Copyright 0 1977 AmericanSociety forMicrobiology PrintedinU.S.A.
Inhibition of Bacteriophage M13 and
4X174 Duplex DNA
Replication and Single-Strand Synthesis
in
Temperature-Sensitive
dnaZ
Mutants of
Escherichia coli
WILLIAM G. HALDENWANG AND JAMES R. WALKER* Microbiology Department, University of Texas, Austin, Texas 78712
Received forpublication8October1976
AfunctionaldnaZ product, known to be essentialfor host DNA
polymeriza-tion and for the synthesis of M13 and
OX174
parental replicative-form (RF)DNA, isrequired also for RF replication and single-strand synthesis bybothof
these phages. Allthree stages of M13and4X174DNAreplication (parentalRF
formation, RFreplication, andsingle-strand synthesis) are inhibited in dnaZts
mutants at elevated temperatures. Inaddition, the thermolabile step in M13
parentalRFformationappears to occurafter RNApriming; i.e.,thesynthesisof
M13 RF DNA proceeded when adnaZts mutant, infected at anonpermissive temperature, was transferred to a permissive temperature in the presence of rifampin.
Thesingle-strand (SS) DNA phages M13 and
4X174requirehostDNAreplicativefactors for
the synthesis oftheir replicative intermediate
and progeny viral DNAs (13, 17). Several Esch-erichia coli mutants, withdefectsinhost chro-mosomereplication,aredeficientingene prod-uctsthat arerequiredby these phages in one or more stagesof theirDNAreplication cycle: pa-rentalreplicative-form (RF) synthesis, RF
rep-lication, or the asymmetric synthesis of
prog-enyviral strands (forasummary, see 7).
We havepreviously presentedevidence that
theproduct of the DNAsynthesis gene,dnaZ,
isneededinvivo for the first stage of M13 and
OX174
replication, the conversionofinfecting viral SS DNA into duplex parental RF DNA (10); Wickner and Hurwitz (21) reported that the dnaZ gene product is required in vitro for DNA polymerase mI activity on primed SS M13,4bX174,
andST-1templates. The observa-tionthat the dnaZfunctionisrequired for DNApolymerase III-catalyzed DNAsynthesis in
vi-trosuggeststhat the dnaZproductmay partici-pate in vivo in all stages of M13 and
OX174
replicationthat have been shown to beeffected
byDNApolymerase III,i.e., RFreplicationand
SSsynthesis (3, 8, 18) (in additiontoparental
RFformation).
In this communication, we report that the replication of M13 and
4X174
RF DNA and the synthesis of progeny SS DNA are inhibitedin dnaZts mutants at nonpermissivetempera-tures. In addition, the dnaZ function in M13
parentalRFformationinvivoappears tooccur
at a step after RNA priming. These data
sug-gest a function for the dnaZ protein in the elongation phase ofinvivoDNAsynthesisorin
aninitiationstepthat occursafterthepriming
reaction.
MATERIALS AND METHODS
Bacterial and phage strains. K-12 strain AX727
dnaZts2016 and the 4X174-sensitive K-12 strain
GN727dnaZts2016 were describedpreviously (10). H727 is adnaZts2016recombinant between H5274
(obtainedfrom D. Ray) andHAX727(Hfrof AX727
constructedby F'lac+ integration)and has the geno-type K-12 F- dnaZts2016 thy rpsE tsx. C727 is a
dnaZts2016 recombinant between E. coli C
(ob-tained from R. McKee) and HAX727. It has the
genotypeC F-dnaZts2016rpsEtsx. F'101/C600was obtained from B. Bachmann, the male specific phage M13 from D. Ray,4)X174h8p(retarded lysis)
from C. Earhart, and 4X174am3 (lysis defective)
from R. L. Sinsheimer. AX727 TS+, GN727 TS+,
H727TS+,andC727TS+werespontaneous
tempera-ture-insensitiverevertantsofAX727, GN727, H727,
and C727. F'lac+ derivativeswereobtainedby mat-ing withF'lac/RV (obtainedfrom M. Malamy).
Media. Yeast extract-tryptone (YET) broth has
been described (20). YET brothwas supplemented
with [3H]thymidine at 10 ,uCi/0.12 ,tg per ml for
radioactive labeling. Minimal phosphate medium
was 0.8% nutrientbroth (Difco), 1.5 mM NH40H,
and2.5mMMgSO4(pH 7.0).
