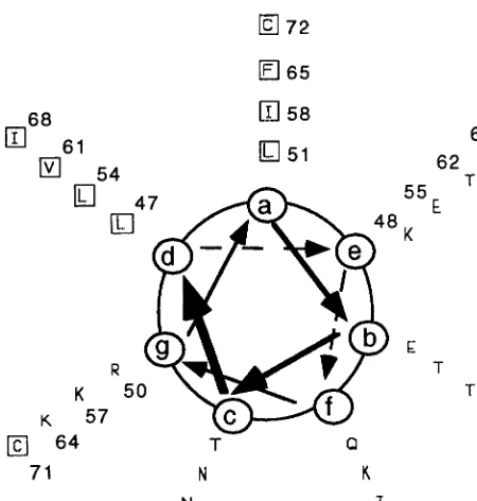
The Vaccinia Virus 14-Kilodalton (A27L) Fusion Protein Forms a Triple Coiled-Coil Structure and Interacts with the 21-Kilodalton (A17L) Virus Membrane Protein through a C-Terminal α-Helix
Full text
Figure

Related documents
In support of these speculations, the results presented in this report show that viruses defective in Us9 internalization and TGN retention, such as those with the acidic
CAV vectors were replication defective in all cell lines tested, transduced human-derived cells at an efficiency similar to that of a comparable human adenovirus type 5 vector, and
We also compared HIV-1 and SIV Nef binding to Src family SH3 domains in coprecipitation studies using recombinant GST fusion proteins (9, 12). Jurkat cells were transfected with
2) Madhav S.Barve, Roopa Sharma compared intubating conditions and time course of action of Rocuronium & succinyl choline in paediatric patients and concluded that Rocuronium
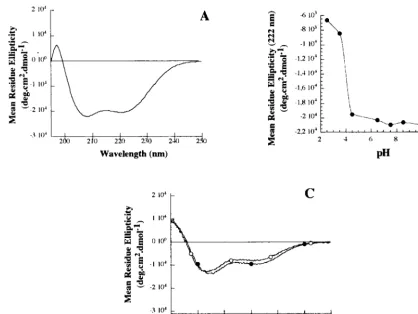
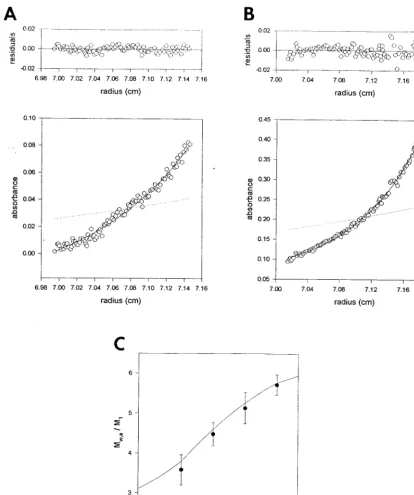
Virions (intracellular mature virus parti- cles) were purified on sucrose gradients from HeLa S3 suspension cultures (14), except for the virions used for binding assays (see Fig.
This ability to have multiple interactions with the promoter complex may be crucial for transcriptional activation, since the IE proteins cannot activate promoters having only a
iining activity of the reverse reaction was only slightly in5247-12 and bi5349 were inactive for integration (data not ished by NEM (Fig. Interestingly, preincubation shown). 4, lane
To see whether this C/EBP factor can activate HBV gene expression in liver cells, we cotrans- fected a reporter plasmid, SK, containing a tandem dimer of the wild-type HBV genome,


