JOURNALOF VIROLOGY, Aug. 1972, p. 288--296
Copyright ©T 1972 AmiiericanSocietyforMicrobiology
Vol. 10, No. 2 Printted ill U.S.A.
Rescue
of
Epstein-Barr Virus from Somatic Cell
Hybrids
of Burkitt
Lymphoblastoid Cells
RONALD GLASER AND FRED RAPP
Department of Microbiology, College of Medicinie, Miltont S. Hershey Medical Celnter, Penllnsylvaniia State
Untiversity, Hershey, Pennsylvania17033
Receivedforpublication 23 May 1972
A Burkitt lymphoblastoid cell line, P31-HR-I, was fused and hybridized to a
human sternal marrow cell line. The somatic cellhybridswere negativewhen
ex-amined forEpstein-Barrvirus(EBV)markers. When thehybridcellswereexposedto
5-iododeoxyuridine, both EBV-specific antigensand virusparticleswereinducedas
determined bythe immunofluorescence testand byelectron microscopy. The data
presented suggestthat the EBVgenome canbe transferred froma lymphoblastoid
cellto anothercelltypeduringcellhybridization, that the EBVgenome canpersist in the hybrid cells forlong periods oftime, andthat synthesis ofthe viruscan be
induced in the heterokaryons.
Inactivated Sendai virus has been used to
in-ducecell fusion inseveral laboratories(7, 12, 21). Theprocedurehasbeen appliedtocancerresearch where somatic cell hybridization (3, 15) has
proventobeafruitfultechniquein manystudies. The rescue ofsimian virus 40 from transformed cells after they were fused to potentially suscep-tible cells (6, 16) hasgreatly stimulateduseof the method invirus laboratories. The method, when applied to papovavirus systems, has already al-lowed observations that suggest that, in somatic
cell hybrids of normal and virus-transformed
cells, virus-specifictumorandtransplantation
an-tigens can be transferred intothe hybridandcan
persistinthehybridcells forlongperiodsof time
(5,
26).
Theassociation of a herpesvirus designated the
Epstein-Barr virus (EBV) with many human
lymphoblastoid cell lines has been reported (8).
A small percentage of the cells containthe EBV
(9) as wellasantigens which canbe detected by
the immunofluorescence test (13). There is also
some evidence that theEBVgenomeisintegrated
inthe host cell genome (19, 27).
Recent findings have indicated that Burkitt lymphoblastoid cells can be fused and hybridized
to both mouse and human cells (R. Glaser and
F. J. O'Neill, Science, in press). In this report, further data is presented concerning the proper-ties of the Burkitt lymphoblastoid somatic cell hybrids and the induction of synthesis of EBV-specificantigens and virus particles using the drug 5-iododeoxyuridine (IUDR).
MATERIALS AND METHODS
Cells. The human sternal marrow cell line, D98'
AH-2, (D98) (25) was maintained in Eagle medium
supplemented with
10%c,0
fetalcalfserum, 100units ofpenicillin/ml, 100 ,ug of streptomycin/ml, 1 jAg of
fungizone,/ml, 10unitsof mycostatin/ml, and 0.075%
NaHCO3 in 8-oz (ca. 0.24 liter) glass prescription
bottlesat37C. The Burkittlymphoblastoidcell line,
P3J-HR-1, (HR-1) was maintained in RPM1 1640
mediumsupplemented with
10%-o
fetal calfserum, 100units ofpenicillin/ml, 100,ugofstreptomycin/ml, 1Mg
of fungizone/ml, 10 units of mycostatin/nd, and
0.075c% NaHCO3 in 8-oz (ca. 0.24 liter) glass pre-scriptionbottles at35C.
Fusion andhybridization. Themethod forfusion of
D98cellstoHR-1 cellshasbeenpreviously described
(R. Glaser and F. J. O'Neill, Science, inipress). The
procedure was as follows: 2 X 106 D98 cells were
seededinto tissueculture platesand grownfor 24 hr
at37C.HR-1 cellswereprepared at aconcentration
of 107 cells in0.2ml inEagle medium without serum.
