JOURNAL OFVIROLOGY, Mar. 1995, p. 1907–1912 Vol. 69, No. 3 0022-538X/95/$04.0010
Copyrightq1995, American Society for Microbiology
Virions Released from Cells Transfected with a Molecular
Clone of Human T-Cell Leukemia Virus Type I Give Rise to
Primary and Secondary Infections of T Cells
DAVID DERSE,
1* JUDY MIKOVITS,
2MARIA POLIANOVA,
1BARBARA K. FELBER,
3AND
FRANCIS RUSCETTI
1Laboratory of Leukocyte Biology, Biological Response Modifiers Program,
1Biological Carcinogenesis and Development
Program, PRI/DynCorp,
2and Human Retrovirus Pathogenesis Group, ABL-Basic Research Program,
3National
Cancer Institute-Frederick Cancer Research and Development Center, Frederick, Maryland 21702-1201
Received 16 August 1994/Accepted 19 October 1994
The ability of molecular clones of human T-cell leukemia virus type I (HTLV-I) to direct the synthesis of
infectious virions has not previously been demonstrated. An HTLV-I provirus originating from an adult T-cell
leukemia patient was cloned into a plasmid vector and is designated pCS-HTLV. This molecular clone was
shown to direct the synthesis of viral mRNAs and proteins in transiently transfected cells; in addition, virus
structural proteins were released into the culture medium. Viral proteins were assembled into virions that
sedimented at a buoyant density characteristic of retrovirus particles and whose morphology was verified by
electron microscopy. Virions concentrated from transiently transfected cell supernatants were incubated with
primary cord blood lymphocytes or with transformed T-cell lines to establish that these particles were
infectious. Expression of spliced, viral mRNAs in the T-cell cultures after both primary and secondary
infections with cell-free virus revealed that pCS-HTLV encodes an infectious provirus.
Human T-cell leukemia virus type I (HTLV-I) is the
etio-logic agent of adult T-cell leukemia and is also associated with
progressive, degenerative neurological disorders referred to as
tropical spastic paraparesis or HTLV-I-associated myelopathy
(10, 13, 26, 27, 39). HTLV-I characteristically infects CD4
1T
cells in vivo and in vitro (3, 11, 12, 27–29). However,
HTLV-I-infected B cells and CD8
1T cells have been obtained after
culture of peripheral blood mononuclear cells (PBMCs) from
infected individuals or after coculture of cord blood
lympho-cytes or PBMCs with HTLV-I-producing cells (4, 14, 20, 21, 25,
31, 38). In vitro infections of other cell types, including
fibro-blasts (2), endothelial cells (15, 17), and neural (32),
monocy-toid (14), and microglial (16) cells, have been reported.
Cell-free infection with HTLV-I is inefficient compared with that of
other retroviruses, and it has been calculated that one in one
million virus particles is infectious (5). Therefore, most studies
of HTLV-I infection and T-cell transformation employ
cocul-tivation techniques. It is still unclear whether the virion is
unusually labile, is poorly released from the infected cell, or
inefficiently binds and penetrates the host cell. Expression of
HTLV-I is highly restricted both in vivo and in vitro and is
regulated at transcriptional and posttranscriptional levels by
inducible, cellular transcription factors and by the products of
the viral tax and rex genes (7, 8, 18, 19, 34–36, 40).
In addition to obstacles imposed by the biological properties
of the virus, molecular genetic studies of HTLV-I infectivity
and replication have progressed slowly because of the lack of
an infectious molecular clone. Several molecular clones of
HTLV-I have been constructed and examined in different
lab-oratories, and some of these have been shown to transiently
express mRNAs and proteins in transfected cells (1, 23, 24);
however, their ability to direct the production of infectious
virus particles has not yet been demonstrated. Although
ge-netic defects in the cloned proviruses may abrogate their
in-fectivity, it is more likely that the lack of demonstrable
infec-tivity results from difficulties attributable to (i) poor infecinfec-tivity
intrinsic to HTLV-I virions; (ii) highly restricted expression of
the virus in infected cells, which limits both detection and
spread of the virus through the cell culture; and (iii) potential
cytopathic effects of the virus in productively infected cells.
