JOURNAL OFVIROLOGY, June 1967, p.466-471 Copyright @ 1967 American Society forMicrobiology
Vol. 1,No. 3 Printed in U.S.A.
Effect of
Interferon
on
Deoxyribonuclease Induction
in
Chick
Fibroblast
Cultures
Infected
with
Cowpox Virus
G. BODO AND C. JUNGWIRTH
ChemischesLaboratoriumderArzneimittelforschung, Vienna, Austria,and Institutfiir Virologie derUniversitdt Wiirzburg, Wiirzburg, Germanly
Received forpublication9February 1967
An exonuclease which degrades native
deoxyribonucleic
acid atpH 9.2 wasin-duced in chick embryo fibroblast cultures and in human amnion cells by infection with cowpoxvirus. Highly purified chick embryo interferon suppressed the
induc-tion oftheenzymeinthehomologous cellsystembutnotinthe humanamnion cell cultures. "Mock" interferon prepared from uninfected chicken eggs and purified
inthe samemanner as biologically active interferonpreparations hadno effect on
theinductionof theenzyme.
In
interferon-treated cells,
the messengerribo-nucleic acid (mRNA) of vaccinia virus
cannotcombine with ribosomes to form polysomes (9).
This
effect
isprobably
mediated by a proteinwhichis not interferon, but which is produced by
the host
cell after
exposure tointerferon
(11, 13,25, 26).
Results obtained with Sindbis
virus byMarcus
and Salb (15)
suggest thatthis
protein exertsits
effect by binding
toribosomes, thereby
specifically inhibiting
the processof
translation of
viral
mRNA.Either
of the twomechanisms of
interferon
action would result
in theprevention ofthe
synthesis
of
virus-specific
proteins.
Interferon
hasbeen found
to prevent theinduction of
certain "early"
enzymessuch
asdeoxyribonucleic acid (DNA) polymerase,
thy-midinekinase, and
probably viral
RNApolym-erase
(4-6, 9, 20).
The purpose of this paper isto show
that
interferon
inhibitsthe
formationof
another
early
enzyme, which is induced in chickembryo
fibroblasts after infection with cowpoxvirus, namely, "alkaline"
deoxyribonuclease
(7, 18).MATERIALS AND METHODS
Cells.Chickembryo fibroblasts were prepared by the standard trypsin procedure. Cellsweregrownin either Carrel flasks for interferon assays or in milk dilution bottles for deoxyribonuclease induction
ex-periments. Parker's medium199with 10% heat inac-tivated calfserum was used for cell growth. Mainte-nancemediumconsisted ofParker'smediumwithout serum.
The U line of human amnioncells was grown in milk dilution bottles fordeoxyribonuclease induction experimentsorin Rouxbottles forthe
preparation
of radioactive DNA (22,24).Growthmediumconsistedof Eagle's minimal essential medium, supplemented with 15% inactivated calfserumand 0.3% Tryptose Phosphate Broth
(Difco).
For the maintenance of cells,theserum content wasreduced from 15to2%. Preparation of'4C-labeled
DNA. To one Roux bottlecontainingagrowingculture of human Ucells,
10
J/c
of 14C-labeledthymidinewasadded (The Radio-chemical Centre, Amersham, England; specific ac-tivity, 40 mc/mmole) and was left in contact with cells for 48 hr. DNAwas preparedfrom the cells by the method of Marmur (16). The specific activity of the DNA preparation was between 1,000 and 2,000 countsper min per ug of DNA.Viruses. The LEE strain of influenza B (ATCC) and cowpoxvirus strain Brightonwere propagated in
10- to12-day-oldchick embryos.
Cowpox virus was used after purification by the method of Joklik (8). No deoxyribonuclease activity wasdetectablewhenpreparationscontaining4 X 107 plaque-formingunits (PFU) of viruswereinvestigated under the conditions described below for deoxyribo-nucleaseassay.