The D98 cells were washed with phosphate-buffered
saline (PBS). Sendai virus (400-1,000
hemagglutinat-ing units in0.2mlof PBS) wasinactivated with
ultra-violet light andadded to the center of the cell sheet and to the HR-1 cell suspension. Both theHR-1 and
D98 cellswerecooled onice for 5min. Then thecell
suspensionwasplacedon themonolayer culturesand
cooled for5 min onice, and thecultureswereplaced
at 37 C for3 to4 hr in an atmosphereof
5%7c,
Co2.Threedays afterfusion,thenormalEagle mediumwas
replacedwith the selective HAT medium containing
aminopterin and thymidine (17), supplemented with
10%c fetal calfserum, 100 units of penicillin/ml, 100
lAg of streptomycin/ml, 1 ,Ag of fungizone/ml, 10
units of mycostatin/ml, and
0.225%c
NaHCO3. Thecells were maintained in HAT medium in 100-mm
288
on November 10, 2019 by guest
http://jvi.asm.org/
RESCUE OF EBV
tissue culture plates inanatmosphereof5%OCO2 at
37C.
Somaticcell hybrids. Afterthe D98 cells haddied
and colonies of hybrid cells (D98/HR-1) were
ob-served, thecellsweretrypsinizedandclonedin 35-mm
tissue culture plates. The cells weremaintained
con-tinuouslyin HAT selective mediumat37C in 250-ml
plastictissuecultureflasksthroughoutthestudy.
Treatment withIUDR. The D98/HR-1 cells were
trypsinized and grownon18-mmglasscoverslipsin
60-mmplastictissueculture plates.Whencell
mono-layerswereobtained, the HATmediumwasreplaced
with 5 ml of Eagle medium containing 40 ,g of
IUDR/ml. The cells were incubated at 37 C for 3
days,atwhich time theEaglemediumcontainingthe drugwasreplacedwith normalEaglemedium foran additional3days.
Immunofluorescencetechniques.Both the direct and
indirectimmunofluorescencetestswereusedtodetect
EBV-specific antigens.Coverslipswithmonolayersof
IUDR-treated cells were washed with
tris(hydroxy-methyl)aminomethane (Tris)-buffered saline, pH7.4, air dried, and fixed in acetone for 3 min. For the
directtest, the cellswere rehydratedin Tris and
ad-sorbed with humanserumpositivefor EBV antigens
(by immunofluorescence) previously conjugated to
fluorescein isothiocyanate (FITC) (obtained from
CharlesPfizer &Co.)for 30min,washed three times with Tris, two times with distilled water, air dried,
and mounted in elvanolonglassslides.
Fortheindirecttest,acetone-fixed cells were
rehy-drated in Tris, adsorbed with human EBV-positive
serum (tested on HR-1 cells) for 30 min, washed
three times with Tris, and readsorbed with rabbit
anti-humanimmunoglobulinG(IgG)-FITC(Hyland)
for 30 min. The cells were washed as before and
mounted. Preparations were examined for
EBV-spe-cific fluorescence with a Zeiss microscope with an
ultravioletlightsource.
Serum-blockingexperiments.Coverslipswith
mon-olayer D98/HR-1 cell cultures that had been treated
with IUDRwereprepared, washed,and fixedas
pre-viously described.The cellswereadsorbed with
EBV-specificantiserum for 30min, washedasbefore, and
readsorbedwithEBV-specificantiserumconjugatedto
FITC, washed, dried, and mounted. The cells were
thenexaminedforEBV-specific antigensby usingthe
immunofluorescencemethod.
Electron microscopy. Cultures ofD98/HR-1 cells
weregrownin 250-mlplastictissue culture flasks and
treatedwith IUDRaspreviouslydescribed. At3, 5,7
and 10 daysafter removal ofIUDR, the cell
mono-layers were washed with
PBS, pH
7.3, and scrapedoff the glass with arubber policeman. Any floating
cellsin theculturewerecombined with the cells from
themonolayer andfixedinKamovsky'sfixative (14)
overnight at 4 C. Thespecimens were postfixed for 2 hr with Dalton's chrome-osmium (4) (containing 2%osmiumtetroxide), dehydrated, andembedded in
Spurr's low-viscosityplastic (24).Thin sections were
prepared by using an LKB ultratome; the sections
were stainedwith 0.5% uranylacetatein
50%r0
meth-anolandReynold's lead (22), placed on naked copper
grids, and examined with an Hitachi HU-12 electron microscope at 75 kv.
