Formulation and Evaluation of Diacerein Loaded Microsponges in Capsule
Full text
Figure




Related documents
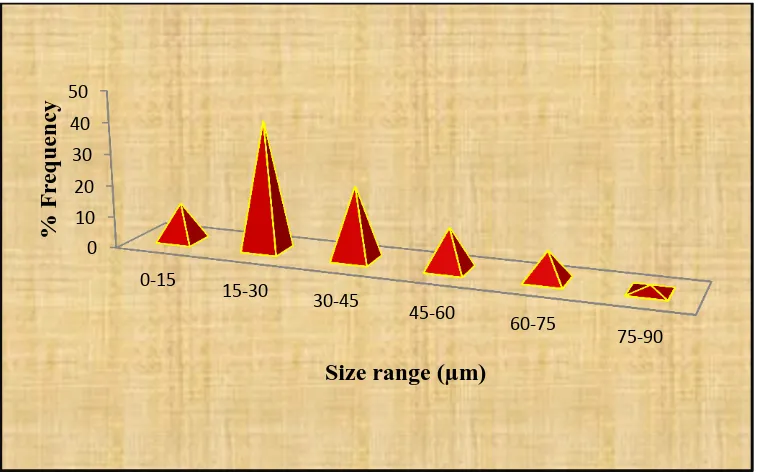
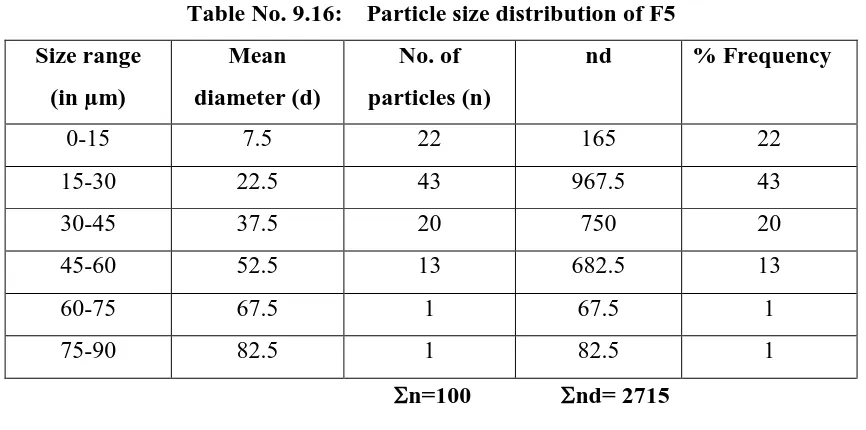
Department of Pharmaceutics, Madras Medical College Page 23 showed that a certain lag time before the drug released generally due to the erosion of the. dry coated
Department of Pharmaceutics, The Erode College of Pharmacy and Research Institute, Erode 110 Table No.48 Drug release of the Domperidone optimised formulation during stability
The percentage yield of copper nanoparticles loaded microsponges of various batches were calculated using the weight of final product after drying with respect to the initial
The in-vitro drug release behavior of optimized acyclovir loaded chitosan nanoparticles (F3) and marketed formulation of acyclovir was investigated in phosphate buffer
Release study show that drug release from microsponges in controlled manner as compared to the pure drug and it was increased with increased in concentration of
Effect of drug: polymer ratio: Drug: polymer ratio had an effect on the production yield, actual drug content, encapsulation efficiency and size of microsponges with
Formulation F7 showed highest drug content and in vitro drug release of 92.74% at the end of 8 h and all the formulations followed zero order kinetics.. Based on the results of
Research Article FORMULATION AND EVALUATION OF FAMOTIDINE FLOATING MICROSPONGES Shireesha Charagonda *, Ramya Deepthi Puligilla, Madhu Babu Ananthula, Vasudha Bakshi Department