These problems are compounded in the analyses of cloned
proviruses since a virus stock generated from transfected cells
must be produced in sufficient quantities for subsequent
infec-tion experiments. Here, we show that cells transfected with the
provirus clone pCS-HTLV released HTLV-I virions that were
able to infect primary or transformed T cells in vitro.
Further-more, virus produced by these infected T cells was capable of
secondary infections of primary, cultured T cells. pCS-HTLV
should therefore prove valuable in future examinations of the
biological and biochemical interactions of HTLV-I with the
host cell.
pCS-HTLV was constructed by transferring the HTLV-I
provirus from a bacteriophage lambda clone containing one of
the three proviruses resident in CS-1 cell DNA to a plasmid
vector. The transformed T-cell line CS-1 was originally
ob-tained by cocultivation of cord blood lymphocytes with
HTLV-I-infected cells from an American adult T-cell leukemia
pa-tient (20, 21). In the process of constructing pCS-HTLV, all
flanking cellular DNA was removed; in addition, 30 bp from
the 5
9
end and 70 bp from the 3
9
end of the provirus were
deleted (these sequences would be regenerated after infection
and replication). pCS-HTLV was previously shown by
North-ern (RNA) blotting, reverse transcription PCR (RT-PCR), and
cDNA cloning experiments to direct the synthesis of major and
minor viral mRNAs in transiently transfected cells (1). In
ad-dition, functional activities of viral Tax and Rex proteins
en-coded by pCS-HTLV have been demonstrated (reference 6
and unpublished information). Enzyme-linked immunosorbent
assays revealed that p24
gagprotein was released into the
growth medium shortly after transfection of both HeLa and
* Corresponding author. Mailing address: Laboratory of Leukocyte Biology, National Cancer Institute, NCI-FCRDC, Frederick, MD 21702-1201. Phone: (301) 846-1504. Fax: (301) 846-6107.
1907
on November 9, 2019 by guest
http://jvi.asm.org/
FIG. 1. Electron micrograph showing mature HTLV-I particles released from transiently transfected HLtat cells and persistently infected MT-2 cells. HLtat cells (HeLa cells stably transfected with human immunodeficiency virus type 1 Tat) in four 60-mm-diameter plates were transfected with 10mg of pCS-HTLV DNA. Two days later, cells were collected by scraping, pooled, and pelleted by centrifugation. At the same time, virus in the cell culture supernatant was concentrated by ultracentrifugation. The cell pellet was washed twice with phosphate-buffered saline and suspended in 5 ml of 1.25% glutaraldehyde. (A and B) HTLV-I particles associated with transfected HLtat cells; (C) virus particles pelleted from the culture supernatant; (D) HTLV-I particles produced from the HTLV-I-infected cell line MT-2. Magnification,381,000.
1908 NOTES J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
FIG. 2. Sucrose density gradient centrifugation of virus released into the supernatant growth medium from 293 cells transfected with pCS-HTLV. (A) Density of fractions; (B) immunoblot of sucrose density gradient fractions. Two days after transfection of 293 cells in 10-cm-diameter dishes with 10mg of pCS-HTLV, 10 ml of growth medium was collected from one plate and clarified by low-speed centrifugation. Virus was pelleted by centrifugation in a 50Ti rotor at 35,000 rpm for 2 h. The virus pellet was suspended in 0.5 ml of 50 mM Tris-HCl–100 mM KCl–1 mM dithiothreitol, layered on a 15 to 60% sucrose gradient, and centrifuged at 21,000 rpm for 16 h in an SW41 rotor. Fractions of approximately 100ml were collected, and the refractive index was determined for each fraction. Fifty microliters of each fraction was resolved by electrophoresis on a 12% polyacrylamide–SDS gel. After electrophoresis, proteins were electrophoretically transferred to an Immobilon-P (Millipore) polyvinylidene difluoride membrane and incubated with HTLV-I-infected human serum (Scripps) and then with horseradish peroxidase-conjugated goat anti-human immunoglobulin antibody. Specific HTLV-I bands were visualized by chemiluminescent staining (ECL; Amersham).