RESULTS
Generalpropertiesofsomatic cell hybrids. When D98 cells were grown in selective HAT medium, the cells died within 2 to 3 weeks. Briefly, D98
cells do notcontain the enzyme IMP
pyrophos-phorylase and are not able toutilizehypoxanthine in the "scavenger" pathway of deoxyribonucleic
acid (DNA) synthesis. The HAT medium
con-tains aminopterinand thymidine which selectively
inhibits de novo purine synthesis but allows cells
capableofsynthesizingDNA, utilizing preformed
purines, to grow (25). However, after fusing the
D98 cells to the Burkitt lymphoblastoid cell line
HR-1 (acellline which only grows insuspension
invitro),theresultingheterokaryons were able to
growinthe selectivemedium, due to the synthesis
ofthe enzyme IMP pyrophosphorylase mediated
by the genome of the HR-1 parent. The fused
cells developed into somaticcell hybrids and were
cloned. Detailed chromosome studies were
per-formed. The modal numbers of the D98/HR-1
clonesexaminedwere approximately equal to the
sumof the modal numbers of the D98 andHR-1
parental cell lines (R. Glaser and F. J. O'Neill,
Science, inpress).
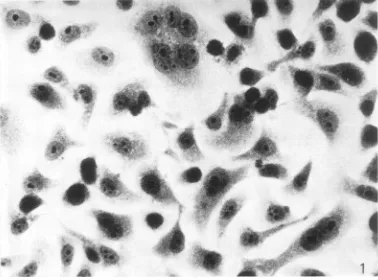
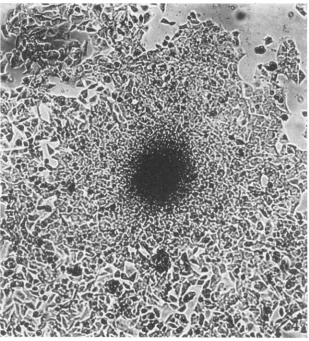
Themorphology of the D98/HR-1 cells, which growasmonolayers, was unlike the morphology
of theD98 orthe HR-1 cells, presumably due to
thecomplement of chromosomessupplied by both
parental lines. The hybrids were larger in size,
and thegrowthpatterns on aglass or plastic
sur-face weredifferent (Fig. 1 and 2). The D98 cells
were contact inhibited (Fig. 3). In contrast, the D98/HR-1 cells were observed to grow frequently
inmultilayered foci (Fig. 4).
EBV specific antigens. When cover
slips
of D98/HR-1 cells were fixed in acetone and testedfor EBV-specific antigens by the direct and
in-direct immunofluorescence test, no evidence of
virus-specific antigenswasfound. These tests were
performed at2, 3,4and 8monthsafter fusion.
Induction ofEBV-specific antigens after treat-ment with IUDR. Coverslip cultures of D98,
hu-manembryoniclung(HEL),and D98/HR-1 cells
clone (cl) 1, 2, 3,and 8 were prepared. Attempts
were made to induce EBV-specific markers by
using 40 ,ug of IUDR/ml in Eagle medium. The
cells weretreated for 3 days after which the
me-diumcontainingIUDR wasreplaced with normal medium for an additional 3 days. Acetone-fixed D98, HEL, and D98/HR-1 cells were tested for EBV-specific antigens by using the direct and
in-direct immunofluorescence tests. The results are
VOL. 10, 1972 289
on November 10, 2019 by guest
http://jvi.asm.org/
._1
_
A;f
t
tai,st:
d
d
.. w
: -:::.
:t
^
tw
w
_
F _
.:- e':.
.:S ",
.-r. y
.;.
, FE __r j_
_SF'
w
ws,
w..
#. w *i
_-t d: ..
.w: ^
[image:3.499.74.452.55.335.2]FIG. 1. Photornicrograplhof D98 cellsstainied with hematoxylini antd eosini. X527.
FIG. 2. Pliotomnicrograph ofD98/HR-1 cellsstainied with hematoxylintandeosini. X527. 290
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.499.74.455.367.642.2]RESCUE OF EBV
''9~~~~~~~~~~~~~O 1
Va JL~~~~~~~~~~~~~~~
IO ~ %* p
* ,.. ; * 4
"q~~~~'%.PA-~~~~~~
Sw~~b~~: 4P~~~
"4*'4t
40a~
-1-.1
41%*
~ ' -~.