Preparation ofphagestocks and marker DNAs. Nonradioactive M13 was prepared by infecting F'101/C600 (4 x 108cells/ml)inYETbroth with M13
at a multiplicityofinfection of50. Aftera 30-min
adsorption periodat30°C, the culture wasdiluted
1:50and growninYET broth at30°C to acelldensity of7 x 108cells/ml. Cellswereremovedbylow-speed
centrifugation, and the phagewasconcentratedby
23
on November 10, 2019 by guest
http://jvi.asm.org/
24 HALDENWANG AND WALKER pelleting as described previously (10). 32P-labeled M13 wasgrown similarly in minimalphosphate me-dium supplemented with 20
tiCi
of carrier free32p043- per ml. After concentration as above, the
phages were purified on high-salt, 5 to 20% neutral sucrose gradients (10) and the viral DNA was iso-lated by cold phenol extraction (2).
OX174am3
and bX174h8pweregrown in E. coli C inYET brothsupplemented with 1.5 mg ofCaCl2per ml. E. coli C cultures (2x 108 cells/ml) were infected with either4X174am3orOX174h8p
at amultiplicity ofinfection of 3 and grown for 2.5 h at 370C. The cultures were concentrated 25-fold into 0.1 M Tris-hydrochloride (pH 8.1) containing 0.5 mg of lyso-zyme per ml and 10 mM EDTA. After a 60-min incubation on ice, lysis was completed by shaking with a fewdrops of chloroform. To decrease viscosity and separate phage from debris, the lysate was drawn through a 0.5-inch (about 1.3-cm), 26-gauge needle. Debris wasremoved by low-speed centrifu-gation. Phage that were to be used as a source of marker DNA (4X174h8p) were gradient purified, and the DNA was extracted by the method of Brown and Dowell (2).Analysis of M13 and
OX174
DNAsynthesis. Ter-mination of isotopeincorporation and lysis of M13-infected cells were accomplished as described by Ray etal. (15). 4X174am3-infectedcells were processed by the protocol of Dumas et al. (4), except that the lysate was incubated with Sarkosyl for 10 min at370Cbefore Pronase treatment, rather than for 16 h at 0°C. Crude lysates of M13- and
OX174am3-in-fected cells were analyzed on high-salt, 5 to 20% sucrose gradients (10). 32P-labeled M13 DNA and unlabeled 4X174h8pDNA wereapplied to the gra-dients as SS markers for M13- andOX174am3-in-fected cell lysates, respectively. The position of the
OX174marker wasassessed by itsbiologicalactivity
in the spheroplast infectivity assay (9). Gradient fractions were collected from the bottom. Samples of 100
Al
were spotted onto GF/A glass fiber filters,which, after drying, were washed with 3 ml of 5%
trichloroacetic acid, dried, and counted.
RESULTS
Role of dnaZ in M13 parental RF forma-tion. An active dnaZproductisrequired for the synthesis of both M13 and
OX174
parental RFDNA(10). InasmuchasdifferentRNA priming
mechanisms are used by each of these phages
(16), itmight be assumed that the dnaZ
prod-uctwouldnotbe involvedinthepriming step of RFsynthesis. This assumption could be tested
inthe M13system. RNA priming on M13
infect-ingSSDNA isbelievedto beeffectedby
rifam-pin-sensitive RNApolymerase (16). Therefore,
ifinfectionofadnaZts mutant by M13 at a
non-permissivetemperature rendered parental RF formationresistant torifampin when the
tem-perature subsequentlywaslowered, itmaybe
concluded thattherifampin-sensitivestep, i.e.,
priming,could be accomplishedintheabsence
ofanactivednaZproduct. Itwaspossibletodo this experiment because the product of the dnaZts2Ol6 allele regains activityimmediately upon temperature reduction even in the
ab-senceofproteinsynthesis (Chuetal.,in
prepa-ration). A culture of F'lac+/AX727 was grown at
300C
and divided into threesubcultures, all of whichwerethenincubated at410Cto inacti-vatethe dnaZproduct. Rifampinwasadded totwoofthese cultures either 5 min before or 5 minafterinfection by M13. AsdescribedinFig. 1, the cultures were then transferred to 30°C
and pulse-labeled with [3H]thymidine. The
third culture wasinfected andpulse-labeledat
41°C to demonstrate that no RF synthesis
oc-curredatthistemperature.
