JOURNAL OFVIROLOGY,June1980, p. 650-657 Vol. 34, No. 3 0022-538X/80/06-0650/08$02.00/0
Relationship
Among Tau Antigens Isolated from Various
Lines of
Simian Virus
40-Transformed
Cells
DANIEL T. SIMMONS,`* MALCOLM A.MARTIN,2PETER T. MORA,3 ANDCHUNGMING CHANG3t SchoolofLifeand HealthSciences, University ofDelaware, Newark,Delaware19711';and Recombinant DNAResearch Unit, National InstituteofAllergyandInfectiousDiseases,2andMacromolecular Biology
Section, Immunology Program,National CancerInstitute,3Bethesda,Maryland20205
Inadditiontothevirus-specified tumorantigens,simian virus 40-transforned
cells containatleast oneotherprotein which canbeimmunoprecipitated with
serumfromanimalsbearing simian virus 40-inducedtumors.Thisprotein,which
isdesignated Tauantigen, hasanapparentmolecularweightof 56,000 as
deter-minedbyelectrophoresisonacrylamidegels. Therelationshipamong Tau
anti-gensisolated from different lines of simian virus 40-transformed cellswas
exam-ined bycomparing the methionine-labeled tryptic peptides of theseproteinsby
two-dimensional fingerprintingonthin-layer celluloseplates. Inthisfashion,we
initially determined that the Tau antigens isolated from three different lines of
transformedmouse cellswerevery similar. Second, we found that Tauantigen
isolated fromaline ofrattransformantswascloselyrelated, butnotidentical,to
the mouse cell Tau antigens. Approximately 70% of their methionine peptides
comigrated intwodimensions.Finally,weshowed thatTauantigen isolated from
aline of transformed human cellswasonly partially relatedtothemouseandrat
proteins. About 40%of the methioninepeptides of the humanproteinwere also
contained in the Tauantigens from the othertwospecies. These resultsstrongly
indicate that the Tau antigens isolated from these various simian virus
40-transformed cell lines containcommon amino acid sequences.
Monkey cells infected with simian virus 40
(SV40) synthesize two proteins (94,000 and
20,000 daltons) which can be
immunoprecipi-tated with serum from animals bearing
SV40-inducedtumors (anti-Tserum) (1,4,29). These
proteinsarethelarge and smalltumorantigens
(T-Ag's) andarecoded by the region oftheSV40
genomethat isexpressed earlyinlytic infection
and invirus-transformed cells (22,23).
In addition to the T-Ag's, cells transformed
bySV40 synthesizeoneotherprotein (50,000 to
56,000 daltons) which can be
immunoprecipi-tated withanti-Tserum (5, 9, 10, 16, 17, 19, 21,
28). This protein is probablycoded by the cell
DNA since it contains very few (zero to two)
methionine-labeledtrypticpeptidesincommon
with either large or small T-Ag (5, 16, 28) and
sinceuninfectedmouseembryo carcinoma cells
manufacture a protein that is very similar or
identicalto the 50,000- to 56,000-dalton protein
isolated from SV40-transformed mouse cells
(19). We havepreviouslycalledthis protein Tau
antigentodistinguishitfrom thevirus-coded
T-Ag's (5). Smith et al. (28) have recentlycalled
thisprotein nonviral T-Ag.
tPresent address: Department of Microbiology, National Yang-MingMedical College and Taipei Veteran General Hos-pital, Sheh-Pei, Taipei, Taiwan.
Tau antigens have been detected inall lines
ofSV40-transformed rat, human,hamster,and
mousecells that have been examined(5, 16, 21,
28),although transformed hamstercells appear
tomake smallerquantities of these proteins (28).
In addition, cells transformed by other viruses
(Moloney murine leukemiaor sarcomavirus)or
by chemicals (methylcholanthrene) synthesize
similarly sized proteins whichmaybe Tau
anti-gens(7). Theseproteinsareapparentlynot
pro-ducedinspontaneouslytransformedmousecells
(5), in SV40-transformed cells which have
re-vertedto aT-Ag-negativestate(5),orin normal
untransformedcells (7, 19,28).Aportion of the
Tauantigen in transformed cellsmaybe found
in acomplex withlargeT-Ag (17).