Preparation of chick interferon. Interferon was prepared in 11-day-old chick embryos with influenza BLeevirus (1). The crude allantoicfluidwaspurified bytreatmentwithperchloricacid andbyprecipitation ofinterferon with zincacetate atpH7.2,accordingto the method of Lampson et al. (10), followed by chromatography on CM-Sephadex C-25 (19). Inter-feronwaselutedbyraisingthepHfrom 6.0to8.0 with 0.1 MKphosphate buffer. Chromatography on CM-Sephadex C-25 was repeated on a smaller column; this time the interferon was eluted between pH 6.0 and 7.0. This repeated CM-Sephadex chromatog-raphy gavehigher yields ofinterferon than the
one-stepgradientelutionemployedbyMeriganetal. (19). Final purification was effected by molecular sieve chromatographyon acolumn
(150
by2cm) ofBiogel
P-200 (Bio-Rad Laboratories,
Richmond,
Calif.),
by 466on November 11, 2019 by guest
http://jvi.asm.org/
useofabuffer containing0.25 Mboric acidand0.038 M NaOH with apH of 8.1 (Bodo, in preparation). Samples were concentrated by precipitating the interferon with zinc acetate at pH 7.2. Finally, con-centrated interferon samples were dialyzed against phosphate-buffered saline and were stored in poly-propylene tubes at 4 C. The interferon preparation for the deoxyribonuclease experiments had atiter of 110,000 units/ml andexhibited aspecific activity of 0.010
jLg
ofprotein per unit of activity. Disc electro-phoresisin polyacrylamide atpH 4.3 bythe method ofReisfeldetal. (23) and at pH 8.9 by themethodof OrnsteinandDavies(21, 21a)showed that thepurified interferonpreparationscould beresolved intoseveral proteinbands.Preparationz
of"mock" chick interferoni. "Mock"interferon was prepared from the allantoic fluid of uninoculated chick embryos andwas purifiedexactly in thesamemanner asactiveinterferonpreparations. Disc electrophoresisinpolyacrylamide at pH 4.3and 8.9 (see above) gave protein patterns exhibiting the sameprotein bands as theactive interferon prepara-tions (Bodo,inpreparation).
Interferon assay. Growthmediumwas replacedby 5ml of Parkermedium 199 5 to 6 hr before the test. In the interferon test, each Carrel flask received 2.0 mlof Parker's medium, 0.3 ml of interferon dilution in Parker's medium containing 0.05% bovine serum albumin, and 0.2 ml of gelatin saline (2) containing about 200 PFUofcowpoxvirusstrainBrighton (12). Plaqueswerecounted after 44 to 48 hrofincubation at37C.
One unit of interferon was defined as theamount of the material that inhibited 50%,Co of the plaques foundin thecontrols.
Proteini determiniationi.
Protein determinations weredoneby the method of Lowryetal. (14).
Inductioni
of deoxyribonuclease. For infection,growth medium was withdrawn from confluent monolayers, and thecellswere infected with cowpox virusat a multiplicity ofapproximately 5 to 10 PFU per cell. Uninfected controls received gelatin saline only. Adsorption wasallowed toproceed for 90 min at37 C. Cellswerethen washedwith theappropriate maintenancemedium and were incubated for various amounts oftimeat 37C with 7.5 ml of maintenance mediumperflask.
Tostudy the effect of interferon on enzyme induc-tion, each flask received 1.0 ml ofinterferon diluted in Parker medium 199 containing
0.05%0
bovine serum albumin and 6.5 ml of the appropriate main-tenance medium (controls received dilution medium only). After 24 hr at 37 C, the overlay medium was withdrawn, and the adsorption of virus was carried out as described above. After infection, interferon was again added to the cell cultures. For harvest, cells were scraped from the glass, washed twice in cold0.01 Mtris(hydroxymethyl)aminomethane buffer (pH 8.0) containing 0.15 M KCl and 0.003 M 3 mercaptoethanol, suspended in 1 ml of the same buffer,andkeptfrozen at -80 C until use.Deoxyribonuclease assay. Frozen cells were dis-rupted by three cycles of freezing and thawing, fol-lowedby sonic treatment for 2 min with an MSE 60-w
ultrasonic disintegrator; care was taken to avoid warming of the samples above 10 C. Extracts were centrifugedat30,000 XGfor1hr.Deoxyribonuclease activity in the supematant fluid was measured as
described by McAuslan (17) within 1 day after pre-paring the extract. Of each reaction mixture, 300 ,uliters contained 20 ,uhters of 0.5 M glycine-NaOH buffer (pH 9.2), 20 ,liters of 0.1 MMgCl2, 50
gliters
of "C-labeled native DNA
(equivalent
to about 60,000 counts per minand 30to60,ugofDNA), and different volumes of enzyme extract(usually
25 to200
Muliters).
Afterincubationat 37Cfor40min, thereaction was stopped by chillingin anice bath and adding50 Alitersof40% trichloroaceticacidsolution. Aftercentrifugation, acid-soluble radioactivity of the supematantfluidwasdetermined inaTri-Carbliquid scintillationspectrometer.
