase converted acidic forms of PrP 27-30 to four
majorspecies having isoelectricpH values between pH6.5
and 7.5. Digesting PrP 27-30 with neuraminidase and
endo-glycosidaseHalsoproduced four major charge isomers, but
thesehadisoelectricpointsbetweenpH 6.8 and 7.9. We also found that heating PrP 27-30 in 0.1 N HCl appeared to
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.612.134.474.73.332.2]convert the acidic species of the protein to four major isomers having isoelectric points between pH 6.8 and 7.9. Contrary to the effect produced by digestion with glyco-sidases, the charge shift produced by heating in acid was also accompanied by a significant decrease in the apparent molecular weight of the protein as measured by SDS-PAGE. The remarkable similarity between the charge isomers
ob-tained by these different procedures suggests a common
chemical basis for their generation. This hypothesis is
sup-ported by reports that terminal sialic acid residues are
hydrolyzed by heating in mild acid (10). It is possible that heating in acid may remove the bulk of the oligosaccharide chains in addition to neuraminic acid. However, it remains to be determined whether the acid treatment alters the charge characteristics of PrP 27-30 by removing sialic acid residues or whether a fundamentally different type of
mod-ification produces a qualitatively similar result (14-16, 23).
Thepresence of oligosaccharides on PrP 27-30 may
influ-ence estimates of its molecular weight. PrP 27-30
consist-entlymigrateswith anapparent molecularweight of
approx-imately 27,000 to 30,000 in SDS-PAGE systems (4, 31, 37,
38, 40; Bolton et al., in press). The protein has been
analyzed ongels of uniform total acrylamide concentration
ranging from13 to 20%andlineargradient gels of5to20%
total acrylamide. When the protein was separated by
high-pressure liquid size exclusion chromatography, however, it eluted at aposition suggestingan Mrof19,500 to 21,000 (38).
Subsequent analysis of the chromatographed PrP 27-30
showedthatit continuedtomigrate with an Mrof27,000 to
30,000 when analyzed by SDS-PAGE (38). Glycoproteins
have been reported to migrate anomalously in SDS-PAGE
systems, oftenresulting inartificially highestimates of their
Mr (27, 35). However, in most cases the deviation of the
observed Mr from the actual value was significant only in
gels with acrylamide concentrations below 10%. The cause
of thediscrepancybetween theseestimatesof Mrremainsto
be established (25, 38).
Equally intriguing is our observation that digestion with
neuraminidase andendoglycosidase H,aloneorin
combina-tion,resultedincharge modification ofPrP27-30, but didnot
alter itsapparent Mr as measuredbySDS-PAGE.Thismight
indicate that an insignificant mass was removed from the
protein during digestion by these enzymes or that the
residues removed have little effect on either the Stokes
radius or the amount of SDS bound to the protein (27).
Assuming that the apparent shift observed indicates the
removalof a minimumoffoursialic acid residues from PrP 27-30, the decrease in mass should be approximately 1,200
daltons. In the absence of other counteracting effects, it
seems likely that this decrease would be observable by
SDS-PAGE. Leach et al. foundthat oligosaccharides retard
the migration of glycoproteins during
electrophoresis
inSDS-PAGE systemsprimarily by decreasingtheamountof
SDS bound to theproteinand thusdecreasingthe netcharge
of the SDS-protein complex (27). However, the overall
effectoftheoligosaccharides onthe migrationof
glycopro-teins in SDS-PAGE gels was not a direct function of the amount of carbohydrate attached. This is probably due to
theunpredictable effect oftheoligosaccharide portiononthe
Stokes radius ofthe SDS-protein complex(27, 35). Inactivation of thescrapie agent by periodate (20, 21, 32, 43) has been reported. This property lent support to specu-lation that the agent might be a replicatingpolysaccharide,
among otherpossibilities(8). Thedemonstration of
oligosac-charides attached to PrP 27-30 provides a logical basis for
interpreting those earlier results. Further studies will be
requiredto determine whether the oligosaccharides on PrP
27-30 areessential forexpressionofbiologicalactivity. Itis clear fromstudies of thescrapie prionand PrP 27-30 thatneither theprionnor theproteinishighly immunogenic
(7, 21, 24, 30). However, with large amounts of PrP 27-30
isolated from infected hamster brain it recently has been
possible to produce antisera to the protein in rabbits (1).