-FIG. 3.Photomicrographof D98 cellsgrowingincell culture. X280.
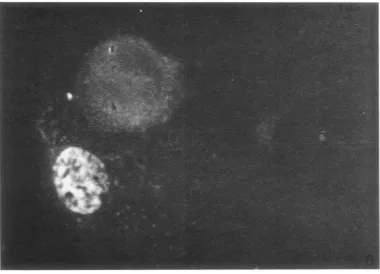
shown in Table 1. All4clonesofD98/HR-1 cells containedEBV-specificantigens bythe direct and indirect immunofluorescencetestwithavarietyof human sera (pretested on HR-1 cells). The sera
werefrompatientswith infectiousmononucleosis
or with nasopharyngeal carcinoma. Cytoplasmic
and nuclear fluorescence was observed (Fig. 5
and
6)
infrom1 to20% of thecells. Normal anddrug-treated
D98 and HEL cells under thesametest conditions were
negative.
Serumblockingtests.Toobtain additional data
onthe
specificity
of the reactions observed,serumblocking tests were performed. D98/HR-1 cells,
grown on cover slips and treated with IUDR,
were exposed to serum containing antibodies to
EBV. This was followed by exposure to serum
containingantibodiestoEBV
conjugated
toFITC(used in the direct immunofluorescence
test).
Re-actions wereobserved incellswhere theprimary
(blocking)
serum was used at adilution of 1:10,but no EBV-specific antigens were observed
by
fluorescence when undiluted EBV antiserum was used to block thereaction.
Todetermine if the EBV antiserum used in the serum blocking experimentswould block the im-munologic reaction in a nonspecific manner, it was tested on rabbit kidney (RK) cells infected with herpes simplex virus type 2 (HSV-2). The EBV-positive serum was adsorbed on acetone-fixed monolayercell cultures ofRKcells infected with HSV-2 (pretested with rabbit anti HSV-2 antiserum and found positive for HSV-2-specific antigens by immunofluorescence). The cells were then adsorbed with rabbit anti-HSV-2 antiserum followed by horse anti-rabbit antiserum conju-gated to FITC. The EI3V-specific antiserum did
not interfere with the detection of the
HSV-2-specific antigens.
Synthesis of virus particles. When cultures of D98/HR-1 cells were examined at 3, 5, 7and 10
days afterseedingin tissue cultureflasks, novirus
particles were observed. However, when D98/
VOL."
10, 1972 291on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.499.91.398.77.414.2]GLASER AND RAPP
FIG.4.Potom aWh o growti-ng in ce re. X280n,
FIG.4.Photomicrograph of
D981HR-1
cellsgrowiniginl cell culture. X280.HR-1 cells previously treated with IUDR were
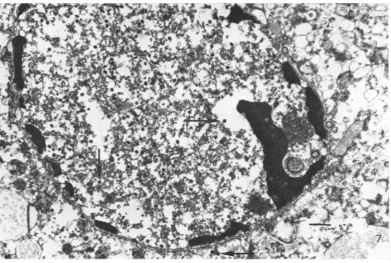
examined,virusparticleswereobservedinthe
nu-clei ofdegeneratingcellsondays7and 10(Fig.7).
Both enveloped (inthe cytoplasm) and
unenvel-opedparticleswerefound in many cells examined.
In asecondsetofexperiments, D98/HR-1 cells
werepooledfromseveralflasksandeitherseeded
intotissue culture flasksorgrownoncoverslips.
One set offlasks and cover slip monolayer cell
cultureswastreated withIUDR; the otherset was
notexposedtothedrug. Seven days after removal of IUDR from the treatedcultures, both sets of cell cultures were fixed for electron microscopy
examination. Thecoverslip monolayercellswere
fixed and examined by the direct
immunofluores-cencetest,by the methodalready described.
The D98/HR-1 cells treated with IUDR
con-tainedvirusparticlesin the nucleiof about
10%l,
of the cells examined and some cells exhibitedparticlesin thecytoplasm;however,novirus
par-ticleswereobserved in the untreatedcells. When
the specimens prepared for immunofluorescence
were examined, the cells treated with IUDR con-tained EBV antigens as described previously. No EBV antigens or particles were detected in the untreated cells (Table2).