REsuLTS
Induction
of
adeoxyribonuclease
inchick embryofibroblast cultures by
cowpox virus. Extractspre-pared from uninfected chick embryo fibroblast
cells
exhibited
verylittle
activity
when tested for"alkaline"
deoxyribonuclease
with "4C-labelednative DNA
assubstrate
(Fig. 1, 2, and 3).
Insome extracts from uninfected
cells,
theexistenceof
the enzymecould
not be demonstratedun-ambiguously.
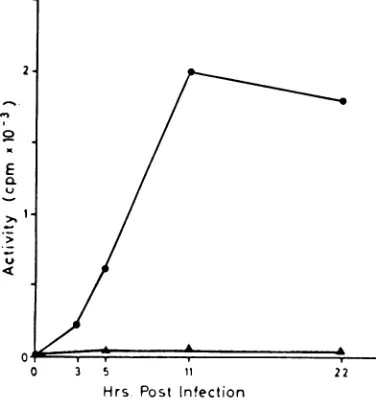
Induction of deoxyribonuclease activity by
cowpox
virus became detectable
atabout
3 hrafter infection
andincreased
rapidly
thereafter.The
specific
activity
was veryhigh
even aslate
as 22 hrpostinfection
(Fig. 1).
At 22 hrpostinfec-tion,
severedestruction of the infected cells
wasobserved, and, consequently,
no furthersamples
were
collected.
As a
rule,
each extract wastested
atdifferent
dilutions
to assure alinear
relationship
betweenactivity
andprotein
content up to about 100 ,ugof
protein
per testsample.
There was, however,some
variation
in the extent ofthis linear
region
among
different
setsof
experiments.
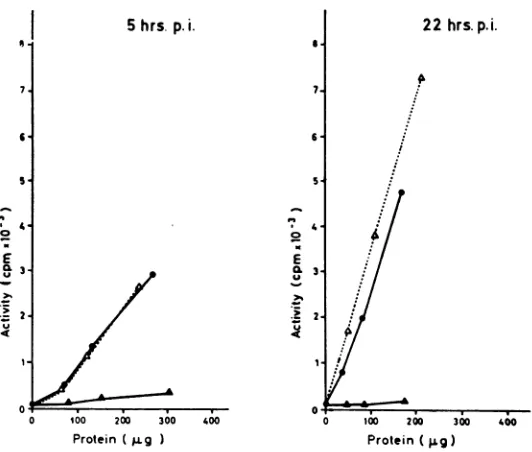
Suppression
of deoxyribonuclease induction by
interferon.
Figure
2 shows theeffect
ofinterferon
on the
induction of
deoxyribonuclease
at twodifferent
times after infection. Interferon
wasusedin
threedifferent
concentrations.
Thehighest
doseof interferon
(1,000 units
perflask) completely
suppressed
theinduction
of"alkaline"
deoxyribo-nuclease
at 5 hrpostinfection.
At 22 hr, thesup-pression
wasstill considerable.
The lowest doseof
interferon
(10units
per flask) caused only avery
slight
inhibition ofdeoxyribonuclease
induc-tion.
Comparison
of the effect of interferon at 5andat 22hr
postinfection indicated
thatsuppres-sion of enzyme induction was more pronounced
at 5hr
postinfection.
Thisis
best shownwith theintermediate interferon concentration (100 units
per
flask).
on November 11, 2019 by guest
http://jvi.asm.org/
BODO AND JUNGWIRTH
To compare the
effect of interferon on
deoxy-ribonuclease
induction
with
its protection against
viral
cytopathogenic
effects, monolayers were
in-spected
microscopically
for cell
destruction
at
3 5 11
Hrs Post Infection
FIG. 1. Time course of increase in "alkaline" deoxyribonucleaseactivityin chick embryo fibroblasts
infected
with cowpoxvirus. Specific activity ofdeoxy-ribonuclease plotted against time after infection. Ab-scissa, hours postinfection; ordinate, enzyme activity expressed as countsperminute rendered acid-soluble per 50 ,ug of protein under the assay conditions used.
Symbols:A, uninfected;*, infected.
regular
intervals. Interferon concentrations of
1,000
units
perflask completely
protected themonolayers up to 22 hr after infection. In
con-trast,
interferon concentrations of 10 units per
flask had
nodetectable protective effect.