FIG. 3. Map of pCS-HTLV showing genetic organization, transcription pattern, and locations of PCR primers. Sequences of the PCR primers were as follows: HT460 (sense), 59TGTGGTGCCTCCTGAACTGC39; HTex1-2 (sense, covers the splice junction of exon 1-exon 2 [ex1-2], positions 482 to 493 and 5015 to 5024), 59TCCGCCGTCTAG/CTTCCTGGTC39; HT5050 (sense), 59ACAGGAGGCTCTCCAAGAAG39; HT5275 (antisense), 59CTGGGGCTGTAATCACCGAAGAT GAG39; HT7356 (antisense), 59AAAGAAGACTCTGTCCAAACCC39; and HT7875 (antisense), 59CATTGGTGAGGAAGGCCCCGAGC39. LTR, long terminal repeat.
1909
on November 9, 2019 by guest
http://jvi.asm.org/
293 cells with pCS-HTLV; levels of p24 peaked at 2 days
posttransfection and decreased with time and cell passage to
low but detectable levels (data not shown). To determine
whether viral proteins released into the growth medium were
assembled into virions, transfected cells and supernatants were
examined by electron microscopy (Fig. 1). HLtat cells (a HeLa
cell line expressing human immunodeficiency virus type 1 Tat)
were transfected with pCS-HTLV, and 2 days later medium
was collected and virus was concentrated by
ultracentrifuga-tion; at the same time, cells were collected by scraping, washed
with phosphate-buffered saline, and fixed in 1.25%
glutaralde-hyde. Mature HTLV-I particles were observed both associated
with transfected cells (Fig. 1A and B) and in the supernatant
(panel C). The morphology of these particles was identical to
that of virus produced from the HTLV-I-infected T-cell line
MT2 (panel D). In subsequent experiments human 293 cells
were used, since they have a high transfection efficiency and
transiently expressed pCS-HTLV at high levels. Supernatant
from 293 cells transfected with pCS-HTLV was concentrated
by ultracentrifugation, applied to 15 to 60% sucrose density
gradients, and centrifuged in an SW41 rotor. Sucrose gradient
fractions were then analyzed by electrophoresis on 12%
poly-acrylamide–sodium dodecyl sulfate (SDS) gels, transferred to
polyvinylidene difluoride membranes, and developed with
anti-HTLV-I antisera (Fig. 2). Bands corresponding to anti-HTLV-I
p24
gagand p19
gagproteins and gp46 envelope surface
glyco-protein were observed with peak intensities centered around
fraction 10. Thus, HTLV-I proteins in the supernatant
[image:4.612.57.553.65.263.2]sedi-mented at a buoyant density of approximately 1.15 gm/ml,
characteristic of retrovirus particles. The immunoblot shown in
Fig. 2 reveals that the profiles of the gag and env protein bands
are not superimposed, reflecting either a loss of envelope
dur-ing the course of centrifugation or heterogeneity in the virus
population related to the extent of virion maturation.
To determine whether the virus particles released into the
medium were infectious, 293 cells in 15-cm-diameter plates
were transfected with 20
m
g of pCS-HTLV DNA. Medium was
changed 2 days after transfection, and supernatants were
col-lected 1 day later. For each infection, virus was concentrated
from one 15-cm-diameter plate of transfected 293 cells.
Super-natants were underlaid with 3 ml of a 10% glycerol, and virus
was pelleted by centrifugation at 35,000 rpm in a 50Ti rotor for
2 h. Virus pellets were suspended in 0.2 ml of serum-free
RPMI 1640 medium containing DEAE-dextran (10
m
g/ml) and
DNase (80 U/ml; Boehringer) for 2 h at 37
8
C. For each cell
type infected, a matching virus suspension was incubated at
65
8
C as a negative control. MOLT4 cells, CEM cells, and
phytohemagglutinin–interleukin-2-activated cord blood
lym-phocytes were maintained as previously described (30). Prior
to infection, 10
7T cells were suspended in 1 ml of RPMI 1640
containing 10
m
g of DEAE-dextran per ml and mixed with 0.2
ml of virus suspension. Cells and virus were incubated for 2 h
at 37
8
C, and then 10 ml of complete medium was added.
