JOURNALOFVIROLOGY,Mar. 1993, P. 1315-1321 0022-538X/93/031315-07$02.00/0
Copyright
© 1993,AmericanSocietyforMicrobiologyRelevance
of
Cysteine
Residues for
Biosynthesis
and
Antigenicity
of Human
Hepatitis
B
Virus
e
Protein
GEORG WASENAUER, JOSEF KOCK, AND HANS-JURGEN SCHLICHT*
Department of Virology, University of Ulm, Albert Einstein Allee 11, W-7900 Ulm, Germany
Received 28September 1992/Accepted 10 December1992
The mature form of the secretory core protein (HBe protein) of human hepatitis B virus contains four
cysteineswhicharelocated atamino acidpositions -7, 48, 61, and 107 relativetothe HBcstartmethionine. In addition, there is acysteine, Cys-183, located in the C-terminal domain of the HBe precursor, which is
cleavedduringHBe maturation.Here,thesignificanceof thesecysteinesforbiosynthesisandantigenicityofthe HBeproteinwasexamined. Thecysteinesatpositions -7 and 61werefoundtobe crucial for HBebiosynthesis. As hasalreadybeendescribed, if theCysatposition -7 ismutated, disulfide-linked HBehomodimerswhich have both HBeantigenicity and HBcantigenicity areexpressed. Herewe showthat thesedimersare due to
Cys-61-Cys-61 disulfide bridgeswhichareformedonlyif theCysatposition-7isnotpresent.Inthewild-type protein,thisdimerizationappearstobe inhibitedbyformation of intramoleculardisulfide bridges betweenthe Cysat -7 andoneofthe internalcysteines. Moreover, Cys-61 isimportant forHBebiosynthesis ingeneral since mutation ofthis amino acid results inproduction of HBeproteinswhichareeitheronlypoorly secreted
orpossessadifferentantigenicity.
One interesting aspect of hepatitis B virus (HBV) gene
expression is that two different core gene products are
produced byinfected cells. One of these proteins, theHBc protein, isthecoreproteinproper,which is locatedprimarily in thecytoplasmand thenuclei of the cells. It self-assembles intonucleocapsidsin avarietyof celltypesand isexported bythe cells onlyaspart ofavirusparticle (4).
The function of the second protein, which is called the HBeprotein,is stillenigmatic.Thisproteinis encodedbyan
mRNA which isslightly larger than the mRNA from which the HBc protein is translated. For this reason, the HBe
precursor contains a small extra sequence, the so-called pre-Csequence, atits Nterminus which isnotpresentinthe HBc protein. This sequence acts as a signal sequence for secretion and mediates translocation of the HBeprecursor into the lumen of theendoplasmicreticulum(8, 9).Aftertwo processingstepsduringwhich 19 of the 29 amino acids of the signal sequence, as well as a strongly basic C-terminal
domain, are proteolytically removed, themature HBe pro-tein is secretedbythe cells(5, 15, 16). Thus,withrespectto itsprimarysequence, theHBeproteindiffers from the HBc proteinin that it contains 10 additional amino acids atits N
terminus and lacks the stronglybasic C-terminal domain. Although the HBc and the HBe proteins share most of their primary amino acid sequence, they exhibit greatly differentbiophysical behaviors. The HBcprotein efficiently assembles intoparticlesand bindsto antibodies whicharise invirtuallyall infected individualsvery early during infec-tion. These antibodies, termed anti-HBcAg, recognize a
linearsequenceonthe HBcprotein (aminoacids 74through 85) which, however, is accessible only on particulate core
protein (10, 11). The HBe protein normally does not form
particles and contains other private epitopes which are
recognized byantibodies(anti-HBeAg)which ariseonlylate
duringinfection andwhoseappearancecorrelates withvirus
elimination(3, 6, 7, 12).
Recentlywehave shown that thedifferences betweenthe
* Correspondingauthor.
HBc and HBeproteins are due mostlyto the shortpiece of the pre-C sequence which remains attached to the mature HBe protein aftercleavage of the signal sequence (14, 17).
One important element in this sequence was found to be a
cysteine. When this amino acid was mutated, an HBe-like
protein was synthesized which differed from the authentic HBeprotein with respect to quaternary structure and anti-genicity.We haveextended these studies by mutatingone or
moreof the internalHBe cysteines. The data show thatall of the cysteines are involved in the formation of disulfide
bridgesbut that onlyCys-61 is crucial forHBebiosynthesis andantigenicity.