Since the synthesis and processing ofthe oligosaccharides
found on PrP 27-30 areundoubtedly performed by the host
cell, the structure of the oligosaccharide chains probably
resembles those found on many other host cell
glycoprot-eins. If these
oligosaccharide
chains cover a substantialportionof the surface of theprotein,theirsimilaritytoother
host structures may effectively protect the protein from
recognition bytheimn-iinesystem.
Our resultsindicatethatneuraminicacids areresponsible forsomeofthe observedchargeheterogeneity ofPrP27-30,
but they do not exclude the possibility that other charge
modifications may also contribute. Serine and threonine
phosphates are reported to be removed from proteins by
heating in alkali under the conditions we used but are
relatively stable to degradation by heating in acid (17).
Removal of serine or threonine phophates
by heating
inalkali wouldbeexpectedtoproducedashift toward alkaline
pH. Thisappears to be indirectcontrast to ourobservations
withPrP27-30, where heatingin analkali resulted inashift
toward forms
having
an acidicisoelectricpoint.
Thisobser-vationwassomewhat obscuredbythe apparentmodification
and degradation of the protein which occurred
during
thetreatment.Covalent modification ofproteinsheatedin strong alkali is well documented (46), but the relevance of the
modifications to the
charge
heterogeneity
of PrP 27-30remains uncertain.
Heating PrP 27-30 in the presence of acid reduced the
charge variation concomitant with a reductionin molecular
size. It has been
reported
thattyrosine phosphate
bondsarecleaved under these conditions
(17).
Whetherboiling
in 0.1N HCl affects the net
charge
of PrP 27-30by
removing
tyrosine
phosphates orby cleaving
terminal sialic acidresidues is uncertain. DigestingPrP27-30withtwodifferent
alkaline
phosphatases
hadno effect oncharge
distribution.These enzymes have been reported to be more active in
cleaving
tyrosine
phosphates than serine and threoninephosphates (44). However, radioiodination at
tyrosine
resi-dues of PrP 27-30 may have inhibited
cleavage
oftyrosine
phosphatebondsby CIAPand HPAP.
Amino acid analyses of homogeneous PrP 27-30 indicate
the presenceof18methionine residues withinthe
molecule,
assuming
that itcontains240amino acid residues(38).
Aftercleavage
ofPrP 27-30 withCNBr,
we detected onemajor
fragmentand two or threeminor
fragments.
Failuretodetectany PrP 27-30after
cleavage
indicatesthatatleastoneof themethionine sites intheproteinis cleaved
uniformly.
Itisnotclear whether the
discrepancy
between the numberofme-thionineresidues and the numberofCNBr
fragments
is dueto close
spacing
of methionine residuesresulting
infrag-mentsof small sizeor to a
complete
lackofcleavage
atsomeof the methionine residues. Our data indicate thatat least
someofthe groups
responsible
forcharge
heterogeneity
in PrP 27-30 are stable to incubation in formic acidduring
cleavage with CNBr and reside on the
major peptide
frag-ment
generated by
this chemicalcleavage procedure.
Thesecharged
groups alsoarefoundonPrP23-26,
themajor
CNBrfragment ofthis
protein,
andasmallerCNBrfragment
thatisnot seen after CNBrcleavage of PrP 27-30. These similari-ties between the CNBr
fragments
of PrP 23-26 and PrP 27-30on November 10, 2019 by guest
http://jvi.asm.org/
support our
hypothesis
that these proteinsarerelated (Bol-ton etal.,
inpress).
Other studies have shown that theseproteins
possesscross-reacting antigenic
determinants (1)and
closely
relatedpeptide
maps (D. C. Bolton and S. B.Prusiner, unpublished experiments).
An
understanding
ofthecauseofthechargeheterogeneity
of PrP 27-30should
provide
abasis formorerapidorefficientmethodsfor
purifying
PrP27-30.Determining
thecomposi-tion of the
oligosaccharides
ofthis unusualprotein
shouldfacilitate structural studies.