Weinvestigated the relationshipamongTau
antigens isolated from various lines of
SV40-transformed cells to determine whether these
proteins areidentical. For this purpose, we
ex-aminedthemethionine-labeledtrypticpeptides
of Tau antigens isolated from three different
linesofSV40-transformedmousecells,oneline
oftransformedrat cells, and one line of
trans-formed human cells. We found that these
pro-teins were not all identical, and yet they all
contained some peptides in common. In
addi-tion, we noticed that the mouse-derived Tau
650
on November 10, 2019 by guest
http://jvi.asm.org/
SV40 Tau ANTIGENS 651
antigens were nearly identical to one another,
that the ratprotein was significantly related to,
butclearly distinguishable from, the mouse
pro-teins, and that the human protein was only partially related to the others.
MATERIALS AND METHODS
Cells. SV40-transformed BALB/c and AL/N mousecells(lines 315 and 215, respectively) have been described previously (5). BALB/c (line 11A8) trans-formants were obtained from G. Todaro. Human
transformedcells (line SV80) were isolated originally
byTodaroetal. (30), and rattransformedcells were
isolatedby W. Topp anddescribedbyBotchan et al. (3).
Immunoprecipitation and gel electrophoresis. Transformedcells were grown in150-cm2culture flasks and labeled for3hwith50uCi ofL-['S]methionine
perml,aspreviouslydescribed (5). The cells in each flask were washed three times with ice-cold 0.01 M Tris-0.001 MNa2HPO4-0.137 MNaCl, pH 7.4 (Tris-bufferedsaline),andlysedduring a 15-min period at 00C in the presence of2mlof extraction buffer (5).
Thelysatewascollected andcentrifuged at 150,000x
gfor 40 min at2MC. The supernatantwascarefully removed andincubated at00C for 1hwith 25piof either normal oranti-Thamster serum. The anti-T serum was obtained from hamsters carrying SV40-inducedtumorsandwasprovided by J. Gruber, Na-tional Cancer Institute.
Protein A-bearingStaphylococcus aureus (Cowan Istrain; NCTC 8530) was prepared by the method of Jonsson andKronvall (14). The bacteriawerewashed three timesat00Cwith extractionbuffer, resuspended in thesamebufferto afinal concentration of 10% (wt/ vol), and added (300
pl)
tothe reaction mixture to precipitate the immunecomplexes.After 1hat0°C, the bacteriawerewashedonceinextractionbuffer, sixtimesin0.1MTris-0.5MLiCl-1%2-mercaptoethanol,
pH9.0(26), andoncein Tris-bufferedsaline (pH 7.4)
andsuspendedin 150
pl
of 0.075 M Tris-sulfate (pH8.6)-2% sodium dodecyl sulfate-2%
2-mercaptoetha-nol-0.002%bromophenolblue-15%glycerol (20).
Gel electrophoresis and chromatography of
tryptic peptides. Protein sampleswere heated and
subjectedtoelectrophoresisfor2.5 to 4hat25mA in
gels containing 13% acrylamide and0.26%
bisacryl-amideasdescribedbyTegtmeyeretal.(29).Proteins tobe characterized furtherwere extracted from the
gelanddigestedwithtrypsinaspreviouslydescribed
(27). Trypticpeptideswere separatedintwo dimen-sions onthin-layercelluloseplatesessentially as de-scribed byGibson (11). The peptides in water-pyri-dine-acetic acid (300:10:3,vol/vol), pH5.4 (12),were applied near the corner of a thin-layer plate and
subjectedtoelectrophoresisat40Cinthesamebuffer
for 3 h at 300 V. Chromatography in the second
dimensionwasperformedin butanol-pyridine-water-acetic acid(97:75:60:15,vol/vol) pH5.3(11),for5.5to 6h at roomtemperature.Afterthoroughdryingina
fumehood,theplatesweredippedinmolten(4000)
2-methylnaphthalene containing0.4%
2,5-diphenyloxa-zole,asdescribed byBonner andStedman (2).Kodak
XR2 film was exposed to the plates for 1 to 3 weeks at -70°C before being developed.