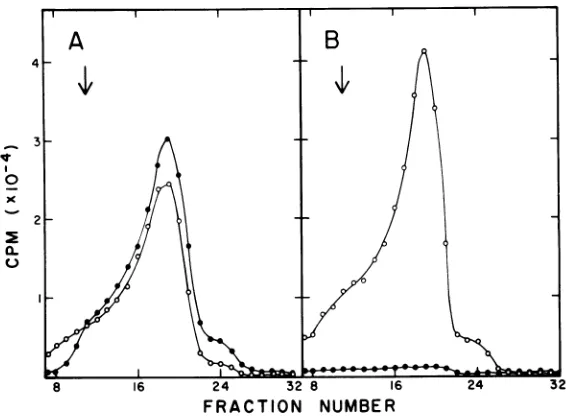
InfectionofadnaZtsculturebyM13at410C resultedin asubstantialamountof RF
synthe-sis upon return ofthe culture to 30°C in the
presenceof rifampin(Fig. 1A).Culturestreated with rifampin before infection yielded no de-tectable peaks ofphage DNA (Fig. 1B). There
was no apparent RF synthesis in the culture
infected andpulse-labeledat41°C(Fig. 1C). We
infer that RNAprimingofinfectingM13
single
strands canoccurinvivo in the absence ofan
activednaZ product.The culture infected with
M13 before rifampin treatment (Fig. 1A)
con-tainedtwopeakswithsedimentationproperties
consistentwith RF IandRF II. The
magnitude
of the RFIIpeak(fraction27)was
unexpected.
Formationof RF II DNAnormallyrequires the
gene 2protein of M13 (5), andthesynthesis of
this protein should have been inhibited in a
rifampin-treated culture. The amount of
la-beled material thatsedimentedasRF IIvaried fromexperimentto experiment; this variation might have been aresult of the length ofthe
410C
preincubation period rather than anin-complete rifampinblockonM13gene 2protein
synthesis. Chloramphenicol, atconcentrations
thatareadequatetopreventRF IIformationin
M13-infected cells(10), failedtoeliminate RF II material fromlysatesofF'lac+/AX727 cultures
thatwerepreincubatedat
410C
andthentrans-ferred to 30°C (data not shown). It is possible
that thematerialformingthe RF IIpeak
repre-sented abnormalDNAsynthesizedinthe
pres-enceofapartiallyreactivated dnaZproductor,
alternatively, RFIbreakdownproductsformed
as aresult ofanucleolytic activitypresentafter the 41°C incubation.
Requirement forthednaZ function in M13
and 4X174 growth afterparental RF
forma-tion. The effect of the dnaZ mutation ofM13
and
4X174
phage growth after parental RF formation was tested by shifting dnaZts andTS+ cultures, which were infected and
incu-J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
M13 AND
OX174
DNA SYNTHESIS IN dnaZts HOSTS M13 RIF 3H 30C5" 4 1I 10 41*C 3HM13
C
300i I"1 1IS"Io
FRACTION NUMBER
FIG. 1. Formation of M13 parental RFat410C byadnaZts culture in thepresence of rifampin. F'lac+I
AX727dnaZtswasgrowntoadensity of3.5 x108cells/ml in YET brothat30°C. Three sampleswerethen
transferredto41°C. Culture A (0)wasincubated for 30 minat41°C andinfected with M13 (multiplicity of infection =100).Five minutesafter infection, rifampinwasaddedtoaconcentrationof200 pg/mland the
incubation of 41°C was continued for an additional 5 min. The culture was thenpulse-labeled with
[3H]thymidine for 10 minat30°C. Culture B (@)wassimilarly treated;however, rifampinwasadded5min
before infection by M13.Culture C(U)wasnotexposedtorifampin butwasinfected and pulse-labeledat41°C. Cellswereharvested, lysed, and sedimented through high-salt neutralsucrosegradients. Sedimentation in
this andall othergraphs wasfrom righttoleft. The verticalarrowindicates the sedimentation position of
P2P]labeledM13 SS DNA.
bated at a permissive temperature (to allow
parental RF formation), to either 41 or 40°C
(nonpermissive for DNA synthesis).
Cultures of F'lac+/H727 dnaZts and TS+
were grown and infected with M13 at 300C.
After 30min,half of each culturewasshiftedto
41°C. Although phageproduction in the dnaZts
and TS+ cultures was similar at 30°C, the
dnaZts culture terminated phage synthesis
after 30min at410C (Fig. 2A). M13 growthwas
slowed in the revertant culture at 410C;
how-ever, phage production continued throughout
the course of the experiment. (The previously
reported[201 immediate cessation of M13 phage
production in another dnaZts strain [AX727]
appears to result from an immediate halt of
M13 SS synthesis, by temperature elevation,
whichprobably is unrelatedtothe dnaZ
muta-tion. M13 SS synthesis alsowas inhibited,
al-though transiently, after a shift ofan AX727
TS+ cultureto 41°C [unpublished data].)
Shifting C727 dnaZts and TS+ cultures to
400C after they were infected with qbX174am3
andincubated at350C (selectedasthe
permis-sive temperature) for 15 min inhibited phage
growth only in the dnaZts culture (Fig. 2B).