DISCUSSION
The results presented in this report and in a preliminary report (R. Glaser and F. J. O'Neill, Science, in press) suggest that Burkitt lympho-blastoid cells have been fused andhybridized with
both mouse and human cells. The chromosome
data, along with the alteration in cell morphology and ability to grow ina selective medium, were the criteria used to support evidence of somatic
cellhybridization.
The data obtained from chromosome analysis
from earlierwork suggested that most, ifnotall,
the HR-1 chromosomes were in the nuclei of the D98/HR-1 cells. In addition, EBV-specific
anti-292 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
FIG. 5. Inmunofiluorescencephotomicrograph cf EBVantigenislocalized in the cytoplasm inD98/HR-J cells
treated with40 ,ug ofS-IUDR/ml
f.
r3 daysand thenexaminted3dcays afterthe cells weregr.wninnormalme-diumn. Many oftilecellscontainin1gEBVantigeniswereroun1ded. X)527.
FIG. 6. Immunofluorescence photomicrograph cfEBVantigens localizedin the nucleuisofa D98/HR-I cell
treated with40,ugcfS-IUDR mlfor3daysandthen examined3days afterthe cellsweregrowninnormal
me-dium. Note theevendistributionicfEB Vantigensthroughoutthenuclelus. X 840.
293
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.499.62.443.372.646.2]GLASER AND RAPP
TABLE 1. Iniductioni of EBV-specific antigens in
D98/HR-J cells treated withIUDR asmeasured
by the immuniofluorescenice test
Immunofluorescence-Avg percent ofpositivecells Cells
HEL'
HEL + IUDRe
D98
D98 + IUDR
D98/HR-1
Clone I
Clone 2 Clone 3 Clone 8
D98/HR-l + IUDR
CloneI Clone 2 Clone 3 Clone 8 Directa test 0 0 0 0 0 0 10-20 10-20 10-20 10-20
Indirecttestsera
la 2b 3'
0 0 0 0 0 0 0 0 0 0 0 0 0 0 5-15 5-15 5-15 5-15 0 0 0 0 1-2 1-2 1-2 1-2
a Serums(diluted1:10)frompatientswith
naso-pharyngeal carcinoma.
bSerum (diluted 1:5) from a patient with in-fectious mononucleosis.
cSerum (diluted 1:2) from a normal patient knowntobenegativeforantibodies againstEBV.
dHumanembryonic lungcells.
e 40jig/ml.
gens, as determined by the immunofluorescence
tests, have beeninduced in thehybrid cellswhen
the cells weretreated with IUDR. Thereare
pre-liminary results (Glaser, unpublished observations)
that induction can also be accomplished with
5-bromodeoxyuridine (BUDR). The data suggest
thatatleastpart ofthe EBVgenome was
trans-ferred from the HR-1 cells to the hybrids and
has persisted for 10 months without expression
of EBV markers, as determined by electron
mi-croscopy and by immunofluorescence. These
re-sults support current concepts on the intgerated
state of the EBVgenome (11). Cloning and
nu-cleic acid hybridizationdatasupportthe concept
thatthe EBVgenomecanpersistwithout
expres-sion for long periods oftime in what had been
presumed to be EBV-negative cells (19, 20). To
furthersupport thisconcept,preliminary data
in-dicate that thereareseveral EBVgenomesineach
D98/HR-1 cell,asdetermined by nucleic acid
hy-bridization experiments now being performed
(M. Nonoyama, personal communication).
An important conclusion can be drawn from
thedatapresented. Namely,the EBVgenomecan
persist and can be activated to synthesize virus
in a cell type other than a lymphoblastoid cell. The possibility exists that the lymphoblastoid cells, in which the EBV is found invivo, maynot
be the sole site of infectious virus replication, an
observation already made in the case of the Marek's disease herpesvirus (2).
Otherinvestigators have reported the induction of EBVantigens in "normal" lymphoblastoid cell lines with BUDR (10). In thepresenceofBUDR, both nuclear and cytoplasmic antigens were de-tected. In addition, ribonucleic acid (RNA)
vi-ruses have also been induced in cell lines with
BUDR or IUDR (1, 18). Thus, the results
pre-sented here concerning induction of EBV
anti-gensin somatic cellhybrids reveal that induction of virusin hybrids mayfollowa pathway similar
to that invirus-transformed cells.
