Vol.44,No.2 JOURNALOFVIROLOGY, Nov. 1982, p. 658-665
0022-538X/82/110658-08$02.00/0
Copyright © 1982, American Society for Microbiology
Localization of Viral Structural Proteins in
the
Cytoplasm
and
Nucleus of Rous-Associated Virus-2-Infected Chicken Embryo
Fibroblasts
PAULA J. ENRIETTOt* ANDRAYMOND L. ERIKSON
DepartmentofPathology, School ofMedicine, UniversityofColorado HealthScienceCenter,Denver, Colorado 80262
Received 5May1982/Accepted 26 July 1982
The cellular location of viral structural proteinswascarried outby
immunohis-tochemistry and by cell fractionation. Antibody against the structural protein p27
wasused inimmunohistochemical reactionstodemonstrate thepresenceof viral
proteins in thecytoplasm and nucleus of Rous-associated virus 2-infected chicken
cells. Localization in the nucleuswasfoundoverheterochromatic regions; in the
cytoplasm itwasfound indiscrete particulatestructures.These observationswere
extended incellfractionation studies in which cytoplasmic and nuclear fractions
wereimmunoprecipitated with antibody against the viral structural proteins.
In an effort to better understand the
interac-tions of the viral structural proteins of avian leukosis sarcoma viruses with the host cell, a
study was initiated to localize these proteins within the cell. The structural proteins of the virus are synthesized from35S viral RNA as a
polyprotein precursor Pr76, which is then
cleaved to give the mature structural proteins found in the virusp27, p12, p15, and p19 (fora
review, see reference 10). The location ofthese
proteins withinthevirusparticle has been stud-ied (17), and it has been
proposed
that p19makes up the inner coat ofthe virion and
p27
andp15 thecore
shell,
andthatp12
isa constitu-ent of theribonucleoprotein
core ofthe virion(2). Several functions have been
assigned
tothese
proteins.
Ithas beenpostulated
thatp19,
aphosphoprotein (11), binds
specifically
to viralRNA,
suggesting
aregulatory
function(15).
Inaddition, p15
has been shown to be a proteasewhichcancleavePr76saeto
give
risetothe viralstructuralproteins (9, 25).
The site ofsynthesis ofPr76 in the host cell
has been studied by Purchio et al.
(22),
whoshowed that
85%
of Pr76 was synthesized onfree polyribosomes, whereas 15% was synthe-sized on membrane-bound polysomes. It has
been postulated that some oftheprocessing of
Pr76 occurs at the membrane, since agents
known to affect membrane protein interactions
block the cleavageof Pr76 invitro
(24).
Tofurtheranalyzetheinteraction of theviral
structural proteins with the host cell, and
per-hapsidentifysitesofsynthesisorprocessing,we
tPresent address: Imperial Cancer Research Fund, Lin-coln'sInnFields,London WC2A3PX, England.
began by carrying out ultrastructural
localiza-tion of the viral structural proteins by using
antibody against them in immunohistochemical
reactions. Cell fractionations of labeled cells
were thencarriedout, followedby
immunopre-cipitation with antibody against the structural
proteins.
MATERIALS ANDMETHODS
Cells and viruses.Chicken embryo fibroblasts (CEF)
wereobtained from10-to 11-day old chicken embryos
(Spafas, Inc.) andweregrown in IMEMZO with zinc
and insulin (Associated Biomedical Systems, Inc.)
containing 10%tryptosephosphate and5%calf serum.
Cellswere platedat adensity of 8 x 105on a 60-mm
petri dish, infected with an appropriate dilution of
stock Rous-associated virus 2 (RAV-2) or
Schmidt-Ruppin D (a generous gift of H. Hanafusa), and
maintained in culture with 10% tryptose
phosphate-5% calfserum.
Preparation ofantibodies.Antibodyagainstthe viral
internal structural protein p27 purifiedby
polyacryl-amide gel electrophoresis was prepared as described
previously(21).