MATERUILS ANDMETHODS
Polymerase chain reaction mutagenesis and generation of vaccinia virus recombinants. The vaccinia virus
recombi-nantsthat encode thewild-type (WT) HBe protein and the
mutant that lacks thecysteine in thenoncleaved portion of thepre-C region have alreadybeen described (13, 14). The othermutants, containing one ormore cysteine mutations, were generated by recloning of restriction fragments
ob-tained from constructs containing arecombinant HBegene
in which the pre-C sequence was replaced by the signal
sequence of the influenza virus hemagglutinin. Single
cys-teine mutations had been introduced into this construct by polymerase chain reaction mutagenesis (17). In all of the
mutants, the cysteines were replaced with serines, except forCys -7/61mutantM7,inwhich thecysteineinthe pre-C region was replaced by a proline. A schematic representa-tion of themutants used in this studyisgiven in Fig. 1.
Sera anddiagnostictestkits.(i)Monoclonal antibodies. The four monoclonal antibodies used for immunoprecipitation
werekindly provided byM.Noah, Behringwerke, Marburg,
Germany. One of these, antibody 03, was specific for the HBelepitope;twoantibodies, 152 and158,werespecificfor
the HBe2 epitope; and one antibody, 312, was specific for
the HBc epitope. All of the monoclonal antibodies were
producedin mice. Theepitopes recognized byantibodies 312 and 152areknown atthe amino acid level (10-12).
1315
Vol. 67, No. 3
on November 9, 2019 by guest
http://jvi.asm.org/
1316 WASENAUER ET AL.
-7 48 61 107 183
[image:2.612.60.296.72.439.2]..;!
,
u
,
1:::::::::::::::s~~~~~~~...
... .. ...
FI. 1. Sceai rersntto oth teins
anl
yze
in thistuy
Thport--'ion
M*
an M12 theposibe-ntamoecla
shown...'
~~~~~~~~~~~~~~~~~~~~~~~...1.'''''
'
...
~~~~~~~~~~~~~~~~~~~~~~~~...
.......-i
sa!-
,
1"'''''''''"'~~~~~~~~~~~~...
'
at
~~~~~~~~~~~~~~~~~~~~~...
1,=iFIG.1.Schematicrepresentation of...
teinsanalyzed in this study. Theporti... which. clavdduig.B.mtrai...
lines.The10-amino-acid portion of thepre...
attached ...atrH eprti...
FniG.t t.Shemposticnrersnttoofsthiersdes
Mll,andM12,thepossibleintramolecular
shown.
(ii) Polyclonal anti-HBc-positive hum anti-HBc-positive humanserum was o ically HBV-infected patient. It was 4
Recombinant Mutated tories (rDNA enzyme immunoassay) and Sorin Biomedica Cysteine (Saluggia,Italy) (enzyme-linked immunosorbent assay)were
wT
HBe used.Allanalyses
weredone inaccordance with the instruc-tions of the manufacturers.Expression of HBV core gene products in tissue culture.
Ml
-7 Cultivation and infection of HepG2 cells were done essen-tially as previously described (13, 14). Inbrief, for standard M2 48 analyses, HepG2 cells (1) (ca. 2 x 106cellsgrown in a20-cm2
dish with 3 ml of Dulbecco's minimal essential medium containing 10% fetal calf serum at 5% C02) were infected M3 61 with recombinant or WTvaccinia virus at a multiplicity of infection of 10 in 1 ml of serum-free medium. After 90 minat M4 107370C,
the inoculum was removed and 2 ml of fresh mediumcontaining 10% fetal calf serum was added. Analyses were done 16 to 36 h after infection.
M5 183 Isolation and
analysis
ofHBVcoregeneproducts
by immu-noprecipitation and Western blotting (immunoblotting). The techniques employed for isolation and analysis of HBV core M6-7/48
gene products were essentially as previously described (13, 14). In brief, cells were grown and infected in20-cm2 dishes M7 -7/61 as describedabove,
themediumwasremoved,
and the cellswere lysed in 1 ml of PBS containing 1% Triton X-100. Nonsoluble material was pelleted by centrifugation, and the M8 -7/107 cleared lysatewasused forimmunoprecipitation. The whole lysate or 2 ml of medium was used to prepare a single M9 -7/183 Western blot
sample.