RESULTS
Immunoprecipitation of Tau antigens.
Tau antigensarecellularproteins that are
syn-thesized inavariety ofSV40-transformed(5, 16,
17, 19, 28) and non-SV40-transformed (7, 19)
cells. However, they havenotbeen detected in
normal, untransformed cells (7, 19, 28). Since
the Tau antigens isolated from various
trans-formedcellshave thesameapproximate
molec-ularweight (50,000to 56,000),we were interested
in determining whether the same protein was
made in all cells transforned by SV40 or
whethertheproteins variedamong different cell
lines.Furthermore, if differenceswere found,we
wanted to determine the relationship among
Tauantigensisolated fromvarious lines of cells
of thesamespecies and ofdifferent species. We
therefore examined the peptides of theTau
an-tigens isolated from three lines of mouse
trans-formants(lines 1lA8,315,and215;derivedfrom
BALB/c, BALB/c, and AL/N mouse strains,
respectively), one line oftransformed rat cells
(line 14B), and one line oftransforned human
cells(line SV80). Theseproteinswere
immuno-precipitated from extractsofcells labeled with
L-[3S]methionine and subjectedto
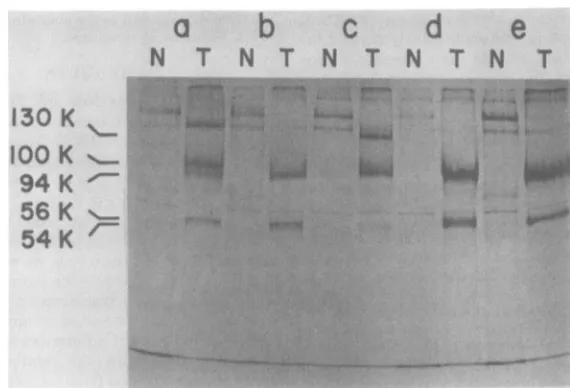
electropho-resis (Fig. 1) in acrylamide gels. Figure1 shows
that each of these cell lines contained one or
more forms of Tau antigen (54,000 to 56,000
daltons) that were immunoprecipitated with
anti-T serum but not with normal serum. In
particular, lines 215, 315, and SV80 containedat
leasttwoforms ofimmunoprecipitable Tau
an-tigens (Fig. lb through d). All cell lines also
containedalargeT-Agwithanapparent
molec-ularweightof 94,000.Largerforms ofT-Agwere
alsoapparentin lines 11A8(100,000daltons)and
315(130,000daltons) (Fig. laandc).These and
other high-molecular-weight forms oflarge
T-Ag have been detected previously in various
linesofSV40-transformedcells(5, 15, 16, 18, 24,
28). The small T-Ag of SV40was not readily
detectedin theseimmunoprecipitates, partially
because the anti-Tserumused waschosen for
maximumprecipitationof Tauantigensand
par-tiallybecause theamountsofanti-Tserumused
weretoosmalltoprecipitate small
T-Ag
quan-titatively (6).
Two-dimensional
fingerprinting
ofme-thionine-labeled tryptic
peptides.