Although phage productioninthe dnaZts
cul-ture wasdetectable at 400C foras longas 105
min, the rate ofphage production, relativeto
that in therevertantculture, appearedto slow
after 15min atthis temperature. Total phage
yield in the dnaZts culture after 165 min at
400C was approximately 1/100 the yieldinthe
revertant culture. Phage growth in these cul-tures was comparable at 350C. Inhibition of
4X174growthwaspreviouslyobservedby
Tak-eto (19) in AX727 (dnaZts) spheroplasts that
weretransformed with 4X174 RFDNA.
41*C
30a
41C
RIF M13 3H 30C25 5 4 1"
A
B
16
cm 12
1 0
X 8
04 0 4
25
VOL. 22, 1977
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.508.54.454.53.359.2]26 HALDENWANG AND WALKER
-J
2
0J
C,
0
-j
ho
I-0
cl
K
[image:4.508.113.400.69.274.2]
I-MINUTES
FIG. 2. Growth of M13 and 4X174am3 in dnaZtsand TS+ cultures. (A) M13. Culturesof F'lac+/H727
dnaZts and TS+were grown at300CinYETbrothsupplemented with2 pgofthymine per ml to a densityof2
X108cellslmlandconcentrated to 2 x 109 cells/ml in buffer (0.01 Mpotassium phosphate[pH7.0] and 0.01 M
MgSO4).M13 was added(multiplicityof infection =50),and the suspension was incubated for 30 min at
300C. Thecells were then washed twice inbufferand diluted to aconcentrationof 2 x 103cells/mlin YET broth (plus 2 pgof thymine per ml) at300C. After30 min at thistemperature,halfofeach culture wasshifted
to41°C.Samples were taken at the times indicated andimmediatelyplatedonF'101IC600.dnaZts cultureat 30°C (A) and at 41 °C(A); TS+ culture at 30°C (0) and at 410C (@). (B)OX174. Culturesof C727 dnaZts and TS+ were grown at 30°C in YET brothsupplementedwith 1.5 mgof CaCl2 per ml to a celldensity of2 x108
cells/ml, concentrated10-fold into 0.01 MMgSO4and 0.01 MCaC12, andinfectedwith
OX1
74am3(multiplic-ity ofinfection =5)for 15 min at30°C. The cells were thenwashedtwice inthe same solution and diluted to2 x 107cellslml in YET broth(containing 1.5 mg ofCaCl2per ml) at35°C. After 15min, halfof each culture wasshiftedto40°C.Samples of0.1 ml weretransferredto1.9 mloflysis buffer(0.1 M Tris[pH8.11,0.5 mgof
lysozymeperml, and 10mMEDTA) at the indicated times.Samplesinlysis bufferwereincubatedfor60 min
onice, vortexed with adropofchloroform,and titered on G727 TS+ (a host that can suppress the
OX1
74am3 mutation). Symbols are as in (A).We conclude that the dnaZ function is
re-quired by both M13 and
OX174
for continualphageproduction after the formation of
paren-tal RF DNA. It is unclear whether the
differ-ences in the rate of termination of M13 and
X174am3 phage growth at nonpermissive
temperatures represent differentrequirements
for the dnaZ product. Given therapid rate of
4PX174
growth relativetothatofM13, however, prolongedOX174
phage production at 400Cmight reflect packaging of previously
synthe-sized 4X174viral DNAorpossibly slower
inac-tivation of the dnaZ product at 400C than at
410C.
Requirement for the dnaZ function inM13
and
OX174
RF replication. To determinewhich stages ofphage DNA replication, after
parental RF formation, were inhibited in the
dnaZts mutants, we firstexamined RF -- RF
replication. F'lac+ derivatives ofdnaZts and
TS+ cultures were infected at 350C with M13
and heldatthistemperaturefor 10min.
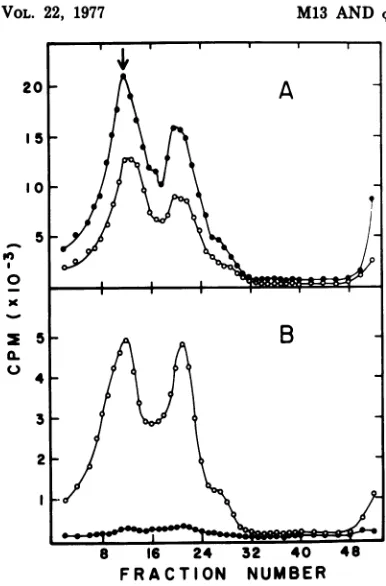
Pulse-labelingduring this perioddemonstrated (Fig.
3A) the formation of RF DNAinboth the mu-tant and revertant cultures atthis permissive temperature. Duplicate cultures thatwere sim-ilarly infected andheldat
350C
for10 minwere then incubated for an additional 15 min at either 35 or410C.