Inter-feron doses of
100units per flask
afforded good
protection at
5hr,
but very little protection at 22
hr
postinfection. The extent of cell protection
observed
microscopically
was,
therefore,
paral-leled by the
suppression of deoxyribonuclease
induction.
Since interferon
waspresent
in the overlay
medium until cells were harvested, the possibility
could
notbe
excluded that
asmall amount of
interferon was
introduced into the reaction
mix-ture
of the
enzymatic
test. Torule
outanyeffect
of interferon
ondeoxyribonuclease activity,
interferon was added
directly to the
deoxyribonu-clease
assaymixture.
Nosignificant influence of
purified chick interferon
onthe
estimation of
deoxyribonuclease activity could be detected,
evenat
interferon concentrations of 12,000 units
pertest.
Specificity of interferon
action.Since
the
highly
purified interferon
preparations
were
nothomo-geneous,
experiments
wereconducted
todemon-strate
that
the observed
suppression
of
deoxyribo-nuclease induction
wasdue
tothe
interferon
component
in ourpreparation and
not to anonspecific inhibitor.
One
control
wasused
tostudy the effect of
"mock"
interferon
on theinduction of the
en-zyme.
There was noevidence
that"mock"
5hrs.p.i.
0
. 3
E
cx
a
2
S I
1
a
_
--°
°°
_,o:- --^o 000
100 200 300
Protein (Aug)
400
22 hrs.
p.i.
[image:3.461.47.235.126.326.2]loo 200 300 Protein ( ug)
FIG. 2. Suppression of deoxyribonucleaseinduction in chickembryo fibroblasts by interferon, 5and22 hr post-infection. Enzymeactivitiesplotted against proteincontentofcell extracts. Abscissa,amountsofproteinusedper
assaymixture;ordinate, deoxyribonuclease activity expressedascountsperminute rendered acid soluble. Symbols:
A,uninfected;*, infected;andO,infected, interferontreated. Interferondoseswere 10,100, and1,000 interferon
unitsperflask.
468
J. VIROL.
2-E aL
u
1%I-U.
_- 4
0
E3 a
2
*S
I
'400
on November 11, 2019 by guest
http://jvi.asm.org/
[image:3.461.90.401.418.616.2]interferon had any influence on
deoxyribonuclease
induction, even at a protein level five times as
high
as
that
used
in
the
experiments with the
highest active interferon
concentrations (Fig. 3).
A
second control made use of the known
species specificity of interferon. Thus, chick
interferon
does notprotect
against viral infectionin heterologous cells and, consequently, should
not suppress
the
induction of
deoxyribonuclease
5hrs.
p.i.
6
6 4 _
E.
.5
*_24
100 200 300
Protein( u g )
400
by cowpox virus in human cells. The
interferon-sensitive U cell line derived from human
amnion
(22, 24)
wasused
to testthis
prediction. It can
be seen
from Fig. 4 that cowpox virus
induced
high activities of "alkaline" deoxyribonuclease
inU
cells also.
Incubation of
these cells
with
purified
chick
interferon did not suppress the induction of
the
enzyme.
It
is evident from these
experiments that the
22 hrs.p.i.
S~~~~
7.
CL~~~~~~~~~~~~.
6-40
E 3 1
20.l
100 200 300 Protein(big )
400
FIG. 3. Effect of "mock"-interferon on deoxyribonuclease induction in chickembryo
fibroblasts
5 and 22 hr post-infection. Enzyme activitiesplotted against protein content of cell extracts. Abscissa, amounts ofprotein used per assay mixture;ordinate,deoxyribonuclease activity expressed as counts per minute rendered acid soluble. Symbols: A, uninfected; *, infected; and A, infected, treated with "mock"-interferon (50 ,ug of protein per flask).5 hrs.
p.i.
21 hrs. p.i.20 , 20
0
O-V I 0..
0~~~~~~~~~~~~~~~~~1
~,
I0 .0
0 50 1000 0 SOO 1000
Protein (Ag) Protein ( g)
FIG. 4. Effectofchickinterferonondeoxyribonuclease induction inhuman Ucells, 5and21hrpostinfection. Enzymeactivitiesplotted against protein content ofcell extracts. Abscissa, protein used per assay mixture;ordinate, deoxyribonuclease activity expressedas countsper minute rendered acid-soluble. Symbols: A, uninfected; *, infected; and0, infected, treated with chick embryointerferon (1,000unitsperflask).
on November 11, 2019 by guest
http://jvi.asm.org/
[image:4.461.90.356.171.397.2] [image:4.461.64.384.455.621.2]BODO AND JUNGWIRTH
observed
suppression
ofdeoxyribonucleaseinduc-tion by interferon
was not caused byimpurities
in
the interferonpreparations,
but must havebeen
due
tothe
interferon
component itself.DISCUSSION
Our
experiments confirm
data onthe
induction
of
an"alkaline"
deoxyribonuclease in chick
embryo fibroblasts by viruses of the vaccinia
group
(18). They show, in
addition,
that an enzymewith similar
properties is
induced in
human amnion cells after infection with
cowpoxvirus.