Charge heterogeneity
in themajor scrapie prion protein
may have mechanistic andtheoretical
implications
aswell. Post-translationalmodifica-tion could be a
required
step in theconversion of inert PrP27-30 to a
biologically
activeprotein
or may playa role inprotecting the protein from degradation during the long
incubation
period
ofthe disease.Glycosylation
as well asother
possible
covalent modifications may beimportant
inregulating
theexpression
ofPrP 27-30 orothermacromole-cules that
play
arole inthe disease process.ACKNOWLEDCMENTS
Wegratefully acknowledge T. May, M. Braunfeld, E. M. Hen-nessey, E. F. Espanol., D. F. Groth, S.P. Cochran, and K. A. Bowmanfor excellent technical assistanceaswellasF. T.Elvin and L. F. Gallagher for editorial and administrative help. We thank M. P.McKinley,P. E.Bendheim,C. G.Bellinger,R. A.Barry, and I. Leiberbergforhelpfuldiscussions.
Thisresearch was supportedbyPublic Health Service research grantsAG02132 andNS14069 from the National Institutes of Health
as well as by giftsfrom R.J. Reynolds Industries, Inc., Sherman Fairchild Foundation, and W. M. Keck Foundation. D.C.B. was supported byPublic HealthServicepostdoctoral fellowshipNS06849 from the NationalInstitutes ofHealth,andR.K.M. wassupported by postdoctoral fellowship 83.201.0.84 from the Swiss National Foundation.
LITERATURE CITED
1. Bendheim,P.E.,R. A.Barry,S.J. DeArmond,D. P.Stites,and S. B. Prusiner. 1984. Antibodies to a scrapie prion protein.
Nature(London)310:418-421.
2. Boersma, D. P., F. Saleh,K. Nakamura,and R. W. Compans. 1982. Structure and glycosylation of Tacaribe viral
glycopro-teins.Virology 123:452-456.
3. Bolton,A.E.,and W. M.Hunter.1973. Thelabellingofproteins
tohigh specificradioactivitiesby conjugation to a 1251-contain-ing acylat1251-contain-ingagent.Applicationtotheradioassay.Biochem. J. 133:529-539.
4. Bolton, D. C., M. P. McKinley, and S. B. Prusiner. 1982. Identification ofaprotein tht purifies with the scrapie prion.
Science218:1309-1311.
5. Cahnmann, H.J., R. Arnon, andM. Sela. 1966. Isolation and characterization of active fragments obtained by cleavage of
immunoglobulin G with cyanogen bromide. J. Biol. Chem. 241:3247-3255.
6. Dietzschold,B.,T.J. Wiktor,R.MacFarlan,and A.Varrichio. 1982. Antigenicstructureof rabiesvirusglycoprotein: ordering andimmunologicalcharacterization of thelarge CNBr cleavage
fragments.J. Virol. 44:595-602.
7. Fraser, H. 1976. The pathology of natural and experimental scrapie, p. 267-305. In R. H. Kimberlin (ed.), Slow virus diseases of animalsandman. Elsevier/North-Holland, Amster-dam.
8. Gibbons,R. A., andG. D. Hunter. 1967. Nature of the scrapie agent. Nature(London)215:1041-1043.
9. Glossman, H., and D. M. Neville. 1971. Glycoproteins ofcell surfaces. J.Biol. Chem. 246:6339-6346.
10. Gottschalk,A.1972.Glycoproteins:theircomposition,structure
andfunction. B. B.A. Libraryvol. 5,parts A and B. Elsevier
Publishing Co., Amsterdam.
11. Gross, E., and B. Witkop. 1962. Nonenzymatic cleavage of
peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J. Biol. Chem.237:1856-1860.
12. Gullick,W.J.,S.Tzartos, and J. Lindstrom. 1981.Monoclonal antibodies as probes of acetylcholine receptor structure. I. Peptide mapping. Biochemistry 20:2173-2180.
13. Hilmert, H., and H.Diringer.1984. Arapidand efficient method toenrichSAF-protein from scrapie brains of hamsters. Biosci. Rep. 4:165-170.