Tocom-pare thepeptidesof Tauantigensisolatedfrom
various lines ofSV40-transformedcells,labeled
immunoprecipitated
proteins
wereapplied
topreparative acrylamide gels. The bands
corre-sponding to the
slower-migrating
Tauantigen
VOL. 34,1980
on November 10, 2019 by guest
http://jvi.asm.org/
652 SIMMONS ET AL.
a
b
c
d
e
N
T
N
T
N
T
N
T
N
T
130K
100
K
94K
056 K
54K
'-FIG. 1. Acrylamide gelelectrophoresis ofproteins immunoprecipitated fromvariouslinesof
SV40-trans-formedcells: labeledproteinsprecipitatedwith eithernormal(N) oranti-T(T)hamsterserumfrom
SV40-transformedline 11A8 (a),215(b), 315(c), SV80(d), and 14B(e)cells. SV40-transformedmousecells (lines
11A8, 315, and215), ratcells (line 14B), and human cells (lineSV80) were labeledfor3h with L-[35S]-methionine. Extractswerepreparedandincubated with either normaloranti- T hamster serum,followedby incubation withproteinA-bearingS.aureus.Proteins in the washedprecipitatesweresubjectedtoacrylamide
gelelectrophoresisfor2.5hat25mA,and the labeledproteinsin thegelweredetectedbyexposuretoX-ray
film. Molecularweights wereestimated bythe relativeratesof migration ofmarkerproteinswithknown molecularweights(5). 130K=130,000 daltons.
species of each cell line (Fig. 1) were excised
from thegels,and theproteinwasextracted and
treated withtrypsin.Theresulting peptideswere
separated intwodimensionsbyelectrophoresis
and chromatography (fingerprinting) on
thin-layer cellulose plates (11). Methionine-labeled
peptidesweredetectedontheplates by
fluorog-raphy,using2-methylnaphthalene (2).To
com-pare any two proteins, peptide samples were
analyzedseparatelyondifferentplatesandas a
mixture on a third plate. Figure 2 shows the
fingerprintsof themethionine-containingtryptic
peptides of the Tau antigens isolated from the
mousetransformants 11A8 (Fig. 2A), 315 (Fig.
2B), and 215 (Fig.
20).
The fingerprint of amixture of the Tau antigen peptides derived
fromcelllines 11A8and 215is shown inFig. 2D.
Themethionine-labeled tryptic peptides of the
Tau antigens fromtwoofthesecell lines (lines
11A8and 215) were indistinguishable (Fig. 2A,
C,andD). Those derivedfromthe third mouse
cell line (line315) (Fig.2B) were slightly
differ-entinthat one peptide (peptide a, Fig. 2A and
C) wasabsent. The appropriate mixing
experi-mentshowedthat theremaining peptides of that
protein corresponded to the peptides derived
fromlinesllA8and215(data not shown). Since
lines 11A8 and 315originatedfrom theBALB/
cstrain ofmice, whereas line215originatedfrom
theAL/N strain, the observeddifferences can-not be due simply to a difference in strains.
Rather, they suggestthatmouse Tau antigens
mightvaryslightly from oneanother,
irrespec-tive of the strains from which the transformed
celllineswerederived. The similarities of these
fingerprints do indicate that the Tau antigens
isolated from mouse transformants are very
closely related proteins. This is in agreement
with the data of Linzer and Levine (19), Smith
etal. (28), andChangetal.(5).Therelationship
among the peptides of the three mouse Tau
antigensis shownbelow(see Fig. 5a).
In asimilarmanner,the Tauantigens isolated
from the rat and human transformants were
comparedwitheach other and withthose of the
mouse cell lines. Figures 3A through C show
that the rat(line 14B) Tau antigen contained16
methionine peptides (Fig. 3B) that were also
present in mouse (line 11A8) Tauantigen (Fig.
3A)and 7peptidesthat weredifferent.The Tau
antigen from human line SV80, on the other
hand,containedonly eightmethionine peptides
(Fig. 3D) that were also present in the mouse
protein (peptides 1, 3, 4, 6, 12, 13, 14, and 16)
(Fig. 3A, D, and E). These eightpeptideswere
alsopresent in the rat Tauantigen (Fig. 3B, D,
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.504.112.397.70.264.2]SV40 Tau ANTIGENS 653
*':.
ts...