Forimmunoprecipitation,
5 ,u of polyclonal antiserum was adsorbed to 25 pdof preswollen protein A-Sepharose (Pharmacia, Freiburg, Germany).M1o
48/61 When mouse monoclonal antibodies were used, 5RI
of amonoclonal antibody solution (immunoglobulin concentra-tion, 1 mg/ml) was adsorbed to 25,ul of preswollen protein
M1
148/107
A-Sepharose together with 5[lI
of goat anti-mouseimmuno-globulin (Sigma, Deisenhofen, Germany). Immunoprecipita-M12 61/107 tion was carried out for 16 h at 4°C. The samples were washed twice with TNE (20 mM TrisHCl, 100 mM NaCl, 10 mM EDTA, pH 8.0), boiled in sample buffer with orwithout M13 -7/48/61 2-mercaptoethanol for 5
min,
and applied to12.5%
polyac-rylamide gels. In one experiment, reduction wasperformed erecombinant HBe pro- by boiling withdithiothreitol
(end concentration, 20 mM) of the precore protein and blocking of free sulfhydryl groups with iodoacetamideIn
are shown as dashed-C region which remains (15
min,
37°C; end concentration, 100 mM). The samples shaded. The solid dots were separated on12.5%
polyacrylamide gels and blotted For recombinantsM10,
onto nitrocellulose by using standard procedures. After disulfide bridges are also blockingfor 6 hwith 1%bovine serum albumin in PBS, the blots were incubated for >16 h with polyclonal HBc/e-specific rabbit serum (end dilution, 1:2,000). After washing for 30 min withPJ3S-1%
Triton X-100, specifically bound antibodies were detected byincubating the filters for 3 h with tan serum. Polyclonal alkaline phosphatase-labelled protein A(Sigma)andstaining btained from a chron- with a commercial alkaline phosphatase detection system strongly HBe protein (Boehringer GmbH, Mannheim, Germany)._A . .
positive and had ananti-HBctiter of about10' asdetermined by endpoint dilution using a diagnostic radioimmunoassay (Coreab; Abbott Laboratories, Wiesbaden, Germany).
(iii) HBc/e-specific polyclonalrabbit serum.HBc/e-specific polyclonal rabbitserum wasgeneratedbymultiple intramus-cular injections of recombinant HBc protein (100 ,ug per dose) denatured by boiling for 5 min in phosphate-buffered saline (PBS) containing 1% sodium dodecyl sulfate and 1% 2-mercaptoethanol. No adjuvant was used. This serum re-actsefficientlywith allknown HBVcore geneproducts. The suitability of theseantibodiesforanalysis ofHBVcore gene products by immunoprecipitation has already been de-scribed (14, 17).
(iv) Diagnostic test kits. For analysis ofHBe antigenicity, the HBe-anti-HBe diagnostic testkitsfrom Abbott
Labora-RESULTS
As is shown in Fig. 1, the HBe precursor which is expressed by recombinantWT HBe, also referred to as the precore protein, contains eight cysteines. Four ofthese are located in the pre-C region: three inthe 19-amino-acid distal portion which is cotranslationally cleaved and one in the 10-amino-acid proximalpiecewhichremains attached tothe mature HBe protein. As has been described byus recently (17), the three cysteines in the cleaved portion have no obvious effect on HBe biosynthesiswhereas the cysteinein the remaining 10 amino acids is very important. If this cysteine is mutated, the HBe protein, which usually is a monomer with only HBe antigenicity, becomes a
disulfide-J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
HBe PROTEIN BIOSYNTHESIS AND ANTIGENICITY 1317
linked homodimer with both HBe antigenicity and HBc antigenicity. However, how this cysteine couldhave such a
drastic effect on HBe structure remained unclear. The four other cysteines are located internally, three in the region
which makes up the mature HBe protein and one in the
C-terminal portion which is cleaved during HBematuration. To analyze the relevance of these cysteines for HBe biosynthesis, wefirstconstructedfourmutantsin whichone
ofthe internalcysteineswasreplaced withaserine (mutants
M2 to M5; Fig. 1). In these and all other experiments, the mutatedgeneswere recombinedinto vaccinia virusvectors and the recombinant viruses thus obtained were used to
expresstheproteinsin the human hepatomacell lineHepG2.
Core gene products were isolated from the tissue culture
supernatant orfromthe cell lysates by immunoprecipitation
and analyzed by sodium dodecylsulfate-polyacrylamide gel electrophoresis andWestern blotting either withorwithout
reductionwith 2-mercaptoethanol. For comparison,weused
samplesobtained from cells infected with recombinants that encode either theWT HBe protein or the mutant in which thecysteine in the noncleaved portion of the pre-Csequence
had been replaced with a serine (mutant Ml; Fig. 1). For
each mutant, at least two different recombinants were
tested. If no or only low-level protein expression was
observed, recombinationwasrepeatedand againatleasttwo
recombinantswere examined.