14. Hsu, C.-H., and D. W.Kingsbury. 1982. Contribution of oligo-saccharide sulfation to the charge heterogeneity of a viral glycoprotein. J. Biol. Chem. 257:9035-9038.
15. Hsu,C.-H.,and D. W.Kingsbury.1982. NSphosphoprotein of vesicular stomatitis virus: subspecies separated by electropho-resis andisoelectric focusing.J. Virol.42:342-345.
16. Hsu, C.-H., and D. W. Kingsbury. 1982.Topographyof phos-phate residues in Sendai virus proteins. Virology 120:225-234. 17. Huang, K.-P., J. C. Robinson, and J. Y. Chou. 1976.
Phosphopro-tein-phosphatase activity associated with human placental alka-line phosphatase. Biochem. Biophys. Res. Commun. 70: 186-192.
18. Hughes, E. N., A. Colombatti, A., and J.T. August. 1983. Murine cell surface glycoproteins. Purification of the roly-morphic Pgp-1 antigen and analysis of its expressionon macro-phages and other myeloid cells.J. Biol. Chem. 258:1014-1021. 19. Hughes, R. C. 1976. Membrane glycoproteins: a review of
structureandfunction. Butterworth & Co. Ltd., London. 20. Hunter, G. D. 1979.Theenigma ofthescrapieagent:
biochem-icalapproachesand theinvolvement of membranes and nucleic acids, p. 365-385. In S.B. Prusiner and W.J. Hadlow (ed.), Slow transmissible diseases ofthe nervous system. Academic Press, Inc.,New York.
21. Hunter, G. D., R. A. Gibbons, R. H. Kimberlin, and G. C. Millson. 1969.Further studies of the infectivity and stability of
extractsandhomogenates derived from scrapie affectedmouse
brains. J. Comp. Patho. 79:101-108.
22. Hunter, W. M., and F. C. Greenwood. 1962. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature(London) 194:495-496.
23. Katoh, I., Y. Yoshinaka, and R. B. Luftig.1984. Murine leukae-mia virusp30heterogeneityasrevealedby two-dimensional gel electrophoresis isnot anartifact of thetechnique.J.Gen. Virol. 65:733-741.
24. Kimberlin, R. H. 1976. Scrapie in the mouse: a model slow disease.MeadowfieldPress, Shildon, UnitedKingdom. 25. Kopaciewicz, W.,andF. E.Regnier.1982. Nonideal
size-exclu-sion chromatography of proteins: effects of pH at low ionic strength. Anal. Biochem.126:8-16.
26. Laemmli,U. K.1970.Cleavageof structuralproteins duringthe assembly of the head of bacteriophage T4. Nature (London) 227:680-685.
27. Leach,B.S., J.F.Collawn, Jr.,and W. W. Fish.1980.Behavior ofglycopolypeptides with empirical molecular weight estima-tion methods. I. In sodium dodecyl sulfate. Biochemistry 19:5734-5741.
28. Markwell,M. A. K. 1982. A newsolid-statereagent toiodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal.Biochem. 125:427-432.
29. Marsh,R.F., and R. H. Kimberlin. 1975.Comparison ofscrapie andtransmissible minkencephalopathy inhamsters.II. Clinical
signs, pathologyandpathogenesis. J. Infect. Dis. 131:104-110. 30. McFarlin, D. E., M. C. Raff, E. Simpson, andS. H. Nehlsen. 1971. Scrapie in immunologically deficient mice. Nature
(Lon-don)233:336.
31. McKinley, M. P., D. C. Bolton, and S. B. Prusiner. 1983. A protease-resistant protein is a structural component of the scrapie prion.Cell 35:57-62.
32. Millson, G.C., G. D.Hunter, and R. H.Kimberlin. 1976. The physico-chemical nature ofthe scrapie agent, p. 243-266. In R. H.Kimberlin(ed.), Slow virus diseases ofanimals and man. Elsevier/North-Holland, Amsterdam.