Pulse-labeling the culturesthat wereheld at
350C
revealed (Fig. 3B) RFand SS DNAsynthesisinbothtsand TS+
cul-tures;however, incubation at41°C terminated
M13-specific DNA replication in the dnaZts
mutant(Fig.
30).
The asymmetric synthesis of M13 SS DNA beginsatapproximately10minafter infection (13). Therefore, itispossible thatasignificant
amount ofRF DNA present in Fig. 3B and C
represented intermediatesinSSsynthesis. The inhibition ofDNAsynthesisinthe ts culture at
410C
may thusreflectadnaZproductrequire-ment in asymmetric synthesis and not RF -*
RFreplication. This ambiguitywasresolved by
treatingM13-infected dnaZts and TS+ cultures
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
4X174 dnaZts
1.5 I.0 0.5
I.
0
x
0.
-20 1 5
50
5
10
8 16 24 32 40 48
[image:5.508.56.246.64.316.2]FRACTION NUMBER
FIG. 3. Replication of M13 RF DNA in dnaZts andTS+ cultures. F'lac+/H727 dnaZts and TS+
cul-turesweregrownto3.5 x108cellslml inYET broth
supplementedwith 2pgof thymidine, transferredto
35°C,andinfectedwithM13(multiplicity of infection
=100). The verticalarrowindicates thepositionof
32P-labeled M13 SS DNA marker. (A) dnaZts (@) andTS+ (0) cultures pulse-labeled for 10min after infection at35°C. (B) dnaZts (@)andTS+ (0)
cul-tures, infectedasabove, were incubatedat35°C for 25 minafter the addition of M13 andpulse-labeled for an additional 5 min with [3H]thymidine. (C)
dnaZts (@) and TS'(0)culturesweretransferredto
410Caftera10-min infection periodat35°C. Fifteen minutesafterthetemperatureshift, thecultureswere
pulse-labeled foranadditional 5 min.
with chloramphenicol before pulse-labeling.
Chloramphenicol, atconcentrations of10 to 30
,ug/ml, selectively inhibits M13 SS DNA
syn-thesis while permiting the replication of M13
RF DNA (14). Cultures of F'lac+ derivatives of
dnaZtsand TS+ strainswereinfected with M13
at 350C and, after 10 min, a portion of each
culturewasshiftedto400C.Afteranadditional
10min (20 min postinfection), chloramphenicol
(30 ,g/ml) was added to each of the cultures
and the incubation was continued for 10 min.
Pulse-labeling after chloramphenicol
treat-mentdemonstrated the synthesis of RF DNA in
dnaZts and TS+ culturesat35°C (Fig. 4A) and
in the TS+ culture at 40°C (Fig. 4B) but
vir-tuallyno M13 RFsynthesis in the dnaZts
cul-ture at400C (Fig. 4B).
Similar results were obtained with
OX174am3-infected
cells. Chloramphenicol, at aconcentration of 30,g/ml,
permits the forma-tion and replication ofOX174
RF DNA while inhibiting SS DNA synthesis (11). Chloram-phenicol-treated (30 ug/ml) dnaZts and TS+ cultures of E. coli C were infected withOX174am3
at 350C. A portion of each culture wasshiftedto400C after5min. Duringthis 5-mininfection intervalat350C,RF Iand IIwere formed (datanotshown). After a20-min incu-bation periodat 35 or400C (25 min post-infec-tion), the cultureswerepulse-labeled for5min with[3H]thymidine. Incubation of the TS+cul-ture at40'Cresultedin a
twofold
increase of 3HincorporationintoRF DNAoverthatobtained
at350C, whereas incubation of the dnaZts cul-ture at 400C decreased incorporation to less than3% ofthe level in a 350 C control culture (Table 1).
The dnaZ productis, therefore, required for RFreplicationbyboth M13and
OX174.
Requirement for thednaZ function in M13 and gX174 SS DNA synthesis. To investigate the requirement for the dnaZ productin M13 SSsynthesis, F'lac+ derivatives of dnaZts and TS+ cultureswereinfectedat300C andheldat
thistemperaturefor120min, followedby
trans-fer of half of eachcultureto410C. After15min ofadditional incubation, the cultures were la-beled witha 15-min [3Hlthymidinepulse. Incu-bation at 410C enhanced the incorporation of [3H]thymidine into both SS and RF DNAinthe TS+ culture over that obtained at 300C (Fig. 5A), but this treatment dramatically reduced
the amount of incorporation into these DNA
species in the dnaZts culture (Fig. 5B). The small amount of labeled SS and RF in the dnaZts culture thatwasincubated for15min at 410C was completely eliminated by a 30-min incubation period at this temperature. DNA
synthesis in the revertant culture was
un-changed bythis additional incubation at410C
(datanot shown).