Infected T cells were collected at various times after infection,
and total cellular RNA was extracted, converted to cDNA by
reverse transcriptase with random hexamer primers, and then
FIG. 4. RT-PCR analysis of RNA expressed in MOLT4 cells, CEM cells, and cord blood lymphocytes (CB) after infection with concentrated supernatants from transiently transfected 293 cells. Approximately 53106
T cells were collected 2 days after infection for preparation of total cellular RNA using RNA STAT-60 (TEL TEST Inc.). One microgram of total cellular RNA was incubated in 20ml of cDNA synthesis reaction mixture with random hexamer primers as previously described (22). Reactions were terminated by addition of 80ml of H2O and heating at 958C for 10 min. RNA was also prepared from transiently transfected 293 cells and converted to cDNA; this cDNA was diluted 1:103
or 1:102
, as indicated, prior to PCR amplification. Lanes i, infections with concentrated virus; lanes m, infections with heat-inactivated virus. The 195-bp tax/rex and the 280-bp env PCR products are indicated. (A) Nested (two-step) PCRs followed by Southern blotting detect Tax/Rex mRNA. Two sequential amplification reactions were used to detect the doubly spliced Tax/Rex mRNA; the first reaction mixture contained primers HT460 and HT7875, and the second contained primers HT5050 and HT7356. The first reaction mixture contained 5ml (5%) of the cDNA reaction mixture, 200 ng of each primer, 200mM (each) deoxynucleoside triphosphate (dNTP), 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, and 2 U of Taq DNA polymerase (Boehringer-Mannheim) in a volume of 50ml. Amplification was achieved with 35 cycles of 948C for 30 s, 608C for 30 s, and 728C for 60 s. A 1-ml aliquot from the first PCR mixture was added to the second reaction and amplified under the same conditions. Twenty-five microliters from the second PCR was run on a 10% polyacrylamide gel, transferred to a nylon membrane, and hybridized with a32P-labelled DNA fragment containing exon 2. (B) Single-step PCRs with cDNA and PCR primers HT5050 and HT7356 were carried out in the presence of [32P]dCTP essentially as described by Saksela et al. (33). Products representing Tax/Rex mRNA were separated on 10% polyacrylamide gels. Reaction mixtures contained, in 50ml, 5ml of diluted cDNA reaction mixture, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 100 mM (each) dNTP, 100 ng of each primer, 0.25ml of [32P]dCTP (3,000 Ci/mmol, 10 mCi/ml; NEN), and 1 U of Taq DNA polymerase (Boehringer). The amplification program consisted of 1 cycle of 948C for 40 s, 608C for 1 min, and 728C for 30 s followed by 32 cycles of 948C for 20 s, 608C for 30 s, and 728C for 20 s. Aliquots (25 ml) from each reaction mixture were run on nondenaturing 10% polyacrylamide gels, dried, and exposed to X-ray film. Molecular size markers (lane pKS) are from
HpaII-digested Bluescript pKS1plasmid DNA (Stratagene) 39end labelled with [32P]dCTP. (C) Single-step PCR mixtures contained cDNA and primers HTex1-2 and HT5275, which amplify cDNAs representing the singly spliced Env mRNA. Reactions were carried out and visualized as described for panel B.
1910 NOTES J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
analyzed by PCR amplification. PCR primers that would
spe-cifically amplify spliced viral mRNAs were chosen (Fig. 3);
neither proviral DNA or viral genomic RNA would be
ampli-fied under these conditions. Initially, a nested PCR
amplifica-tion procedure followed by Southern blot analysis of reacamplifica-tion
products was used (Fig. 4A). This two-step process, using
prim-ers HT460 and HT7875 for the first reaction and primprim-ers
HT5050 and HT7356 for the second, specifically amplified the
doubly spliced Tax/Rex mRNA. Bands corresponding to the
Tax/Rex mRNA, as defined by their size and hybridization to
a Tax/Rex probe, were observed after infections of MOLT4
cells, CEM cells, and cord blood lymphocytes. The cDNAs
were also examined by single-step PCR amplification in the
presence of [
32P]dCTP with either HT5050 and HT7356
prim-ers to detect Tax/Rex mRNA or HTex1-2 and HT5275 primprim-ers
to detect Env mRNA (Fig. 4B and C). Again, bands of the
predicted size were observed in cells infected with virus from
pCS-HTLV-transfected 293 cells; no bands were ever observed
in cells infected with the heat-inactivated virus preparations.