33. Morrissey, J. H. 1981. Silver stain for proteins in polyacryl-amidegels:amodifiedprocedurewith enhanceduniform sensi-tivity.Anal. Biochem. 117:307-310.
on November 10, 2019 by guest
http://jvi.asm.org/
34. O'Farrell, P. Z., H. M. Goodman, andP. H. O'Farrell. 1977. High resolution two-dimensional electrophoresis of basic as
wellasacidicproteins. Cell 12:1133-1142.
35. Poduslo, J. F. 1981. Glycoprotein molecular-weight estimation using sodium dodecyl sulfate-pore gradient electrophoresis: comparison of tris-glycine and tris-borate-EDTA buffer
sys-tems.Anal. Biochem. 114:131-139.
36. Prusiner, S. B. 1982. Novel proteinaceous infectious particles
causescrapie. Science 216:136-144.
37. Prusiner,S.B.,D. C.Bolton,D.F. Groth, K. A. Bowman, S. P.
Cochran, and M. P. McKinley. 1982. Further purificationand characterization of scrapieprions. Biochemistry 21:6942-6950.
38. Prusiner,S.B.,D. F.Groth,D. C.Bolton,S. B.Kent,andL.E. Hood. 1984. Purification and structural studies of a major
scrapie prionprotein. Cell 38:127-134.
39. Prusiner, S. B., D. F. Groth, S.P. Cochran, F.R. Masiarz,
M. P. McKinley, and H. M. Martinez. 1980. Molecular
proper-ties, partialpurification andassay by incubation period
meas-urement of the hamster scrapie agent. Biochemistry 19: 4883-4891.
40. Prusiner, S. B.,M.P.McKinley, K. A. Bowman, D. C. Bolton,
P.E. Bendheim, D. F.Groth, and G.G. Glenner. 1983.Scrapie
prions aggregate to form amyloid-like birefringent rods. Cell 35:349-358.
41. Raghow, R., A. Portner, C. H. Hsu, S. B. Clark, and D.W.
Kingsbury. 1978. Chargeheterogeneity of polypeptides of
neg-ative strand RNA viruses. Virology90:214-225.
42. Rohwer, R. G.1984. Virus-likesensitivityof thescrapieagent to heatinactivation. Science 223:600-602.
43. Rohwer, R. G.1984.Scrapie infectiousagentisvirus-likein size andsusceptibilitytoinactivation.Nature(London) 308:658-662. 44. Swarup, G., S. Cohen, and D. L. Garbers. 1981. Selective dephosphorylation ofproteins containing phosphotyrosine by alkalinephosphatases.J. Biol. Chem.256:8197-8201.
45. Tarentino, A. L., and F. Maley.1974.Purification andproperties ofanendo-,-N-acetylglucosaminidasefromStreptomyces
gris-eus.J. Biol. Chem. 249:811-817.
46. Whitaker, J. R.1980. Changes occurringinproteinsinalkaline solution, p. 145-163. In J. R. Whitaker and M. Jujimaki (ed.),
Chemical deterioration of proteins. American Chemical
Soci-ety,Washington, D.C.
47. Wood, F. T., M. M. Wu,and J. C. Gerhart.1975.The radioac-tivelabeling ofproteinswithaniodinatedamidinationreagent.
Anal. Biochem. 69:339-349.
![FIG.1.brainfordimension25°Cal.,chargemethyl-3,5-di-['25I]-iodo-p-hydroxybenzimidatenormalwere3,000SDSsuspended NEPHGE-SDS-PAGE of scrapie-infected and normal sucrose gradient fractions](https://thumb-us.123doks.com/thumbv2/123dok_us/1412245.94103/3.612.332.546.439.615/brainfordimension-chargemethyl-hydroxybenzimidatenormalwere-sdssuspended-scrapie-infected-gradient-fractions.webp)
![FIG. 3.radioiodinatedexposedpelletpurifiedindicates25°Cmethanolofmethanolfor2.Na1251[1251]iodophenyl) The proteinase Protease digestion and radioiodination do not alter charge heterogeneity](https://thumb-us.123doks.com/thumbv2/123dok_us/1412245.94103/4.612.136.476.77.335/radioiodinatedexposedpelletpurifiedindicates-cmethanolofmethanolfor-iodophenyl-proteinase-protease-digestion-radioiodination-heterogeneity.webp)