Tumor-bearing rabbit serum which recognizes the
viral internal structuralproteins was prepared as
de-scribedby Brugge and Erikson (3).
Cellfractionations.Preparations ofclean nucleifree
ofcytoplasmic material were made in the following manner.Cellswerewashed threetimeswithcold STE
(0.15MNaCl, 0.05MTris [pH 7.2],0.0001 MEDTA),
after whichtheywere removed from theplate by brief
treatmentwith0.05% trypsininsalinD(0.05% trypsin,
0.1% glucose, 0.15 M NaCl, 0.001 M KCl, 0.1 M
sodium phosphate buffer). After trypsinization, cells
werewashed twice incold STE, pelleted at 2,000 rpm
for5min,andlysedin 0.01 MTris-hydrochloride(pH
7.2)0.15MNaCl-0.5% NonidetP-40withblendingat
high speed inaVortex mixer forapproximately15 s.
658
on November 10, 2019 by guest
http://jvi.asm.org/
LOCALIZATION OF VIRAL PROTEINS 659
Nuclei were pelletedfrom the lysate by centrifugation
at5,000 rpmfor 5 min. The cytoplasmic fractionwas
removed and kept onice; nucleiwerewashedtwo to
three times with lysisbuffer to remove allcytoplasmic
material.Nuclei preparationsweremonitoredateach
step by light microscopy. Washed nucleiwere then
lysed in RIPA (13) lysis buffer (0.01 M
Tris-hydrochlo-ride [pH 7.2], 0.15 M NaCl, 1% Triton X-100, 1%
deoxycholate,0.1% sodiumdodecyl sulfate[SDS]).A
general proteaseinhibitor, Trasylol (FBA
Pharmaceu-ticals, New York, N.Y.), was added to nuclei lysis
buffer andRIPAlysis bufferto afinal concentration of
1% (1). Cytoplasmic fractionswereadjustedtoRIPA
lysis conditions. All stepswerecarriedout at4°C.
Radiolabeling cells and immunoprecipitations. RAV-2-infected CEF were radiolabeled and
immunoprecipi-tatedessentially as previously described (3).Analysis
of immunoprecipitates wascarriedout on10%
SDS-polyacrylamide gels as described by Laemmli (14).
Gels were then soakedfor 30 min in a1 Msolution of
salicylic acid (4),dried,and exposed at-70°C (Kodak
X-Omat X-rayfilm).
Preparations of antibody forimmunohistochemistry.
Antibody against the viral internal structural protein
p27 was used for immunohistochemical staining reac-tions. Immunoglobulin G (IgG) fractions of normal and immune sera made as described previously (23) were absorbed with normal CEF which had been fixed in
acetone for 10 min and washed three times in
phos-phate-buffered saline (PBS) (0.15 M NaCl, 0.0042 M
KCI, 0.01 M sodium phosphate buffer [pH 7.2], 5 x
10-4M MgCl2, 9 x 10-4 M CaCl2). Absorption was
carried outovernightat4°C, and absorbed serawere
clarified at 100,000 x gfor 30 min. Absorbed antisera
were used in staining reactions at dilutionsof 1:16 in
PBS-10% sucrose.
Preparation of horseradish peroxidaseconjugatesof
IgG. Sheep anti-rabbit IgG was kindly provided by
AntonioMartinez-Hernandez(Universityof Colorado
Health ScienceCenter, Denver) andwasconjugatedto
horseradishperoxidase bythemethodof Nakane and
Kawaoi(18).