4*~~~~~~~~~41
*
FIG. 2. Fingerprintsof methionine-labeledtrypticpeptidesofmouse Tau antigens isolatedfromline11A8
(A),315(B),215(C),andl1A8plus 215(D) cells. SV40-transformed mouse cell lines11A8,315,and 215 were labeledwith L-[3SJmethionine,andthe extracts of these cells were incubated with hamster anti-T serum in separate immunoprecipitation reactions. Tau antigensfrom each of these cell lines were isolated from preparativeacrylamide gels and treated withtrypsin,and the resulting peptides were applied near the corner ofathin-layer cellulose plate (11). Electrophoresis was carried out at 300 V for 3 h at4°C. Thedirection of electrophoresis wasfromleft to right near the bottom of eachfingerprint. This was followed by ascending thin-layerchromatography (frombottom to top in thefingerprints). Plates were dipped in 2-methylnaphthalene andexposedtoX-ray filmat-70°C for about 3weeks.
and F) and were therefore a subset of the 16
peptides common to the mouse and rat Tau
antigens. These dataaresummarized below(see
Fig. 5b).
The relationship betweenthe different
elec-trophoretic forms (56,000and 54,000daltons) of
theTauantigens isolated fromtransformed
hu-mancells (Fig. ld)wasinvestigatedby
compar-ing their methionine-labeled tryptic peptides.
For preparative purposes these proteins were
separated from one another by subjecting the
samplestoelectrophoresisforalonger periodof
time than for thegelshown inFig.1.Figures4A
and B show thefingerprints of the
methionine-containingtrypticpeptidesof the slower (56,000
daltons) and faster (54,000 daltons) species of
lineSV80 Tauantigen,respectively.The results
ofthemixingexperiment areshown in Fig.4C.
These two proteinscontained thesame 18
pep-tides. The 56,000-dalton form of the protein
containedanadditionalpeptide(Fig.4AandC,
peptideb).Thisresultissummarized below(see
Fig. 5c).
DISCUSSION
Ananalysis ofmethionine-labeled tryptic
pep-tidesshowed that the Tauantigens isolated from
three different linesoftransformedmousecells
were very similar. This is in agreement with
previous observations madebyus(5),by Linzer
andLevine (19),and mostrecently by Smithet
al. (28). We noticedin this study, however, as
did Smithetal.(28),thatallmouseTauantigens
were not identical. The Tau antigen isolated
fromline315lackedoneofthepeptides (Fig. 1,
peptide a)presentintheproteinsfrom the other
two mouselines(lines llA8and215).
Bycomparingthepeptidesofmousecell Tau
antigen (line 11A8) with those ofrat(line 14B)
and human (line SV80) transformed cells, we
found that the three proteins apparently
con-tained thesameeightmethionine-labeledtryptic
peptides (Fig. 5b). Furthermore,themouseand
rat Tau antigens appeared to be more closely
related, sharing an additional eight peptides.
The moststraightforwardinterpretationof these
VOL. 34,1980
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.504.114.399.70.318.2]654 SIMMONS ET AL.
'A 16
*
~~~~~1
*14
~13
12
*;::'sV;0 ....
~~~24 $~~~~~~
-'~~~~~~-g
16
*14
13
12
3
..
:
*
1 a:?. .16
#16
#14
t12
3 :
FIG. 3. Comparison ofmethionine-labeledtryptic peptides ofTauantigensisolatedfrommouse,rat,and humantransformants: fingerprints ofmethionine-labeledpeptides ofTauantigensisolatedfromlines 11A8 (A), 14B (B), 11A8plus14B (C),SV80(D), SV80plus 11A8(E), andSV80plus14B(F). Tauantigenswere
isolatedfrommethionine-labeledtransformedmouse(lineMMA8),rat(line 14B),andhuman(lineSV80)cells by immunoprecipitation and gel electrophoresis. Tryptic peptides wereprepared and separated in two dimensionsonthin-layercelluloseplates.Thenumberedpeptidesin(A),(B),and(C) refertopeptidescommon
tomouseandratTauantigens. Those in(D), (E),and(F) refertopeptidespresent inmouse,rat,and human
Tauantigens.
results is that these Tau antigens are
descend-antsofasingle proteinwhich has evolved
differ-ently in mice,rats,and humans. Thissituation
would be analogous to the evolution of
cyto-chrome cor hemoglobin (8), in which proteins
from closely related species are more similar
than those fromdistantly related species. This
wouldexplain the observedsimilarity in the Tau
antigens of mouse and rat transformants and
their more distant relationship to the human
protein. The eight peptides thatwerefound in
all oftheTauantigens thatweexaminedmight
correspond to regions in the proteins that are
conserved to maintain proper structure and
function.