Mutationof cysteine 61severelyimpairs HBe secretion.The
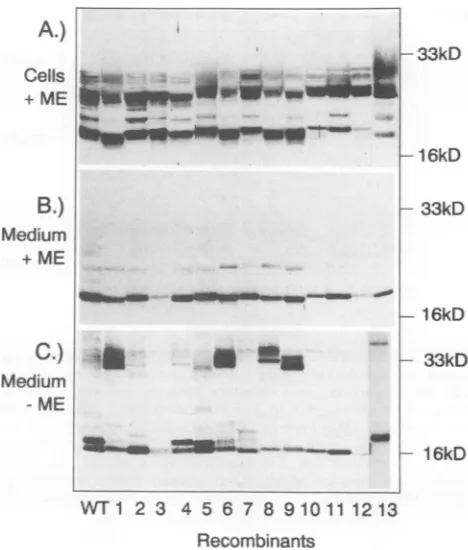
Western blot results obtained with these and several other mutants are shown in Fig. 2A to C. The Western blot in
panel A shows the cell lysate samples after reduction with 2-mercaptoethanol, the blot in panel B shows the secreted
proteins after reduction, and the blot in panel C shows the
secreted HBe proteins without reduction. The cell lysate sampleswithout reduction are not shown because the band pattern wastoo complex.
Asis shown in Fig. 2A, lanesWT and 1to5, theWTand themutatedcore geneproducts lackingonecysteine
(recom-binants 1through5)werewell expressedwithin the cells. In
accordance with previous findings, at least two different proteinspecieswith sizes of about 22 to23kDa and 17kDa, correspondingtothepartially processed and thematureHBe proteins, were observed. In this experiment, only small
amounts of the full-length precore protein (25 kDa), whose
abundance is highly variable, were detected.
However, analysis ofthemedium samples withorwithout
reduction with 2-mercaptoethanol revealed someinteresting
differences. (i) Mutation of cysteine 61 resulted in
produc-tion of an HBe protein which was only poorly secreted
(compare recombinant3 with theothermutants). (ii)
Muta-tion of either cysteine 48, 107,or183(recombinants 2,4,and
5, respectively) influenced the formation of intramolecular disulfide bridges, an effectwhich became visible only when
the sampleswere analyzed without reduction (Fig. 2C).
Cysteines 48 and 107 are involved in intramolecular
disul-fide bridge formation. As is evidenced by the two closely
spacedprotein bands inFig. 2C, lane WT, therewereatleast two different HBe species in the nonreduced sample. Since
these two HBe proteins migrated faster than the reduced
HBe protein, which showed up as a single band (Fig. 2B,
lane WT), theymost likelyrepresent different tertiary
struc-turesofoneprotein whichareduetodifferentintramolecular
disulfide bridges. Both cysteines 48 and 107 appear to take part in these disulfide bondings, since mutation of either
amino acid significantly altered the ratio between the two
species, whichare usually present in about equal amounts
(comparethe WTwith recombinants 2 and4). Interestingly,
A.)
Cells
+ ME
B.)
Medium
+ ME
C.)
Medium ME
-33kD
-1 6kD
- 33kD
-16kD
- 33kD
WT1
23 4567 8910111213
Recombinants
FIG. 2. Western blot
analysis
of HBV core gene productsex-pressed
inHepG2
cells. The cellswere infected with recombinant vaccinia viruses that encode either theWT HBe protein ormutantproteins lacking
one or more cysteines. Core gene products wereisolatedeither fromacell
lysate
(A)orfromthe culture medium(B andC)
by using
HBc/e-specific
polyclonal rabbit serum. Thesam-ples
wereseparated
on a 12.5%polyacrylamide
gelwith (+ME) orwithout
(-ME)
reduction with 2-mercaptoethanol. After Westerntransfer, core gene
products
were detected by using the sameHBc/e-specific
rabbit serum and alkaline phosphatase-labelledpro-tein A.The numbers of the lanescorrespondtothenumbers ofthe recombinants shown in Fig. 1.
we also observed a
slight
butreproducible
alteration of the bandpattern
whencysteine
183wasmutated (Fig. 2,recom-binant 5; see also
Fig.
5).
Twoprotein
bands were stillvisible,
but these were more narrowly spaced. This findingsuggests
thatcysteine
183,
which isnotpresentin thematureHBe
protein
because of the C-terminal cleavage ofthe HBeprecursor, influences HBe folding.
The disulfide-linked HBe dimers are exclusively due to
Cys-61-Cys-61
disulfide
bridges.