The env-specific primers amplified additional cDNAs in CEM
cells, but the virus-specific band was seen only in virus-infected
cells (Fig. 4C). Band intensity was maximal at 2 days after
infection and began to decrease at day 4 (data not shown).
Thus, de novo viral mRNA synthesis in the infected cells
sug-gested that the virus had successfully completed the early steps
of infection, including penetration of the cell, RT, integration,
and transcription.
In order to determine whether the infected T cells released
infectious virus, MOLT4 cells were infected with cell-free virus
from 293 cells transfected with pCS-HTLV as described above.
Two days later, the infected MOLT4 cells were washed three
times with complete medium and placed in 3.5-cm-diameter
dishes (2
3
10
6cells per dish). Into each dish was placed a
transwell (3-
m
m pore size) containing either cord blood
lym-phocytes or PBMCs (2
3
10
6cells) previously activated for 4
days with phytohemagglutinin. An additional set of dishes that
contained uninfected MOLT4 cells as negative controls was
prepared. This cell culture method allows fluid exchange
with-out cell contact or mixing. Two days after initiation of the
transwell cocultures, cells were removed and RNAs were
ex-tracted, converted to cDNA, and then PCR amplified with
primers specific for either Tax/Rex or Env mRNA as described
above. Bands of the predicted size were observed with cDNAs
from both cord blood cells and PBMCs cultured in transwells
with the infected MOLT4 cells (Fig. 5). No HTLV-I-specific
PCR products were observed in cells cultured with uninfected
MOLT4 cells. Thus, the provirus encoded by pCS-HTLV is
infectious in secondary infections and therefore competent in
both afferent and efferent phases of the infectious cycle.
Cord blood cells from three independent donors were
ex-amined for their ability to be transformed with cell-free virus
produced from transiently transfected 293 cells. Although
in-fected, none of the cord blood cell cultures proliferated for
periods longer than 4 to 6 weeks in the presence of
interleu-kin-2. There are several interpretations of these preliminary
results. First, it is possible that the virus titer is simply too low
and too few cells were productively infected. It is important to
note that T-cell transformation mediated by cell-free
prepara-tions of HTLV-I is much less efficient than that mediated by
coculture with infected cells (3, 37). Second, it is possible that
pCS-HTLV is infectious but not transforming. We are
cur-rently trying several other approaches to infect and transform
T cells, including modification of the cell-free infection
proto-col and development of cells that persistently express
pCS-HTLV for transformation mediated by cell contact.
Although molecularly cloned HTLV-I proviruses have been
reported to express viral proteins and to release virus-like
particles after transfection into cells, their ability to produce
infectious virions has not been established. It was therefore the
primary objective of these studies to determine whether the
virus produced from cells transfected with pCS-HTLV was
infectious. The observation that both primary lymphocytes and
transformed T-cell lines expressed spliced viral mRNAs after
exposure to virus from transiently transfected cells indicated
that pCS-HTLV encodes an infectious provirus. Moreover, the
ability of infected MOLT4 cells to pass the virus to primary T
cells indicated that the virus had successfully completed all
steps of the infectious cycle. The cell preferences and kinetics
of infection observed here with virus from transfected cells are
consistent with results from cell-free infections using virus
from MT-2 cells (5). In that report, MOLT4 cells also gave a
greater signal (more HTLV-I DNA) than CEM cells after
cell-free infection and the signal deteriorated rapidly after
infection.
[image:5.612.60.296.73.275.2]Many fundamental questions pertaining to the biology and
biochemistry of HTLV-I have not been addressed because of
the lack of an infectious molecular clone. pCS-HTLV now
provides a reagent to study the molecular genetics of HTLV-I
and to test the effects of mutations in structural and regulatory
genes and cis-acting elements on the various stages of the
infectious cycle. For example, it should now be possible to
directly examine determinants of HTLV-I infection, virion
as-sembly, replication, gene expression, and T-cell transformation.