Some lines of SV40-transformed celLs
con-tained more than one electrophoretic form of
Tauantigen(Fig. 1). This observation has been
madepreviously byus (5) and others (9, 16, 21,
28). Fingerprint analysis of the
methionine-la-beledtryptic peptides of the 56,000- and
54,000-daltonforms of theTauantigens isolated from
SV40-transformed human cells showed that
these proteins were closely related (Fig. 4 and
5c). Therefore theremaybeaprecursor-product
relationship between these two forms of Tau
C
..D
.:I
E
..
::.;...ft 16
.:...
F
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.504.114.401.68.435.2]SV40 Tau ANTIGENS
A
0
b
*:
E*'!
%.:w
,:::
g.:..
i .M-
w:.''...
.S x fUN...
.?,,::::
g£ 4.:' ': se, :te .b,,l_-;. .': ::N.:'.:::
?: ..:,
4>a
W...
::§:. ,:^ .:
:,X.'.'";.: :. ssMe,
, .:
t%'*
:..:t,@::.
*.. h.i:;:. |; ...
':
.+,. ,.
': :: ^+!
[image:6.504.110.398.69.333.2]..
FIG. 4. Comparison ofthemethionine-labeledtrypticpeptidesoftwo different forms ofhuman Tau antigen:
fingerprints ofthemethionine-labeledpeptides of the56,O0-dalton (A),54,000-dalton (B), and a mixture of
the 56,000- and54,000-dalton(C) Tauantigens isolated fromlineSV80 cells. Human transformed cells (line SV80)werelabeled withL-[3S]methionine, and extracts of these cells were incubated with anti-T serum in an
immunoprecipitationreaction. The 56,000- and 54,000-dalton forms of line SV80 Tau antigen (Fig. 1) were
separated bygel electrophoresisfor4hat 25mA. The trypticpeptides of these proteins were subjected to
electrophoresisandchromatography on thin-layer cellulose plates.
a IIAS
b
IIAS315
C
svo 56K
sv8o 54K
FIG. 5. Relationships among the methioni beled trypticpeptides ofmouse Tau antigens (a), mouse, rat,and human Tauantigens(b),and human
56,000-dalton(56K)and54,000-dalton(54K)Tau
an-tigens (c). The numbers in the areas where three circlesoverlapin(a) and(b) refertothe numbersof
peptidescommon tothethreespecies ofTauantigen
indicated. The numbersofpeptidescommontoonly twoofthe threeproteinsare indicated inthe other
antigen, or the proteins may be products of
nearly identicalgenes. Pulse-chase experiments
or labeling experimentsperformed in the
pres-ence of protease inhibitors might distinguish
between these
possibilities.
)
Recent datafromourlaboratory (manuscriptin
preparation)
indicatethataTauantigen
pro-SV80 tein can be immunoprecipitated from monkey
cells after infection with SV40. These results
imply that Tau antigens are induced in either
permissive or nonpermissive (i.e., mouse [19])
cells after infection with SV40. An analysis of
the methionine-labeled tryptic peptides of the
Tauantigen isolatedfrom infected monkeycells
showed that thisprotein isveryclosely related
to the Tau antigens from transformed human
ne-la-
cells
(line SV80). Thisobservation supports ourregions where the circles overlap. The numbers of peptides uniquetoindividualproteinsareindicated outsidetheoverlappingregions. In(c),inwhichonly twoproteins are compared, the number within the region ofoverlap refers to the number ofpeptides commontothesetwoproteins.
VOL. 34,1980 655
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.504.57.250.411.567.2]656 SIMMONS ET AL.
contention thatTauantigensareevolutionarily
conservedproteins.