Fixation and staining procedure. RAV-2-infected
CEFweregrownon35-mmpetri dishes andfixed for
20minat4°C with 2% paraformaldehyde in PBS. After
three washings in PBS-100o sucrose (each wash, 10
min), cells were incubated in absorbed normal or
immune serum for 3 h at room temperature, washed
threetimes inPBS-10%sucrose, andincubated for 1 h
insheep anti-rabbit IgG labeled with horseradish
per-oxidase. After postfixing in 2% glutaraldehyde in
PBS-10%sucroseandwashing inPBS-10%sucrose,
the cells were incubated for 30 min in 7x 10-5M
3,3'-diaminobenzidene in 0.05 M Tris buffer (pH 7.2)
(DAB) plus10% sucrose and thenin DABplus 0.05%
H202 for5 min. Cellswereosmicatedin 1%OS04in
0.01 M phosphate buffer afterthree washes in PBS-10% sucrose and were dehydrated in graded ethanols andembedded inEpon-aralditeasdescribed
previous-ly (16). Poprevious-lymerized blockswere thinsectioned,
exam-inedon aPhillips201 electronmicroscope operatingat
80kV, and photographed.
RESULTS
Localization ofinternal structural proteins by
immunohistochemistry. Localization was carried
outby the procedure described in Materials and
Methods by using the IgG fraction from serum
containing antibody against the viral structural protein p27 or normal serum preabsorbed as described above. The specificity of the serum
used was determined by immunoprecipitating
total cell extracts of RAV-2 and CEF labeled with [35S]methionine. Anti-p27 IgG precipitated predominantly Pr76 (which contains p27 anti-genic determinants) and p27, whereas normal
rabbit IgG precipitated no detectable proteins
(datanotshown).
RAV-2-infected CEF and normal CEF were
grown, fixed, and stained. After the embedded
cells were sectioned, they were examined by
electron microscopy.
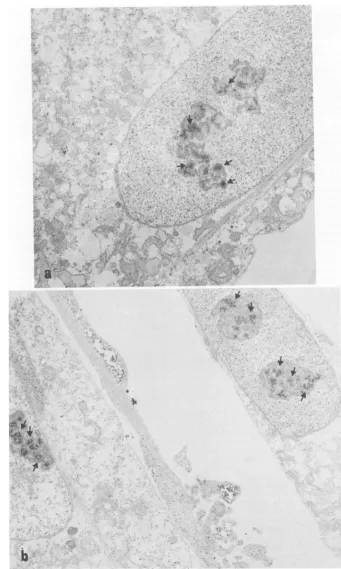
As canbeseeninFig. 1,RAV-2-infected CEF
showed two major stainingpatterns, one
cyto-plasmic,asexpected, and theother nuclear.The
nuclear stainingwasobserved in approximately
85% of the cells examined and appeared to be
concentrated over a heterochromatic region
withinthenucleus that may represent the
nucle-olus. Twoexamples ofthe nuclear staining
pat-tern can be seen in Fig. 1. Diffuse nuclear
staining was also seen, but on examination at
high magnificationit wasfoundtobeparticulate,
with noobvious structure.
Inadditiontothe nuclearstaining observed, a
diffuse cytoplasmic staining was seen which
showed little localizationto structural elements
(Fig. 1). At higher magnification, the staining
was found to be localized in discrete particles
withinthe cytosolwhich ranged insize from 12
to36 nmindiameter (Fig. 2).
Control staining reactions were carried out
(Fig. 3). RAV-2-infected cells stained with ab-sorbed normal IgG and uninfected cells stained with absorbed anti-p27 showednospecific stain-ing and little background stainstain-ing in either the
cytosolorthenucleus.
Immunoprecipitation of cell fractions. To
con-firm the localization ofaviral structuralprotein
to the nucleus, cellfractionations were carried
out. In addition, itwas of interestto determine whether the protein(s) localized to the nucleus
represented only Pr76orp27,orif both could be foundthere, sincetheantibodyusedfor
staining
recognized both.
RAV-2-infected CEF were labeled with
[35S]methionine,
fractionated into cytoplasmicand nuclear fractions, and immunoprecipitated
withanantiserumwhich had a high titer against
all the viral structural proteins and pp6Osr'.
Analysis ofthe immunoprecipitates was carried
outby SDS-polyacrylamide gel electrophoresis.