The reason
for choosing chick
embryofibro-blast cultures
for
ourexperiments is that
inter-feron
from chicken eggs can behighly
purified
and
isby
far thechemically
bestcharacterized
interferon
preparation available.
The amount
of
interferon required for
inhibi-tion of
deoxyribonuclease
induction
wasfound
tobe
considerably higher than the
amountneeded
for
complete suppression of plaque formation by
cowpox
virus.
However,this
does
notnecessarily
reflect
ahigher sensitivity of infectious virus
formation
tothe
action of
interferon; rather, it
may
be
explained
by the different multiplicities
of
infection used in the
twodifferent
typesof
experiments.
The
suppression of viral
cytopatho-genic
effect under the
conditions of
enzymeinduction
was infactapproximately
asstrong asthe
inhibition
by interferon of "alkaline"
deoxyri-bonuclease
induction.
Inspite of
thepresenceof
interferon in
themedium throughout
theexperi-ments,
the
inhibitory effect of interferon
ondeoxyribonuclease
induction
wasusually
moremarked
at 5hr
than at 22 hrpostinfection. This
suggests
that the observed
inhibition by interferon
is
partially
overcomeduring
prolonged
incuba-tion.
The
suppression of deoxyribonuclease
induc-tion by
interferon
wasspecies
specific,
as shownby the failure of chick interferon
toinhibit
deoxyribonuclease induction
in human Ucells.
In
addition, biologically
inactive "mock"
inter-feron from
uninfected chicken
eggs had noeffect
on the
induction
of viraldeoxyribonuclease
inchick
embryo fibroblasts.
Theseexperiments
strongly
support the notion that it is the interferoncomponent rather than an
unspecific impurity
contained
in ourinterferon
preparation which is
responsible
for theinhibition
ofdeoxyribonucle-ase
induction.
The
"alkaline" deoxyribonuclease
inducedby
vaccinia virus in HeLa cells
differs
invarious
properties
from theenzymefound
inuninfected
cells
(17).
Also,
its induction isinhibited
by
theaddition of
puromycin
(18).
Therefore,
the"alkaline"
deoxyribonuclease
induced in HeLacells
is probably
a newprotein formed
in responseto
vaccinia infection.
Whetherthis
new proteinis coded
by the genome of thevirus
or the hostcell
cannotbe
decided
atthis time.
Ourobserva-tion that interferon inhibits the
induction
ofthis
early
enzyme cannotbe
taken
asunequivocal
evidence that "alkaline" deoxyribonuclease
is
aviral
enzyme.ACKNOWLEDGMENTS
We thank H. Tuppy ofthe Institute of Biochem-istry, University ofVienna, and E. Wecker, Institute of Virology, University of Wurzburg, for valuable discussions in the course of this work. The expert technicalassistanceofL.Dantineand P.Hiestandis alsogratefully acknowledged.
This investigation was supported by the Deutsche Forschungsgemeinschaft.
LITERATURE CITED
1. CANTELL, K., M. VALLE, R. SCHAKIR, J. SAUK-KONEN, AND E. UROMAN. 1965. Observations on production, assay and purification ofchick embryo interferon. Ann. Med. Exptl. Fenniae (Helsinki)43:125-131.
2. FAZEKASDE ST. GROTH, S., D. M. GRAHAM, AND I. JACK. 1958. The serology of mumps infec-tions. I. Anewsourceofantigenand a simpli-fied complement fixation test. J. Lab. Clin. Med. 51:883-896.
3. FRIEDMAN, R. M., AND J. A. SONNABEND. 1964. Inhibition of interferon action by p-fluor-phenylalanin.Nature203:366-367.
4. FRIEDMAN, R. M., AND J. A. SONNABEND. 1965. Inhibition by interferon of production of double stranded Semliki forest virus RNA. Nature206:532.