Theinformationpresented in thispaperand
the evidence cited aboveallow us to speculate
about the role of Tau antigens in infected or
transformed cells. Given that Tauantigenscan
beisolatedfromtransformed cells in theformof
acomplex withT-Ag(17),that thesynthesisof
Tauantigens is induced after infection of
mon-keycells withSV40, and that Tauantigensare
conserved proteins, we can hypothesize that
theseproteinsareinvolvedinafunctionusually
attributed toT-Ag (forexample, the induction
ofhost DNA synthesis). It is well known that
theactivation ofhost DNAsynthesis isoneof
the early events that occur in permissive or
nonpermissive cells after infection with SV40
(13, 25). The agent that is responsible for this
process would have to be made in these celLs
early after infection and presumably in
trans-formedcellsaswell. Inadditiontomeeting this
requirement, Tau antigens appear tohave the
abilitytobindtoT-Ag,atleast intransformed
cells (17). We can speculate, therefore, that a
complex ofT-Ag andTauantigen isresponsible
forthis induction.Fromthismodel,it iseasyto
explain the observation that Tau antigens are
conserved proteins since they would have to
interact with SV40 T-Ag, as well as with the
DNA-synthesizingmachinery ofmanydifferent
typesofcells.
ACKNOWLEDGMENTS
WeacknowledgeMargaret Sexauer for her expert technical assistance.
This workwassupportedin partbyPublic Health Service grant CA25942toD.T.S.fromtheNational Cancer Institute.
LITERATURE CITED
1. Ahmad-Zadeh, C., B. Allet,J. Greenblatt, and R. Weil. 1976. Two forms of simian virus40-specificT antigen in abortive andlyticinfection. Proc.Natl. Acad. Sci. U.S.A. 73:1097-1101.
2.Bonner, W. M., and J. D. Stedman. 1978. Efficient fluorography of 3H and "4Conthinlayers.Anal. Bio-chem.89:247-256.
3. Botchan, M., W.Topp,and J.Sambrook.1976.The arrangement of simian virus40sequences in the DNA
of transformed cells. Cell 9:269-287.
4. Carroll,R.B., and A. E. Smith.1976.Monomer molec-ularweightof Tantigen from SV40-infected and trans-formed cells. Proc. Natl. Acad.Sci. U.S.A.
73:2254-2258.
5. Chang, C.,D.T.Simmons, M. A. Martin, and P. T. Mora. 1979. Identification and partial characterization
of new antigens from simian virus 40-transformed
mousecells. J. Virol. 31:463-471.
6. Crawford, L. V.,D.C.Pim, and D. P. Lane. 1980. An immunochemical investigation of SV40 T-antigens. II. Quantitationofantigens and antibody activities. Virol-ogy 100:314-325.
7.DeLeo, A. B., G. Jay, E. Appella, G. C. Dubois, L. W. Law,and L. J.Old. 1979. Detection of a
transforma-tion-related antigen in chemically induced sarcomas andother transformed cells of the mouse. Proc. Natl. Acad.Sci. U.S.A. 76:2420-2424.
8. Dickerson, R. E., and I. Geis. 1969. The structure and action ofproteins. Harper & Row, Publishers, New York.
9. Edwards, C. A., G. Khoury, and R. G. Martin. 1979. Phosphorylation of T-antigen and control ofT-antigen expression in cells transformed by wild-type and tsA mutantsof simian virus 40. J.Virol. 29:753-762. 10. Gaudray, P., M.Rassoulzadegan, and F. Cuzin. 1978.
Expression of simian virus40early genes in transformed ratcells is correlated with maintenance of the trans-formedphenotype. Proc. Natl. Acad. Sci. U.S.A. 75: 49874991.
11. Gibson, W. 1974. Polyoma virus proteins: adescription of the structuralproteins of the virion basedon poly-acrylamide gel electrophoresis and peptide analysis. Virology 62:319-336.