Inall cases, thenucleiweremonitored by
phase-contrastmicroscopyforcytoplasmic
contamina-tion. Ascanbe seen inFig.4a, thepredominant protein precipitated from the nuclear fraction
VOL. 44,1982
on November 10, 2019 by guest
http://jvi.asm.org/
.4.
r~~~~~~~~~~~~~~~~~~~~Ar
4*1~~~~~4
4.
FIG. 1. Electronmicrographsof cells stainedwithabsorbed immuneserum.RAV-2-infected CEFwerefixed
in 2%p-formaldehydeand stained with theIgGfraction ofanti-p27 absorbed with normal CEF. The second
antibodyused in these experimentswas sheepanti-rabbitIgGlabeled withhorseradishperoxidase. (a)Arrows
indicate staining over a heterochromatic area ofthe nucleus. Diffuse cytoplasmic staining can also be seen.
Magnification, x24,684. (b) Arrows showreactionproductwithin thenucleus overheterochromaticareas. In
addition, diffusecytoplasmic stainingcanbe seen.Magnification, x20,401.
660
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.491.80.421.46.615.2]a,''
<',
'"'';-ik ;
4^ r. 9. 1
*-6Z
AL~~~~~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ '
4~~~~~~~~~~~~~~4
cytplsmManiiction
x41,400..
(@: ors~~~~~~~
FI.
.Hih-aniiatonpotmirgrps
fyopamicsann.RV2ifce
E eefxdstiedn
peaedfrelcrn
irscp secibdi tetxt
a Arwidctepriclt
cyolsi
tiigosre
ihntectpamo©ifce
el.Mgiiain
3,0.()Hge
manfiG.io
2.Hg-agenification
phthbomicrogaph
sof
cin
topasi staining.RAV-2-stinfced CEFti
werefixhnted
cytoplasm. Magnification, x410,400.
661
on November 10, 2019 by guest
http://jvi.asm.org/
a
At
oh
a
FIG. 3. Electronmicrographsofstainingcontrols. RAV-2-infected andnormal CEFwerefixed, stained,and
preparedfor electron microscopy as described in the text. (a) RAV-2-infected CEF stained with normal IgG
preabsorbed as described in thetext todetect nonspecific staining. Magnification, x24,684. (b) Normal CEF
stainedwithanti-p27 preabsorbedas describedinthetext todeterminethe level ofnonspecificbackgroundin
normal cells.Magnification, x24,684.
662 0
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.491.84.424.52.620.2]LOCALIZATION OF VIRAL PROTEINS 663
nuc b
SR-D
cyto nuc
Pr-76-
-Pr
76-
p27-1 2
pp60-3 4
_-35k
1 2 3 4 T7
FIG. 4. Cell fractionationand immunoprecipitation of RAV-2- andSchmidt-RuppinD(SR-D)-infectedCEF.
In eachcase,cellswerelabeled with [35S]methionine, fractionated, andimmunoprecipitatedasdescribed in the
text.Theywereanalyzed by SDS-polyacrylamide gel electrophoresisfollowed by fluorography. (a)
Autoradio-gramof RAV-2-infected CEF cytoplasmic (cyto)and nuclear (nuc)fractions precipitated with immune serum
from tumor-bearing rabbits (tracks 1 and 3)ornormal serum(tracks 2and4).(b) Autoradiogram of
Schmidt-Ruppin D-infectedCEF cytoplasmic (cyto) and nuclear (nuc) fractions withimmuneserumfrom tumor-bearing
rabbits (tracks1and3)ornormalserum(tracks2 and4). Molecular-weightmarkersare[35S]methionine-labeled
T7.
was Pr76; Pr76 and p27 were precipitated from
the cytoplasmic fraction.
To determine whether the observation of viral
structuralproteins in the nucleuswasrestricted
to RAV-2 infections (a leukosis virus), CEF
infected with asarcoma virus, Schmidt-Ruppin
D, were labeled with [35S]methionine,
fraction-ated, and immunoprecipitated. Ascanbeseenin
Fig. 4b, the predominant protein precipitated in
the nucleuswas again Pr76.