The sera used in the indirect and direct im-munofluorescence tests were known to be highly specific. They had been tested against cells in-fected with other herpesviruses and RNA tumor
viruses as well as avariety of celllines in this
lab-oratory and at Pfizer & Co. (K. Traul,personal
communication). Thedata fromthe serum-block-ing experiments and the induction experiments with D98 and HEL cells also support the
speci-ficity of the antigens observed in the D98/HR-1
cells.
There was no emperipolesis of the HR-1 cells by theD98 cells asdetermined bybrightfield and phase-contrast microscopy, discounting the possi-bility ofcarry-over oflymphoblastoid cells. To further support this, no evidence of the
persist-ence ofHR-1 cells was found by visual
observa-tions and bychromosome analysis when the cells
were cloned. Furthermore, the cells that are posi-tive in theimmunofluorescence test and by
elec-tronmicroscopy areepithelial-like in morphology. Whether the D98/HR-1 cells are transformed
isstill not clear. Thehybrid cells grow in
multi-layers, indicating a loss of contactinhibition. The D98 cells have never been observed to do this. However, further work is necessary to clarify this point.
Theproduction of EBV particles in the IUDR-treated cells clearly shows that the EBV genome maintained in the hybrid cells are complete enough to code for EBV DNA and capsid pro-teins, since complete virus particles with dense cores were found. Whether enough information is presenttocode for the synthesis of "infectious"
EBV with a higher oncogenic potential than the
EBV presently obtained from lymphoblastoid cells remains to be determined. If infectious, the
propertiesofthis virus and its ability to replicate
or transform various cell types will represent an important extension of this work.
294 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
RESCUE OF EBV
295
FIG. 7.Electronz micrograph of D98/HR-1cells treated with 60 jig of
S-IUDRI'ml
7days after replacemenzt of medium conitaining IUDR with ntormalmedium. Noteparticles with densecoresin the niucleus and cytoplasm (arrows). Barrepresenits I,um.TABLE 2. Iniductionz of EBV-specific anttigenis antd
virus particlesinD98/HR-1 cells treated with
5-iododeoxyuridinie (IUDR)
CellsCells Presence of virus
~~antigensa
Presenceparticlesof virusD98/HR-1 None detected None detected
D98/HR-1 + Present Present
IUDRb
Serum(diluted 1:10)fromapatientwith
naso-pharyngealcarcinoma.
bAt 60
jig/ml
for 3 days; cells were fixed forelectron microscopy 7 days after the removal of
IUDR (performedat37C).
ACKNOWLEDG MENTS
We are grateful to Patricia Nelson and Ross Farrugia for excellenttechnicalassistance. Wethank Karl Traul andGuyde Thefortheseraused in thestudy.
Thisstudy wasconducted under Public Health Service con-tract 70-2024 within the Special Virus Cancer Program of the Nationial Cancer Institute.
LITERATURE CITED
1. Aaronson, S. A., G. J. Todaro, and E. M.Scolnick. 1971. Induction ofmurine C-typeviruses from clonal lines of virus-freeBALB/3T3cells.Science174:157-159.
2. Calnek, B. W., H. K. Adldinger, and D. E. Kahn. 1970. Feather follicle epithe!ium; a source of enveloped and
infectious cell-free herpesvirus from Marek's disease.
Avian Dis. 14:219-233.
3. Coon,H. G., and M. C. Weiss. 1969. Sendai producedsomatic cellhybrids betweenLcell strains and between liver and L cells. Wistarlnst. Symp. Monogr. 9:83-96.
4. Dalton, A. J. 1955. Achrome-osmium fixative for electron
microscopy. Anat. Rec.121:281.
5. Defendi,V., B.Ephrussi, H. Kcprowski, and M. C. Yoshida.
1967. Properties of hybrids between polyoma-transformed
and normal mouse cells. Proc. Nat. Acad. Sci. U.S.A. 57:299-305.
6. Dubbs, D.R.,andS.Kit.1968.Isolation of detectivelysogens
fromsimian virus 40-transformedmousekidney cultures. J.Virol. 2:1272-1282.
7. Engel, E., B. J. McGee, and H. Harris. 1969. Cytogenetic and nuclear studies on A9 and B82 cells fused together by Sendai virus: the early phase. J. Cell Sci.5:93-119. 8. Epstein, M.A.,B. G. Achong,and Y. M. Barr. 1964. Virus
parniclesinculturedlymphoblastsfromBurkitt'slymphoma.