12. Gracy, R. W. 1977. Two-dimensionalthin-layermethods. Methods Enzymol. 47:195-205.
13. Hatanaka, M., and R. Dulbecco. 1966. Induction of DNA synthesis bySV40. Proc. Natl. Acad. Sci. U.S.A. 56:736-740.
14. Jonsson,S., and G. Kronvall. 1974. The use of protein A-containing Staphylococcus aureus as a solid phase anti-IgG reagent in radioimmunoassays as exemplified in thequantitation of a-fetoprotein in normal human adult serum. Eur. J. Immunol. 4:29-33.
15. Kress, M., C. De Vaux Saint Cyr, and M. Girard. 1977. The molecular weight of SV40 T-antigens.INSERM Colloq. 69:79-92.
16. Kress, M., E. May, R.Cassingena, and P. May. 1979. Simian virus 40-transformedcells express new species ofproteinsprecipitable by anti-simian virus 40 tumor serum.J. Virol. 31:472-483.
17. Lane, D. P., and L. V.Crawford. 1979. T-antigen is bound to a host protein in SV40 transformed cells. Nature (London) 278:261-263.
18. Lichaa, M., and E. Niesar. 1977. Characterization of SV40T-antigen present in SV40 transformed cells. IN-SERMColloq. 69:211-222.
19. Linzer, D.I. H., and A. J.Levine. 1979. Characterization ofa54Kdalton cellular SV40 tumor antigen present in SV40-transformedcells and uninfected embryonal car-cinomacells. Cell 17:43-52.
20. Maurer, H. R.,and R. C. Allen. 1972. Useful buffer and gelsystems foracrylamide gel electrophoresis. Z. Klin. Chem. Klin. Biochem. 10:220-225.
21. Melero, J. A., D. T. Stitt, W. F. Mangel, and R. B. Carroll.1979.Identification ofnewpolypeptidespecies (48-55K)immunoprecipitable by antiserum to purified large T antigen and present in SV40-infected and -transformed cells. Virology 93:466-480.
22. Paucha, E., R. Harvey, and A. E. Smith. 1978. Cell-free synthesis of simian virus 40 T-antigens. J. Virol. 28; 154-170.
23. Prives, C., E. Gilboa, M. Revel, and E. Winocour.
1977. Cell-free translation of SV40 early messenger RNAcoding for viral T antigen. Proc. Natl. Acad. Sci. U.S.A. 74:457-461.
24. Prives, C., Y. Gluzman, and E. Winocour. 1978. Cel-lular andcell-free synthesis ofsimianvirus40 T-anti-gens inpermissive and transformed cells. J. Virol.26:
587-595.
25. Sauer, G., and V. Defendi. 1966. Stimulation of DNA synthesis and complement-fixing antigen production by SV40inhuman diploidcellcultures: evidence for "abor-tive" infection. Proc. Natl. Acad. Sci. U.S.A.
56:452-457.
26. Schwyzer, M. 1977. Purification ofSV40 T-antigen by immunoaffinity chromatography on staphylococcal pro-J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
SV40 Tau ANTIGENS 657
teinA-Sepharose.INSERMColloq.69:63-68.
27. Simmo D. T., K. K. Takemoto, and M. A. Martin.
1977.Relationshipbetween the methionine tryptic
pep-tides of simian virus 40 and BK virustumorantigens. J. Virol. 24:319-325.
28. Smith, A. E., R. Smith, and E. Paucha. 1979. Charac-terization of differenttumorantigenspresentincells
transformedby simian virus 40. Cell 18:335-346.
29. Tegtmeyer, P., M. Schwartz, J. K. Collins, and K. Rundeli. 1975.Regulation oftumorantigen synthesis by simian virus 40geneA. J.Virol. 16:168-178.
30. Todaro, G. J., H. Green, and M. C. Swif. 1966. Sus-ceptibility of human diploid fibroblast strainsto
trans-formationby SV40 virus. Science 153:1252-1254. VOL. 34, 1980
on November 10, 2019 by guest
http://jvi.asm.org/