Asimilartemperatureeffectwasobserved in
4OX174am3-infected
dnaZts cultures (Table 2). ts and TS+ cultures were infected and incu-bated for45 min at 3000. Half of each culture wasfurtherincubatedateither30 or400Cfor30 min, followed by pulse-labeling with[3Hlthymidinefor5min. Asaresult of the
30-min incubation at 400C, the number of 3H
countsthatsedimented as SS 4X174 DNA
de-creased, relativetothe300Cincubationdata, by 16%intherevertant culture and by94% inthe dnaZts culture.
It is likely that the terminationofM13 and
OX174
SS synthesis is a direct result ofare-22, 27
on November 10, 2019 by guest
http://jvi.asm.org/
28 HALDENWANG AND WALKER
4,
0-8 16 24 32 8 16 24 32
[image:6.508.115.398.67.275.2]FRACTION NUMBER
FIG. 4.Replicationof M13 RF inchloramphenicol-treateddnaZts and TS+ cultures. Theverticalarrows indicatethe position of M13 SS DNA. (A) dnaZts (O) and TS+ (0) cultures wereinfectedas inFig.3 and incubated at35°Cfor 20 minafterthe addition of M13. Thecultureswere then treated withchloramphenicol
(30pg/ml)for 10min,followedbypulse-labelingfor 5 min with[3H]thymidine.(B) dnaZts (@) and TS+ (0)
cultures were transferred to 40°C 10 min after infection at 350C. Ten minutes after the shift to 40°C,
chloramphenicol(30pg/ml)wasadded and the 40°C incubation wascontinuedfor anadditional10min. The
cultures were thenpulse-labeledfor 5 min with[3H]thymidine.
TABLE 1. Replication of Xl74am3 RF indnaZts and TS+ cultures
Total RF I and II synthesis(cpm)
Host
350C
400C
Ratio40/350C
dnaZts
12,100
300 0.025TS+ 10,700 22,800 2.1
a C727dnaZts andTS+culturesweregrown to 3
x 108 cells/mlinYET broth (containing 1.5mgof
CaCl2perml)at300C,concentrated10-foldinto 0.01
M MgSO4 and 0.01 M CaCl2, and infected with
OX174am3 (multiplicity ofinfection = 3) at
350C.
After 10min, thecells were diluted 1:10 into YET
broth (plus1.5 mgof CaCl2per ml)containing30
jig
ofchloramphenicolperml andincubatedfor 5 min
at350C.Half of each culturewastransferredto400C,
and theincubationwascontinued for20min. Allof
the cultures were then pulse-labeled with
[3Hlthymidine
for 5 min attheirrespectivetempera-tures,harvested,lysed, andanalyzed.Thedata pre-sented foreach culture are the sum of the
trichloroa-ceticacid-insoluble3Hcountsthatsedimented as RF Iand II DNA.
quirement forthednaZproductinthisreaction rather than asecondary effect ofthe dnaZ re-quirementinRFreplicationwith asubsequent depletion ofanecessaryRFpool. M13 RF repli-cation, butnotSSsynthesis, requiresthednaG product (15);arequirementforthednaC
prod-ucthasbeenobserved in
4X174
RFreplicationbut not in SS synthesis (11). In both of these
examples, shifting
phage-infected
temperature-sensitive cultures of these mutantsto nonper-missive temperatures after RFreplication
per-mittedlarge amounts of SSsynthesis thatwere
comparable eitherto thoseobserved in
revert-antculturesatthat temperature(15)or tothose
obtainedinthemutantculturesat apermissive
temperature (11). Shifting M13- or
OX174-in-fected dnaZts cultures to nonpermissive
tem-peratures after RF replication dramatically
curtailedSSsynthesisrelativeeitherto mutant
culturesat a permissive temperatureor to
re-vertantculturesattheelevatedtemperature.
Thus, it appears that the synthesis of M13 and
(PX174
SS DNA requires an active dnaZ product.DISCUSSION
Basedonthedatainthiscommunication and
those previously reported (10), it appears that
thednaZfunction isrequiredby bothM13and
OX174
in all stages of their DNA replication cycle. Conversion of SSDNA toRF,RFreplica-tion, and the synthesis of progeny single
strands are inhibited in dnaZts mutants at
nonpermissive temperatures. Thefindingthat
anactivednaZproductisdispensableforaloss
in rifampin sensitivity in the M13 SS -- RF
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
M13 AND OX174 DNA SYNTHESIS IN dnaZts HOSTS
20
15
10
Ko
04
3
2
24 32 40 48
FRACTION NUMBER
FIG. 5. Synthesis of M13 single-strand DNA in dnaZts and TS+ cultures. F'lac+IH727 dnaZtsand TS+ culturesweregrownto3 x108 cells/ml in YET
broth(containing2pgof thymidineperml)at300C and infected with M13 (multiplicity of infection =
100). Aftera30-min infection period, the cellswere
diluted 1:4 intofresh YET broth (containing 2pgof thymidineperml)andthe incubationwascontinued for 90 min. Theverticalarrowindicates theposition
of M13 SS DNA. (A) TS+ culture. After the 90-min incubationperiod, half oftheculturewastransferred
to410C. Bothsubcultureswereincubatedfor15min
and then pulse-labeled with [3H]thymidine for an
additional15min.Symbols: (0)300C; (a)410C. (B) dnaZts culturetreatedasin(A). Symbols: (0)300C;
(0) 410C.