Furthermore, this molecular clone provides a homogeneous
source of infectious virus, in contrast to many persistently
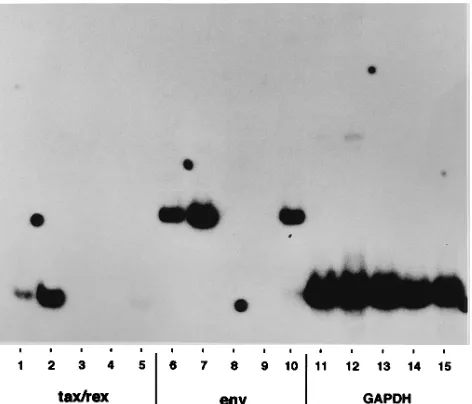
FIG. 5. RT-PCR analysis of RNA expressed after secondary infection of cord blood lymphocytes or PBMCs. RT-PCR analyses were performed essentially as described in the legend to Fig. 4B except that cDNA was prepared by using the superscript preamplification system (Life Technologies) with 5mg of total cel-lular RNA. PCR mixtures contained primers HT5050 and HT7356 for HTLV-I
tax/rex (lanes 1 to 5), primers HTex1-2 and HT5275 for HTLV-I env (lanes 6 to
10), and primers for cellular GAPDH (9) (lanes 11 to 15). Coculture of infected or uninfected MOLT4 cells with cord blood lymphocytes or PBMCs in transwell culture dishes is described in the text. cDNAs were prepared from cord blood cells from transwell cultures with HTLV-I-infected MOLT4 cells (lanes 1, 6, and 11), HTLV-I-infected MOLT4 cells from the bottom of transwell dishes (lanes 2, 7, and 12), cord blood cells from transwell cultures with uninfected MOLT4 cells (lanes 3, 8, and 13), PBMCs from transwell cultures with uninfected MOLT4 cells (lanes 4, 9, and 14), and PBMCs from transwell cultures with HTLV-I-infected MOLT4 cells (lanes 5, 10, and 15).
VOL. 69, 1995 NOTES 1911
on November 9, 2019 by guest
http://jvi.asm.org/
infected cell lines that produce defective viruses that often
interfere with subsequent analyses.
We thank Anne Meyers for expert technical assistance and Matthew Gonda and Kunio Nagashima for electron microscopy.
This project was sponsored in part by funds from the Department of Health and Human Services, NCI, under contracts N01-C0-74102 with PRI/DynCorp and N01-C0-74101 with ABL.
REFERENCES
1. Ciminale, V., G. N. Pavlakis, D. Derse, C. P. Cunningham, and B. Felber. 1992. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J. Virol.
66:1737–1745.
2. Clapham, P., K. Nagy, R. Cheingsong-Popov, M. Exley, and R. A. Weiss. 1983. Productive infection and cell-free transmission of human T-cell leu-kemia virus in a nonlymphoid cell line. Science 222:1125–1127.
3. DeRossi, A., A. Aldovini, G. Franchini, D. Mann, R. C. Gallo, and F.
Wong-Staal.1985. Clonal selection of T lymphocytes infected by cell-free human T-cell leukemia/lymphoma virus type I: parameters of virus integration and expression. Virology 143:640–645.
4. Ehrlich, G. D., F. Davey, J. Kirshner, J. Sninsky, S. Kwok, D. Slamon, R.
Kalish, and B. J. Poiesz.1989. A polyclonal CD41and CD81lymphocytosis in a patient doubly infected with HTLV-I and HIV-1: a clinical and molec-ular analysis. Am. J. Hematol. 30:128–139.
5. Fan, N., J. Gavalchin, B. Paul, K. Wells, M. J. Lane, and B. J. Poiesz. 1992. Infection of peripheral blood mononuclear cells and cell lines by cell-free human T-cell lymphoma/leukemia virus type I. J. Clin. Microbiol. 30:905– 910.
6. Felber, B., D. Derse, A. Athanassopoulos, M. Campbell, and G. N. Pavlakis. 1989. Cross-activation of the Rex proteins of HTLV-1 and BLV and of the REV protein of HIV-1 and nonreciprocal interactions with their RNA re-sponsive elements. New Biol. 1:318–330.