We recently reported thatmutation of the
cysteine
at -7 leads to production of HBe homodimers which are linked via a disulfide bridge. Thiseffectis also visible in
Fig.
2C(compare
lanesWT
and1). Inthiscontextit should be stressed that here, aswell asinour
previous
reports,
the term dimer will be usedto refer toanHBe homodimer which is covalently linked via a disulfide
bridge.
This meansthat thepossibility
that thereare dimerswhichare due to noncovalent linkagesis leftopen.
To
gain
adeeper insight
into the mechanismby whichthecovalently
linked dimers are formed, we prepared fourdouble mutantsin which the
cysteine
at -7 andoneoftheinternal
cysteines
weremutated(recombinants
6through9;Fig. 1). Figure
2,
lanes6to9,
shows theproteinswhichwereobtained after
expression
of these mutants. As is clearlyvisible,
mutation ofcysteine
61completely abrogated HBe-~~~~~~~~~~~~~~~L
-__
a_
_
|
1 k
VOL.67,1993
on November 9, 2019 by guest
http://jvi.asm.org/
[image:3.612.320.554.76.351.2]+ + + +
+-WT1011 121313
Recombinants
FIG. 3. Comparison of the mobility of WT HBe protein and mutant proteins lacking one or more cysteines with and without
reduction. Expression and Western blot analysis were done as
described in the legend to Fig. 1, except that reduction was
performed withdithiothreitol (DIT) and subsequent blocking of free sulfhydryl groups with iodoacetamide. This allows side-by-side
analysis of reduced and nonreduced samples on one gel. Lanes
which show samples which were reduced before analysis are
markedwithaplus sign. The numbers of the lanes correspondtothe numbers of the recombinants shown in Fig. 1.
dimerization (compare recombinant 7 with recombinants 6, 8, and9). On the otherhand, mutationofcysteines 48, 107, and 183 resulted only in some alteration of the complex
pattern ofthe dimerbands. As is also obvious from Fig. 2, mutationofCys-183(recombinant 9)also hadacleareffect.
The cysteine at -7 can form intramolecular disulfide
bridgeswithanyof theinternalcysteines.One mechanismby which the cysteine at -7 could inhibit HBe dimerization would be formation of an intramolecular disulfide bridge
withCys-61. Whether there is such acysteine -7/61
disul-fidebridgecouldnotbe testeddirectly bymutationofCys-61 alone, sincetheresultingproteinwassecreted in onlytrace amountsand the cellularproteinpatternwastoocomplexto be analyzed without reduction (data not shown). To over-come this problem,three double mutantswereprepared in
which not only one but two of the three internal HBe cysteines weremutated(mutants 10 through 12; Fig. 1).
Expression of the recombinant proteins revealed two interesting effects. (i) The two mutants containing the cys-teine at-7butlackingCys-61 (recombinants10 and12)were poorly secreted, and (ii) all proteins migrated faster if not reduced. This suggests that these proteins still contain an intramolecular disulfide bridge. Since, as is shown in Fig. 1, only oneintramolecular disulfide bond ispossible inmutants 10, 11,and12, thismeansthat thecysteineat-7caninteract with any of the threeinternal cysteines.
Toexaminethis point further, we preparedatriplemutant (mutant M13; Fig. 1) which lacked two internal cysteines (Cys-48and Cys-61) aswell asthecysteineat -7. Since in this mutant only one cysteine is left, there can be no intramolecular disulfidebridge. The results of the analysis of thismutant areshown inFig.2(recombinant 13) andin more detail inFig. 3.
In theexperimentwhose results areshown in Fig. 3,we examined side by side themobility of the secreted WT HBe protein and that of the recombinants lacking Cys-48 and Cys-61(recombinant 10), Cys-48 andCys-107(recombinant 11), Cys-61 and Cys-107 (recombinant 12),orthecysteines at -7,48, and 61(recombinant13).Reduction, whichinthis experiment was performed with dithiothreitol and subse-quentblockingoffreesulfhydryl groups with iodoacetamide, was done as indicated in the figure legend. Efficiency of reduction can easily be monitored by the immunoglobulin heavy chains (size, about 45 kDa afterreduction).
Asis shown inFig. 3, only after mutation of three of the four HBecysteineswas aproteinexpressedwhichmigrated equally fast with or without reduction. The mostplausible explanation for this finding is thatnonreduced recombinant proteins 10, 11, and 12 are more compact owingto intramo-leculardisulfide bridgeswhichmustbeformed between the cysteine at -7and any of the internalcysteines.