5. GHOSH, S. N., AND G. E. GIFFORD. 1965. Effect ofinterferononthe dynamicsofH3-thymidine incorporation and thymidine kinase induction in chick fibroblast cultures infected with vac-cinia virus.Virology 27:186-192.
6. GORDON, I., S. S. CHENAULT, D.STEVENSON, AND
J. D. ACTON. 1966. Effect of interferon on polymerization of single-stranded and double-stranded mengovirus ribonucleic acid. J. Bacteriol. 91:1230-1238.
7. HANAFUSA, T. 1961. Enzymatic synthesis and breakdownofdeoxyribonucleicacidbyextracts of L-cells infected with vaccinia virus. Biken's J.4:97-1 10.
8. JOKLIK, W. K. 1962. The purification of four strains ofpoxvirus. Virology18:9-18.
9. JOKLIK, W. K., ANDT. C. MERIGAN. 1966.
Con-cerningthemechanism of action of interferon. Proc.Natl.Acad. Sci. U.S. 56:558-565. 10. LAMPSON, G. P., A. A. TYTELL, M. M. NEMES,
AND M. R. HILLEMAN. 1963. Purification and characterization of chick embryo interferon. Proc. Soc. Exptl. Biol. Med. 112:468-478. 11. LEVINE, S. 1964. Effect of actinomycin D and
puromycin
dihydrochloride
onaction of inter-feron.Virology24:586-588.470
J. VIROL.on November 11, 2019 by guest
http://jvi.asm.org/
12. LINDENMANN, J., AND G. E. GIFFORD. 1963.
Studies on vaccinia plaque formation and its
inhibition by interferon. III. A simplified plaque inhibitionassayofinterferon. Virology
19:302-309.
13. LOCKART, R. Z. 1964. The necessity for cellular RNA and proteinsynthesis for viral inhibition resulting from interferon. Biochem. Biophys. Res. Commun. 15:513-518.
14. LOWRY, 0. H., N. J. ROSEBROUGH, A. L. FARR,
AND R. J. RANDALL. 1951. Protein measure-mentwith folin phenolreagent. J. Biol. Chem. 193:265-275.
15. MARCUS, P. I., ANDJ. M. SALB. Molecular basis of interferon action: Inhibition of viral RNA translation. Virology 30:502-516.
16. MARMUR, J. 1961. A procedure for theisolation ofdeoxyribonucleic acid frommicroorganisms. J. Mol. Biol. 3:208-218.
17. McAUSLAN, B. R. 1965. Deoxyribonuclease
ac-tivity of normal and poxvirus-infected HeLa cells. Biochem. Biophys. Res. Commun. 19:
15-20.
18. MCAUSLAN, B. R., P. HERDE, D. PETT, AND
J. Ross. 1965. Nucleases ofvirus-infected ani-mal cells. Biochem. Biophys. Res. Commun. 20:586-591.
19. MERIGAN, T. C., C. A. WINGET, AND C. B. DIXON, 1965. Purification and characteriza-tion of vertebrate interferons. J. Mol. Biol. 13:679-691.
20. OHNO, S.,ANDT. NoziMA. 1964. Inhibitory effect
of interferon on the induction of thymidine
kinase in vaccinia virus-infected chick
em-bryo fibroblasts. Acta Virol. (Prague) 8:479. 21. ORNSTEIN, L., AND B. J. DAVIES. 1964. Disc electrophoresis.I.Backgroundandtheory. Ann.
N.Y.Acad. Sci. 121:321-349.
21a. ORNSTEIN, L., AND B. J. DAVIES. 1964. Disc electrophoresis. LI. Method andapplicationto
human serum proteins. Ann. N.Y. Acad. Sci. 121:404-427.
22. POHJANPELTO, T. 1961. Two different thermo-stable variants of polio virus. Virology 15: 231-236.
23. REISFELD, R. A., U. J. LEWIS, D. E. WILLIAMS. 1962. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature 195:281-283.
24. STRANDER, H., AND K. CANTELL. 1966. Produc-tion of interferon by human leukocytes in vitro. Ann. Med. Exptl. Fenniae (Helsinki) 44:265-273.
25. TAYLOR, J. 1964. Inhibition of interferon action by actinomycin. Biophys. Biochem.Res.
Com-mun. 14:447-451.
26. TAYLOR, J. 1965. Studies on the mechanism of action of interferon. I. Interferon action and RNA synthesis in chick embryo fibroblasts infected with Semliki forest virus. Virology 25:340-349.