To rule out thepossibility that the structural
proteins precipitated from the nucleus were
boundnon-specificallytothe nuclei during their
isolation,areconstitution experimentwasdone.
RAV-2-infected CEF labeled with
[35S]methi-oninewerefractionatedasbefore,as were
unla-beledinfected cells. The total labeled
cytoplas-micfraction wasthen mixed with the unlabeled
nuclearfraction for 15minat4°C. The unlabeled
nucleiwerethen pelleted and washedoncewith
lysis buffer, aswerelabeled nuclei. The labeled
cytoplasm, the labeled nuclei, and unlabeled
nuclei that had been mixed with labeled
cyto-plasm were then immunoprecipitated with
tu-mor-bearing rabbitserum.AscanbeseeninFig.
5, Pr76 and p27 could be precipitated from the
labeledcytoplasmic fraction from
RAV-2-infect-ed CEF. The predominant protein precipitated
from thelabeled nuclear fraction was Pr76; no
detectable Pr76orp27wasprecipitable from the
unlabeled nuclear fraction which had been
mixed with labeledcytoplasm.
This experiment seemed to indicate that the
Pr76 precipitable from the nuclear fraction of
RAV-2-infected CEFwas notaconsequence of
nonspecific binding of that proteintothe nuclei
during this isolation procedure. Instead, it
ap-peared that Pr76was aspecificcomponentof the
nuclear fraction.
Pr76- _ t 80K
_ l_
~~ _
W
-35K
p27-1 2 3 4 5 6 T7
FIG. 5. Reconstitutionexperiment withcytoplasm
and nucleus. Parallel platesof RAV-2-infected CEF
were fractionated intocytoplasmic and nuclear frac-tions afteroneplatewaslabeled for 2 h with 150,uCiof
[35S]methionine. Labeledcytoplasm was mixed with
unlabelednuclei for15minat4°C. After the unlabeled
nuclei were washed once, three fractions were
immunoprecipitated. Lanes 1 and 4, labeled
cyto-plasm; lanes 2 and 5, labeled nuclei; lanes 3 and 6,
unlabeled nuclei mixed with labeled cytoplasm and
washedoncewith RIPA. Lanes1to3,tumor-bearing
rabbit serum; lanes 4 to 6, normal rabbit serum.
Precipitateswereanalyzed by SDS-polyacrylamide gel
electrophoresis, fluorography, and autoradiography. R AV-2
cyto
VW
-80k VOL.44, 1982
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.491.100.392.77.240.2] [image:6.491.252.448.405.545.2]664 ENRIETTO AND ERIKSON
Quantitation of Pr76 in cytoplasmic and
nucle-ar fractions of RAV-2-infected CEF. To
deter-mine the proportion of Pr76 in each fraction,
labeled RAV-2-infected CEF were fractionated
and immunoprecipitated in antibody excess to
precipitate allof the structural protein present. The bands corresponding to Pr76 were cut from
thegel and counteddirectlyin scintillation fluid.
After subtraction of background counts, it was
calculated thatapproximately 6% of the Pr76 in RAV-2-infected cells was precipitable from the nuclear fraction.
DISCUSSION
Inthis series of experiments, itwasfound that viralstructural proteins could be localized to the
nucleus of CEF infected with avian leukosis
sarcoma virus. The predominant protein found
inthe nucleus byimmunoprecipitationwas Pr76;
however, because of the relative proportions of
Pr76 andp27 in thecytoplasmic fraction,it is not
possible to rule out the presence of p27 inthe nucleus also. It should be said, however, that
intermediate cleavage products of Pr76 (p66,
p60) were never observed in the nucleus by
immunoprecipitation.
A nuclear phase for the viral structural
pro-teins of avian leukosis sarcoma virus has not
been described before. However, regulatory roleshave been proposed for theviralstructural
proteins by several investigators. It has been
suggested that a virus-specific protein is
re-quired for integration of proviral DNA during virusreplication(6, 7, 12), and such a model has
been describedin the murine system to explain
theabrogation of
Fv_lb
restriction (8). Inaddi-tion, it has been suggested that a viral protein
might be involved in the regulation of the
splic-ing of the5'terminalsequence of the genome to
subgenomic mRNA (5, 7).