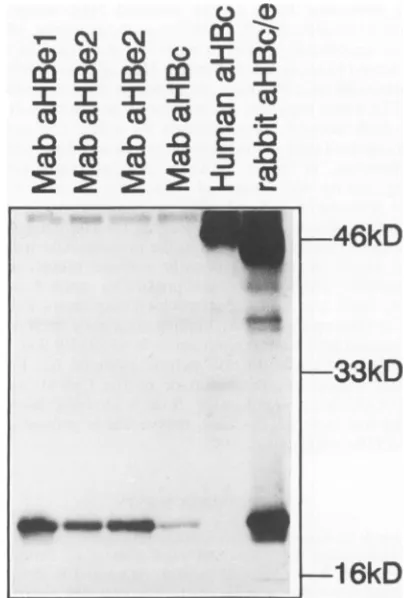
[image:4.612.65.297.60.244.2]Mutation ofCys-61 reduces HBeantigenicity. One impor-tant characteristic of the HBe protein is that it is serologi-cally distinct from the other major core gene product, the HBcprotein. Since formation of the intermolecular Cys-61-Cys-61 disulfidebridgeresulted inanHBeproteinwhich had not only HBe but also HBc antigenicity, we wondered whether the intramoleculardisulfidebridges also contributed
TABLE 1. AnalysisofHBeAg content in medium from cells expressingWTHBeprotein or mutantproteins
lackingone, two, or threecysteinesa
Photometer reading Testkitsource Dilution
WT M2 M4 M5 M7 M1o M11 M13
Abbott Laboratories None >2 1.9 1.9 >2 >2 0.5 1.6 1.6
1:10 1.7 1.6 1.6 1.4 0.8 0.2 1.4 0.2
1:20 1.4 1.7 1.3 1.5 0.5 0.2 1.5 0.1
1:50 1.2 1.4 0.9 1.2 0.4 0.2 0.9 0.1
1:100 0.7 1.1 0.7 0.8 0.2 0.2 0.6 0.1
Sorin Biomedica None 2.7 2.4 2.5 2.5 2.7 0.6 2.5 2.2
1:10 2.7 2.1 2.7 2.4 0.8 0.1 2.5 0.7
1:20 2.4 1.9 2.6 2.4 0.5 0.2 2.3 0.3
1:50 2.4 2.4 2.3 2.3 0.2 <0.1 2.0 0.1
1:100 1.8 2.4 1.2 1.4 0.1 <0.1 1.0 <0.1
aSampleswerediluted asindicatedandtested for HBeAg by using diagnostic test kits from Abbott Laboratories and Sorin Biomedica. The values in the tables shownarethephotometerreadouts which correspond to the enzymatically formed colors. Higher values mean higher HBeAg contents. The plateau values were about 2.0 for the Abbotttestand 2.7 for theSorintest.Negativesamples had a valueof 0.04 in both tests.
DTT-
+WF1011 121313
EIILIN
... --imolimw
on November 9, 2019 by guest
http://jvi.asm.org/
[image:4.612.60.558.566.696.2]HBe PROTEIN BIOSYNTHESIS AND ANTIGENICITY 1319
00a)
m(.
(1X
C:
X
cco
fin
E
co
Xc
co
C
=5
2 2
2
2
I:
co
_0
.mm
33kD
[image:5.612.77.279.78.377.2]*1 6kD
FIG. 4. Analysisof the antigenicity of the HBeprotein lacking cysteines at positions -7, 48, and 61 by immunoprecipitation. HepG2 cellswere infected with recombinant M13, and core gene
products were isolated by immunoprecipitation from the tissue culture medium by using the indicated monoclonal antibodies or
polyclonalsera.Proteinswereseparatedon a12.5%polyacrylamide
gel without reduction,and after Western transfertheyweredetected
by using HBc/e-specificrabbitantiserum and alkaline phosphatase-labelled protein A. Lanes: Mab aHBel and aHBe2, HBe-specific monoclonalantibodies; Mab aHBc, HBc-specific monoclonal anti-body; Human aHBc, patient serumpositive for HBe protein and
HBc-specific antibodies; rabbit aHBc/e, polyclonal rabbit serum
positiveforbothHBe- andHBc-specificantibodies.
to the HBe antigenicity. To test this, we first performed
immunoprecipitation analyses by using polyclonaland
mono-clonal antiseraspecificfor theHBeprotein,theHBcprotein,
orboth. The specificityof thedifferent sera andantibodies
has been demonstratedpreviously (14, 17).