Lancet(London) 1:702-703.
9. Epstein, M.A., G. Henle,B. G. Achong,andY. M. Barr. 1965. Morphological and biological studies on a virus
in cultured lymphoblasts from Burkitt's lymphoma. J.
Exp.Med.121:761-770.
10. Gerber, P.1972.Activation ofEpstein-Barrvirusby
5-bromo-deoxyuridinein"virus-free"humancells. Proc. Nat. Acad.
Sci.U.S.A.69:83-85.
11. Hampar, B.,J. G.Derge,L. M.Martos,and J. L. Walker. 1972.SynthesisofEpstein-Barrvirusafteractivation of the
viralgenomeina"virus-negative"humanlymphoblastoid
VOL. 10, 1972
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.499.48.244.399.511.2]GLASER AND RAPP
cell (Raji) mi-iade resistanit to 5-bromodeoxyuridine. Proc. Nat.Acad.Sci. U.S.A. 69:78-82.
12. Harris, H., J. F. Watkins, C. E. Ford,and G. 1. Schoefl. 1966. Artificial heterokaryons of animalcellsfromdiffi-rent species. J.CellSci. 1:1-30.
13. Henle, G.,and W. Henle. 1966. Immnunofluorescence in cells derived fi-omii BoLrkitt's lymphoma. J. Bacteriol. 91:1248-1256.
14. Karnovsky, M. J., 1965. A formaldehyde-glutaraldehyde fixative of high osmolality for uLsein electron miicroscopy. J.CellBiol. 27:137A-13SA.
15. Klein, G., U. BreguLla, F. Wiener-,and H. Har-r-is. 1971. The analysis of miialignancy by cell fusion. 1. Hybrids betwceni tumoutrcellsandLcellderivativos. J. Cell Sci. 8:659-672. 16. Koprowski, H.,F. C.Jensen,and Z. Steplewski. 1967. Acti-vation ofpr-oduction of infectious tumor virus SV40 in heterokaryoni cultures Proc. Nat. Acad.Sci. U.S.A. 58:127-133.
17. Littlefield, J. W. 1964. Selection ofhybridsfr-ommatingsof
fibroblasts in vitr-o and their presumbed recombiniants. Science 145:709-710.
18. Lowy,D.R.,W.P.Rowe,N.Teich,and J. W.Hartley. 1971.
Murine leukemnia virus: high-frequency activationin rtitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science 174:155-156.
19. MauLrer, B.A., T. Imamiiura, and S. M.Wilbert. 1970. Inci-denceof EBvirus-con!ainingcells in pr-imary andsecondary
clonesof several Burkitt lymphomiia celllines. Cancer-Res. 30:2870-2875.
20. Nonoyamna, M., and J. S.Pagano. 1971. Detectionof
Epstein-BarrviralgenomeinnonproduLctive cells. Nalute(London) 233:103-106.
21. Okada,Y., and F. Murayamiia. 1968. FusionofcellsbyHVJ requirement of concentration of virus particlesat thesite ofcontactoftwocells forfusion. Exp. Cell Res. 52:34-42. 22. Reynolds, E. S. 1963. Theuseof lead citrateathigh pH as an electron-opaque stain in electr-onimicroscopy. J. Cell Biol. 17:208-212.
23. Scaletta, L.J.,and B.EphruLssi.1965.Hybridization ofnotmal
and neoplastic cells in r,itro. Nature (London) 205:1169. 24.Spurr, A. R. 1969. A low-viscosity Epoxy resin embedding medium for electron microscopy. J. UltrastruLct. Res. 26:31-43.
25.Szybalski, W., E. H. Szybalska, and G. Ragni. 1962. Genetic studies with hLLumian cell lines. Nat. CancerInst. Monogr. 7:75-89.
26. Weiss, M. C.1970. Further studiesonloss of T-antigen from
somliatic hybrids between mouse cells and SV40-trans-formiiedhuman cells. Proc. Nat. Acad. Sci. U.S.A. 66:79-86. 27. ZurHausen, H., and H. Schulte-Holthausen. 1970.Presence of EBvirus nucleic acid homology ina"virus-free"lineof
BurkittttlmouLr cells.Natutie (London) 227:245-248.
296 J. VIROL.