Athird regulatory role could beimagined for viralstructuralproteinsin theselection of newly transcribed viral RNA destined to become
ge-nomic RNA in budding virions. The fact that viral structural proteins have been shown to
bindviral RNA andassociatewith it in thevirion
(2, 15) indicates arole for structural
proteins
invirusmorphogenesis atanother level.
Anindication that the viral structuralproteins
may have adifferent role inthe cytoplasmand
nucleus comes from preliminary pulse-chase
experiments. When Pr76 inthe
cytoplasm
andnucleus arefollowedduring variouspulse-chase
conditions, the turnover time of cytoplasmic
Pr76 appears to be longer than that ofnuclear
Pr76, suggesting adifferent roleforthe nuclear
structuralproteins.
The second
pattern
ofstaining observed wascytoplasmicanddiffuseinnature.In
addition,
itwasfoundthatboth Pr76 andp27were
immuno-precipitable from the cytoplasm of infected
cells. Thepresenceof viral structuralproteins in
the cytoplasm wasexpected; however, we were
surprised to find such a diffuse pattern. No
evidencewas seenfor specificassociationof the
viralstructural proteinswithinternal membrane
systems of the host cell or the plasma
mem-brane. Thismayreflect thelimits ofdetection of this technique, since it has been reported that
only 15% of the Pr76 is synthesized on
mem-brane-bound polysomes(22). Some localization at the plasma membrane was also expected; however,werarelysawbudding viruswhere an
accumulation of viral proteins wouldhavebeen
more likely. Again, this may reflect the limita-tions of the technique employed.
Thelocalizationthat was seen athigh magnifi-cation appeared in discrete particles, which
rangedin size from 12 to 36 nm. Thepossibility
existed that these structures represented
poly-somes in the process of translating viral 35S
mRNAintoPr76.This seemsunlikely, sincethe
size of the average ribosome is approximately
200 nm(20). Theseparticlesmay instead
repre-sent viral protein complexes or protein:RNA complexes which will, with maturation, bud from the surface of the cell.
In summary, localization of viral structural
proteins Pr76 and p27 wascarried out, with the finding that a portion of Pr76 is located in the
nucleus oftheinfected cell. Itisnotcompletely clear whether cleavage products of Pr76 are
presentinthenucleus, amatterwhich could be
clarified by using monospecific antisera of
high
titeragainst theseproteins.
ACKNOWLEDGMENTS
Theadvice andhelp of Alan S. Jones and P.K.Nakaneis gratefullyacknowledged.
This workwassupportedby Public Health Service grants CA 15828 and CA 21117 from the National Institutes of Health. P.J.E. wastherecipientofapredoctoral fellowship (training grant CA 09157) from the National Institutes of Health.
LITERATURECITED
1. Biseid,G. 1970. Anassay method forTrasylolRbasedon the in vitroinhibitionofhumanplasma kallikrein. Acta Pharmacol.Toxicol. 28:225-232.
2. Bolognesi, D. P. 1974. Structural components ofRNA tumorviruses.Adv.VirusRes.19:315-360.
3. Brugge, J. S.,and R. L.Erikson. 1977.Identification ofa transformationspecific antigen induced byanavian sarco-mavirus.Nature(London)269:346-348.
4. Chamberlin,W.1979.Fluorographicdetection of radioac-tivity in polyacrylamide gelswith the watersolublefluor, sodiumsalicylate.Anal. Biochem. 98:132-135. 5.Cheung, K.-S., R. E. Smith, M. P. Stone, and W. K.
Joklik.1972.Comparisonof immature(rapid harvest)and mature Rous sarcomavirus particles. Virology 50:851-864.