Surprisingly, no gross alteration ofantigenicity was
ob-served. Whetherone,two,orthreecysteinesweremutated,
theproteins alwaysboundtotheHBe-specificantibodies but not to the HBc-specific antibodies. As an example, the
Western blot obtained with recombinant13,which lacks all butonecysteine, is shown inFig. 4.The weakreactivity of recombinant 13 with theHBc-specific monoclonalantibody shown in the figure is not significant, since we often
ob-servedsomereactivityof HBeproteinswith thismonoclonal
antibody which, however,varied betweenexperiments.The
more reliable reagent was the polyclonal HBc-specific
hu-man serum, which never showed any reactivity with HBe
proteins.
Sinceitappeared possible thatthecysteinemutations had
a moresubtleeffecton antigenicity,weperformed serologic
analyses by using two commercially available diagnostic
46kD
27I
2 4
5
7
WT
1310 11
Recombinant
FIG. 5. Analysis of the expression efficiency of the WT and mutated HBe proteins used for antigenicity testing with two diag-nostic test kits. Portions of the medium samples used for the
diagnostic assays were subjectedtoimmunoprecipitation byusing
polyclonal HBc/e-specific rabbit serum and analyzed by Western blotting withoutreduction,asdescribed in thelegend to Fig. 1.
kits. Compared with immunoprecipitation, these test kits have the important difference that for a protein to react positively in the assays, two different antibodies that recog-nize distinct epitopes on the same protein must bind con-comitantly. With the Abbotttestkit, a polyclonal antiserum isemployed for both the solid and the fluid phases, whereas with the Sorin assay, two different monoclonal antibodies areused.Inaddition, these assays also allow comparison of thestrengths of the antigen-antibody reactions since binding of theantigenscan be testedat different dilutions.
For these analyses, eight different recombinant proteins wereselected: theWTHBeprotein; the three single mutants M2, M4, and M5, lacking either cysteine 48, 107, or 183, respectively; the three double mutants M7, M10, and Mll, lacking either cysteines -7and61, 48 and 61, or48and 107, respectively; and the triple mutant M13, lacking cysteines -7, 48, and 61. HepG2 cellswereinfected with the respec-tive recombinant vacciniaviruses, and after2days thetissue culture medium wascollected. To test efficiency of protein expression, an aliquot of the medium was subjected to immunoprecipitation and subsequent Western blotting by usingthepolyclonal rabbit serum, whichreactswellwith all known coregene products. Forantigenicity analysis, undi-luted medium and four different dilutionsbetween 1:10 and 1:100weretestedwith thetwodifferentdiagnostic test kits. The results of these experimentsareshownin Fig. 5 and in Table 1.
Asis demonstrated by the Western blotin Fig. 5, expres-sion efficiencies didnotvarymorethan aboutfivefold, with mostof the recombinantproteinsbeing expressed in similar amounts. However, in the serological assays differences were observed which could not be explained by different expression efficiencies alone.Asisshown in Table1, when-everCys-61wasmutated, HBe antigenicity dropped signif-icantly. This was especially obvious for the triple mutant, M13. Since mutant Mll, lacking cysteines 48 and 107, exhibited normal HBeantigenicity,wepropose thata disul-fide bridge between the cysteines at -7 and 61, the only possible intramolecular disulfide bridge in mutant Mll, is important forHBeantigenicity.
VOL.67, 1993
on November 9, 2019 by guest
http://jvi.asm.org/
[image:5.612.348.525.78.244.2]DISCUSSION
Afterwediscovered thata secretionaryblock ofa
C-ter-minally truncated HBe precursor could be overcome by
using another leader peptide (14),we decidedto analyze in detail how thepre-Csequence influencesfolding oftheHBe
protein. To this end,wehave shown previously thatatleast
two important functions are exerted by the 10-amino-acid noncleavedportion of the pre-Csequence. (i) A hydrophobic
motif inhibitsassembly of the HBe protein into particles, and (ii) a cysteine prevents formation of disulfide-linked HBe dimerswhichexhibitagrossly altered antigenic profile (17).
In the work described here, we examined disulfide bond formation within and between HBeproteinmolecules. With these experiments, wewanted to answertwo questions: (i) bywhich mechanism does the cysteine in the pre-C region inhibit formation ofcovalentlylinked HBe dimers, and (ii) how important are intramolecular disulfide bonds for HBe
secretion and antigenicity?
Withrespect tothe firstquestion,wefound that therecan
be intramoleculardisulfide bonds between thecysteinein the noncleaved portion of the signal sequence and any of the internal cysteines. Each of these bondings appears to be sufficient to block formation of intermolecular disulfide bridgesbetween Cys-61 residues, theonlyoneof the three
internal cysteines which can form intermolecular bonds. How canthis beexplained?