conversion indicates thatthednaZ function is not neededfor RNA priming andpredicts that it would be required for DNA synthesis on RNA-primed templates. Wickner and Hurwitz have recently demonstrated, by using an in vitro complementation assay, that the dnaZ product is an elongation factor required by
DNApolymeraseIIIforduplexDNAsynthesis
onRNA-primedSSphage templates (21).Inthe
in vitro reaction, using purified proteins, the
dnaZ productwasneeded fornucleotide incor-porationonprimedtemplates byDNA polymer-ase II and III butwasnot absolutely required byDNApolymerase I(21).
Therole of thednaZproductinDNA
polym-TABLE 2. Synthesisof
OX1
74am3
SSDNA indnaZts and TS+ cultures
SSDNAsynthesis (cpm)
HostHost30°C00 40040°C Ratio300C40/
dnaZts 55,600 3,500 0.06
TS+ 66,600 56,200 0.84
a C727dnaZts and TS+cultures were grown and
concentrated as in Table 1 and infected with
4PX174am3(multiplicityof infection = 3) at 300C for
15 min.The cells were diluted1/10 intoYETbroth
(containing 1.5mg ofCaCl2 per ml) and incubated
for 45 min at300C. Half of each culture wasthen
transferred to 40"C, incubation was continued for30
min, andallofthesubcultures werepulse-labeled
with [3H]thymidine for5 min.The3Hincorporation
intoSSDNA wasdeterminedas inTable1.
eraseIII activitydoesnotappear tobeconfined
to parental RF formation. Replication of RF DNA and the asymmetric synthesis of viral single strands, processes requiring an active dnaE product (3, 8, 18), terminate when the dnaZts product is inactivated. As withparental RFformation, however,ourdata donot distin-guish between a requirement for the dnaZ function in ongoing polymerization and in an initiation event in DNA synthesis that may follow RNApriming. 4X174 RF replication and SS synthesis terminate in the absence ofthe E. colidnaG product (12), which has been reported to possess rifampin-insensitive RNA polymer-aseactivity(1). M13 RF replication requires the dnaG product (15) and is also inhibited by ri-fampin (6). M13 SS synthesis does not require the dnaGproduct (15) and is rifampin resistant
for atleasttwosuccessiverounds of SS
synthe-sis (6). It could not be determined, however,
whether a rifampin-sensitive initiation event wasrequired after severalrounds of SS synthe-sis(6).Thus,itispossible that all stages ofM13 and
OX174
DNAreplication require RNA prim-ingevents. Ittherefore follows that the dnaZ product may play a roleinthe initiation of theDNApolymerizationreaction(e.g., facilitating
the formation of a DNA polymerase/primer complex) rather than being needed for the ac-tualpolymerization reactions involved. The
ob-servation that DNA polymerase III, without
elongation factors, is able to incorporate
nu-cleotides on DNase-treated (21) or gapped
du-plextemplates (22) makes this possibility more attractive.
ACKNOWLEDGMENTS Wethank theindividuals indicated for strains. This work wassupported by American Cancer Society grantNP169and,inpart,by Public Health Service grant 29
VOL. 22, 1977
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.508.55.248.49.342.2]30 HALDENWANG AND WALKER
A108286 from the National Institute ofAllergyand Infec-tiousDiseases. W.G.H. wassupported by Public Health Service training grants 5T01 GM00337 and 1 T32 GM07126, bothfrom theNational Institute ofGeneral Medical Sci-ences;J.R.W. wassupportedduringaportionof this work by Public Health Service research career development award GM29413 from the sameInstitute.
LITERATURE CITED
1. Boucht,J. P., K.Zechel, and A.Kornberg.1975. dnaG gene product, a rifampicin-resistant RNA polymer-ase,initiates the conversionofasingle-stranded coli-phage DNA to its duplex replicativeform. J. Biol. Chem. 250:5995-6001.
2. Brown, L.R., andC. E.Dowell. 1968. Replicationof coliphageM13. II.Intracellulardeoxyribonucleicacid formsassociated with M13infectionof mitomycin C-treated cells.J. Virol. 2:1296-1307.