7. Felber, B., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N.
Pavlakis.1985. The pX protein of HTLV-1 is a transcriptional activator of its long terminal repeats. Science 229:675–679.
8. Fujisawa, J., M. Seiki, T. Kiyokawa, and M. Yoshida. 1985. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc. Natl. Acad. Sci. USA 82:2277–2281. 9. Gendelman, H., R. Friedman, S. Joe, L. Baca, J. Turpin, G. Dveksler, M.
Meltzer, and C. Dieffenbach.1990. A selective defect of interferon-alpha production in human immunodeficiency virus-infected monocytes. J. Exp. Med. 172:1433–1438.
10. Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G.
De The.1985. Antibodies to human T-lymphotrophic virus type I in patients with tropical spastic paraparesis. Lancet ii:407–410.
11. Gessain, A., F. Saal, M. Giron, J. Lasneret, S. Lagaye, O. Gout, G. De The,
F. Sigaux, and J. Peries.1990. Cell surface phenotype and human T lym-photropic virus type I antigen expression in 12 T-cell lines derived from peripheral blood and cerebrospinal fluid of West Indian, Guianese, and African patients with tropical spastic paraparesis. J. Gen. Virol. 71:333–341. 12. Hattori, T., T. Uchiyama, T. Toibana, K. Takatsuki, and H. Uchino. 1981. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood 58:645–647.
13. Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K.
Ki-noshita, S. Shirakawa, and I. Miyoshi.1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476–6480.
14. Hiramatsu, K., M. Masuda, and H. Yoshikura. 1986. Mode of transmission of human T cell leukemia virus type I (HTLV-I) in a human promyelocytic leukemia HL60 cell. Int. J. Cancer 37:601–606.
15. Ho, D., T. R. Rota, and M. S. Hirsh. 1984. Infection of human endothelial cells by human T-lymphotropic virus type I. Proc. Natl. Acad. Sci. USA
81:7588–7590.
16. Hoffman, P. M., S. Dhib-Jalbut, J. A. Mikovits, D. S. Robbins, A. L. Wolf,
G. K. Bergey, N. C. Lohrey, O. S. Weislow, and F. W. Ruscetti.1992. Human T-cell leukemia virus type I infection of monocytes and microglial cells in primary human cultures. Proc. Natl. Acad. Sci. USA 89:11784–11788. 17. Hoxie, J. A., D. M. Matthews, and D. B. Cines. 1984. Infection of human
endothelial cells by human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 81:7591–7595.
18. Inoue, J., M. Seiki, and M. Yoshida. 1986. The second pX product p27X-III of HTLV-1 is required for gag gene expression. FEBS Lett. 209:187–190. 19. Inoue, J., M. Yoshida, and M. Seiki. 1987. Transcriptional (p40X) and
post-transcriptional (p27X-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc. Natl.
Acad. Sci. USA 84:3653–3657.
20. Longo, D. I., E. P. Gelmann, J. Cossman, R. A. Young, R. C. Gallo, S. J.
O’Brien, and L. A. Matis. 1984. Isolation of an HTLV-transformed B-lymphocyte clone from a patient with HTLV-associated adult T-cell leuke-mia. Nature (London) 310:505–506.
21. Mann, D. L., J. Clark, M. Clarke, M. Reitz, M. Popovic, G. Franchini, C. D.
Trainor, D. M. Strong, W. A. Blattner, and R. C. Gallo.1984. Identification of the human T cell lymphoma virus in B cell lines established from patients with adult T cell leukemia. J. Clin. Invest. 74:56–62.
22. Martarano, L., R. Stephens, N. Rice, and D. Derse. 1994. Equine infectious anemia virus trans-regulatory protein Rev controls mRNA stability, accumu-lation, and alternative splicing. J. Virol. 68:3102–3111.
23. Mori, K., H. Sabe, H. Siomi, T. Iino, A. Tanaka, K. Takeuchi, K. Hirayoshi,
and M. Hatanaka.1987. Expression of a provirus of human T cell leukaemia virus type I by DNA transfection. J. Gen. Virol. 68:499–506.