6. Coffin, J.M.1979.Structure,replicationand recombina-tionof retrovirus genomes: someunifyinghypotheses.J. Gen. Virol.42:1-26.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
LOCALIZATION OF VIRAL PROTEINS 665
7. Cooper, G. M., and S. Okenquist. 1979. Mechanism of transfection of chicken embryofibroblasts by Rous sarco-mavirus DNA. J. Virol. 28:45-52.
8. Cordell, B., S. R. Weiss, H. E. Varmus, and J. M. Bishop. 1978. At least 104 nucleotides aretransposedfrom the5' terminus of the avian sarcoma virus genome to the 5' termini of smaller virus mRNAs. Cell 15:79-91. 9. Dittmar, K. J., and K.Moelling. 1978. Biochemical
prop-erties of p15-associated protease in avian RNA tumor virus. J. Virol.28:106-118.
10. Eisenman, R. N., and V. M. Vogt. 1978. Thebiosynthesis of oncovirusproteins. Biochem. Biophys. Acta 473:187-200.
11. Erikson, E., J. S. Brugge, and R. L. Erikson. 1977. Phosphorylated and nonphosphorylated forms of avian sarcoma viruspolypeptide p19. Virology 80:177-185. 12. Gallis, B., R. Eisenman, and H. Diggelman. 1976.
Synthe-sis of the precursor toavian RNAtumorvirus internal structural proteins early after infection. Virology 71:302-313.
13. Gilead, Z., U.-H. Jen, W. S. M. Wold, K. Sugawara, H. M.Rho, M. L. Harter, andM.Green. 1976. Immuno-logical identification of two adenovirus 2-induced early proteins possibly involved in celltransformation.Nature (London) 264:263-266.
14. Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature(London) 227:680-85.
15. Leis, G. P., J.McGinnis, and R. W. Treen. 1978. Rous sarcoma virus p19 binds to specific double stranded regions of viral RNA. Effect of p19 on cleavage of viral RNA by RNase III. Virology 84:87-98.
16. Molhehauer,H. H.1964. Plasticembedding mixtures for
useinelectronmicroscopy. Stain Technol. 39:111-114. 17. Montelaro, R. C., and D. P. Bolognesi. 1978. Structure and
morphogenesis oftype-C retroviruses. Adv. Cancer Res. 28:63-89.
18. Nakane, P. K.,and A. Kawaoi. 1974.Peroxidase-labeled antibody. Anewmethod ofconjugation. J. Histochem. Cytochem. 22:1084-1088.
19. Oppermann, H., J. M. Bishop, H. E. Varmus, and L. Levintow. 1977.Ajoint productof genes gag and pol of ASV: apossible precursorof reversetranscriptase. Cell 12:993-1005.
20. Porter, K. R., and M. A. Bonneville. 1973. The fine structureof cells andtissues, 4thed., p. 11-13. Lea & Febiger,Philadelphia.
21. Purchio, A. F., E. Erikson, and R. L. Erikson. 1977. Translation of 35s and ofsubgenomic regions of avian sarcoma virus RNA. Proc. Natl. Acad. Sci. U.S.A. 74:4661-4665.
22. Purchio, A. F., S. Jovanovich, and R. L. Erikson. 1980. Siteofsynthesisof viralproteins in avian sarcoma virus-infected chicken cells. J. Virol. 35:629-636.
23. Sell, S., S. Linthieum, D. Bass, R. Baher, B. Wilson, and P. K. Nakane. 1977. Immunohistologic techniques, p. 272-305. In C. Borek, C. M. Fenoglio, and D. W. King (ed.), Advances inPathobiology, vol. 6. Stratton Interna-tionalMedical Book Corp., New York.
24. Vogt, V. M., R. Eisenman, and H. Diggelmann. 1975. Generation of avianmyeloblastosis virus structural pro-teinsby proteolytic cleavage of precursor polypeptide. J. Mol. Biol.%:471-493.
25. Vogt, V. M., J. Wight, and R. Eisenman. 1979. In vitro cleavageof avian retrovirus gag proteins by viral protease p15. Virology 98:154-167.
VOL.44, 1982