3. Dumas, L. B., and C. A. Miller, 1973. Replication of bacteriophage 4X174 DNA in a temperature-sensi-tive dnaE mutant of Escherichia coli C. J. Virol. 11:848-855.
4. Dumas, L. B., C. A. Miller, and M. L. Bayne. 1975. Rifampin inhibitionofbacteriophage 4X174parental replicative-form DNA synthesis in an Escherichia coli dnaC mutant. J. Virol. 16:575-580.
5. Fidanian, H. M., andD. S. Ray. 1972. Replicationof bacteriophage M13. VII. Requirement of the gene 2 proteinforthe accumulationofaspecificRFII Spe-cies.J.Mol.Biol. 72:51-63.
6. Fidanian, H. M., and D.S. Ray. 1974. Replicationof bacteriophage M13. VIII. Differential effectsof rif-ampicinand nalidixic acidonthesynthesisofthetwo strandsof M13duplexDNA. J. Mol. Biol. 83:63-82. 7. Geider, K. 1976. Molecular aspects of DNAreplication
inEscherichia coli systems. Curr.Top. Micro. Immu-nol. 74:56-112.
8. Greenlee, L. L. 1973.Replicationofbacteriophage OX-174 ina mutant ofEscherichia coli defective in the dnaE gene. Proc. Natl. Acad. Sci. U.S.A. 70:1757-1760.
9. Guthrie, G. D., and R. L. Sinsheimer. 1963. Observa-tions on the infection ofbacterial protoplasts with deoxyribonucleic acid ofbacteriophage OX174. Bio-chem.Biophys. Acta72:290-297.
10. Haldenwang,W.G.,andJ. R.Walker. 1976. Parental RFformation ofphageqbX174 and M13requiresthe dnaZ geneproductofEscherichia coli. Biochem.
Bio-phys.Res. Commun. 70:932-938.
11. Kranias,E.G.,and L. B.Dumas.1974.Replicationof
bacteriophage 4X174 DNA in a temperature-sensi-tivemutantofEscherichia coli C. J. Virol. 13:146-154.
12. McFadden, G.,and D. T.Denhardt.1974.Mechanism ofreplicationof4X174 single-strandedDNA. IX. Re-quirementfor theEscherichia coli dnaGprotein. J. Virol.14:1070-1075.
13. Marvin,D.A.,and B.Hahn. 1969. Filamentous bacte-rial viruses. Bacteriol. Rev. 33:172-209.
14. Ray,D.S. 1970.Replicationofbacteriophage M13. IV. Synthesis of M13-specific DNA in the presence of chloramphenicol.J. Mol. Biol.53:239-250.
15. Ray,D.S.,J.Dueber,andS.Suggs. 1975.Replication
ofbacteriophageM13. IX.Requirementof the Esche-richia colidnaG function of M13 duplex DNA replica-tion. J.Virol.16:348-355.
16. Schekman, R.,A.Weiner,andA.Kornberg. 1974. Mul-tienzyme systems of DNA replication. Science 186:987-993.
17. Sinsheimer,R. L. 1968. Bacteriophage qX174 and re-lated viruses. Prog. Nucleic Acid Res. Mol. Biol. 8:115-169.
18. Staudenbauer,W. L. 1974.Involvement of DNA polym-erasesI and III in the replication ofbacteriophage M13. Eur. J.Biochem. 49:249-256.
19. Taketo,A. N975.Replication ofOAandOX174in Esche-richia coli mutantsthermosensitive in DNA synthe-sis. Mol. Gen. Genet. 139:285-291.
20. Truitt, C. T. and J. R. Walker. 1974. Growth ofphages X,4X174and M13requiresthe dnaZ geneproduct of Escherichia coli. Biochem. Biophys. Res. Commun. 61:1036-1042.
21. Wickner, S., and J. Hurwitz. 1976. Involvement of Escherichia coli dnaZ gene product in DNA elonga-tion in vitro. Proc. Natl.Acad. Sci. U.S.A. 73:1053-1057.
22. Wickner, W., R. Schekman, K. Geider, and A. Korn-berg. 1973. A new form of DNA polymerase III anda copolymerase replicate a long single-stranded primer-template. Proc. Natl. Acad. Sci. U.S.A. 70:1764-1767.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
![FIG.1.AX727P2P]labeled[3H]thymidinethisCellstransferredbeforeinfectionincubation Formation of M13 parental RF at 410C by a dnaZts culture in the presence of rifampin](https://thumb-us.123doks.com/thumbv2/123dok_us/1546341.107164/3.508.54.454.53.359/labeled-thymidinethiscellstransferredbeforeinfectionincubation-formation-parental-dnazts-culture-presence-rifampin.webp)