24. Nicot, C., T. Astier-Gin, E. Edouard, E. Legrand, D. Moynet, A. Vital, D.
Londos-Gagliardi, J. P. Moreau, and B. Guillemain.1993. Establishment of HTLV-I-infected cell lines from French, Guianese and West Indian patients and isolation of a proviral clone producing viral particles. Virus Res. 30:317– 334.
25. Okada, M., Y. Koyanagi, N. Kobayashi, Y. Tanaka, M. Nakai, K. Sano, K.
Takeuchi, Y. Hinuma, M. Hatanaka, and N. Yamamoto.1984. In vitro infection of human B lymphocytes with adult T-cell leukemia virus. Cancer Lett. 22:11–21.
26. Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, M. Matsumoto, and
M. Tara.1986. HTLV-I associated myelopathy, a new clinical entity. Lancet
i:1031–1032.
27. Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C.
Gallo.1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415–7419.
28. Poiesz, B. J., F. W. Ruscetti, M. S. Reitz, V. S. Kalyanaraman, and R. C.
Gallo.1981. Isolation of a new type-C retrovirus (HTLV) in primary uncul-tured cells of a patient with Sezary T-cell leukemia and evidence for virus nucleic acids and antigens in fresh leukemic cells. Nature (London) 294:268– 271.
29. Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G.
Dalgleish.1990. In vivo cellular tropism of human T-cell leukemia virus type I. J. Virol. 64:5682–5687.
30. Ruscetti, F. W., V. S. Kalyanaraman, R. Overton, J. Mikovits, H. Stevenson,
K. Stromberg, R. B. Herberman, W. L. Farrar, and J. R. Ortaldo.1986. Analysis of effector mechanisms against HTLV-I and HTLV-III/LAV in-fected lymphoid cells. J. Immunol. 136:3619–3625.
31. Ruscetti, F. W., M. Robert-Guroff, L. Ceccherini-Nelli, J. Minowada, M.
Popovic, and R. C. Gallo.1983. Persistent in vitro infection by human T-cell leukemia-lymphoma virus (HTLV) of normal human T-lymphocytes from blood relatives of patients with HTLV-associated mature T-cell neoplasms. Int. J. Cancer 31:171–180.
32. Saida, T., K. Saida, M. Funauchi, E. Nishiguchi, M. Nakajima, S. Matsuda,
M. Ohta, K. Ohta, H. Nishitani, and M. Hatanaka.1988. HTLV-I myelitis: isolation of virus, genomic analysis, and infection in neural cell cultures. Ann. N. Y. Acad. Sci. 540:636–638.
33. Saksela, K., E. Muchmore, M. Girard, P. Fultz, and D. Baltimore. 1993. High viral load in lymph nodes and latent human immunodeficiency virus (HIV) in peripheral blood cells of HIV-1-infected chimpanzees. J. Virol.
67:7423–7427.
34. Seiki, M., J. Inoue, T. Takeda, and M. Yoshida. 1986. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 5:561–565.
35. Sodroski, J. 1992. The human T-cell leukemia virus (HTLV) transactivator (Tax) protein. Biochim. Biophys. Acta 1114:19–29.
36. Sodroski, J. G., C. A. Rosen, and W. A. Haseltine. 1984. Trans-acting tran-scriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science 225:381–385.
37. Sugamura, K., M. Sakitani, and Y. Hinuma. 1984. Microplate method for retrovirus-induced transformation of normal human T-cells. J. Immunol. Methods 73:379–385.
38. Yamamoto, N., T. Matsumoto, Y. Koyanagi, Y. Tanaka, and Y. Hinuma. 1982. Unique cell lines harboring both Epstein-Barr virus and adult T-cell leukemia virus established from leukemia patients. Nature (London) 299: 367–369.
39. Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implica-tion in the disease. Proc. Natl. Acad. Sci. USA 79:2031–2035.
40. Zhao, L. J., and C. Z. Giam. 1991. Interaction of the human T-cell lympho-tropic virus type I (HTLV-I) transcriptional activator Tax with cellular fac-tors that bind specifically to the 21-base-pair repeats in the HTLV-I en-hancer. Proc. Natl. Acad. Sci. USA 88:11445–11449.
1912 NOTES J. VIROL.