In 1988, Argos and Fuller proposed a model for the three-dimensional structure of the HBc proteinwhichwas
based on primary sequence homology between the HBc protein and the mengovirus capsid protein. This sequence
comparison was then extrapolated to the known three-dimensional structure of the mengovirus capsid (2). Al-thoughonecanargue about the value of suchcomparisons, in thiscase wefoundaremarkable correlation betweenour
biochemical data and the location ofcysteineresidues in the Argos-Fuller model. In this model, all of the cysteines lie
next to each other within or at the border of a structure
consisting of several 1-strands. A portion of the HBc N
terminus, starting around amino acid 15, isproposed tobe
partof thisstructure.Thus, inthis model the N terminus of the HBc protein is located in the vicinity of the internal cysteines.The additional 10 amino acids of thepre-C region should then belongand flexibleenoughtobindtoanyof the internalcysteines, and thismightbe thewaybywhich HBe dimerization and/or aggregation is prevented. Currently, work is inprogresstorefine this model andto testit with the helpof furthermutants.
With respect to the secondquestion, wefound that HBe
antigenicity is influenced by disulfide bonds. Again, the cysteinesat-7 and61arethe crucialones.Ifthecysteineat
-7 is lacking an HBe dimer linked via a Cys-61-Cys-61
disulfide bridge is formed which has both HBe antigenicity and HBc antigenicity (14). If Cys-61 was mutated, the
proteinstillexhibitedonlyHBeantigenicitybut thestrength ofreactivitywasreduced. Theseantigenic changeswerealso
reflectedbythe differentefficiencies with which theproteins
were secreted. The data suggest that if the Cys at -7 is
present,Cys-61is alsorequiredfor the HBeproteintoadopt
astructurewhich iscompatible with efficient secretion and strongreactivitywithHBe-specificantibodies.Interestingly, Cys-183, which is cleaved during HBe maturation, also
appearstohaveaneffectonHBefolding.Thismight explain why the transient presence of the C-terminal domain is
requiredforefficientbiosynthesis and intracellulartransport of the HBeprecursor(14).
The molecular basis of the reduced HBe antigenicity remainstobe determined. Inimmunoprecipitations,all HBe proteins specificallyboundtomonoclonal antibodieswhich arebelievedtorecognizetwomajorHBeepitopesand which competewith the antibodies contained in thediagnostictest
kits. The same was true if antigenicity was tested at rela-tively high antigen concentrations by using test systems whichrequiredconcomitantbindingoftwodistinct antibod-ies. However, at higher dilutions, a significantly reduced binding affinity was observed. Since mutation of Cys-61 always resulted in reduced HBeantigenicity, we favor the explanation that thelowerreactivityin the clinical assays is duetoconformationalchangesintheprotein. Alternatively, Cys-61 could be part of a major epitope recognized by HBe-specific antibodies. This possibility cannot be
ex-cluded, since it is still debated which sequences form the different HBeepitopes.Twobindingsites have beendefined
atthe amino acid level
(amino
acids 76to89[10]
and 128to133
[12]),
and these do not includecysteine
61. From apractical standpoint,
the behavior of theCys-61
mutantscould be of clinicalsignificance. If thereareinfectious HBV
mutantsthat lackthis
cysteine, they
could be missedduring
clinical HBe
diagnosis.
ACKNOWLEDGMENTS
Wethank U. Koszinowski for continuous support,andwe
grate-fully acknowledge the perfect technical help of C. Ertinger, A.
Luske, and M.Wastl. We alsothank P. Argosand R.Bringasfor
stimulatingdiscussions and M.Noah, Behringwerke Marburg, for themonoclonalantibodies.
This workwas supported by the Deutsche Forschungsgemein-schaft(Schl270/1-4andSchl270/4-1)anda
Forschungsschwerpunkt-forderungof the LandBaden-Wurttemberg.
REFERENCES
1. Aden,D.P.,A.Fogel,S.Plotkin, I.Damjanov,and B.Knowles. 1979.ControlledsynthesisofHBsAginadifferentiated human
liver carcinoma-derived cell line. Nature (London)
282:615-616.
2. Argos, P.,andS. Fuller. 1988. Amodel forthehepatitisBvirus core protein: predictionof antigenic sites andrelationship to RNA-viruscapsid proteins. EMBO J. 7:819-824.
3. Ferns,R.B.,and R.S. Tedder. 1984.Monoclonalantibodiesto
hepatitis B e antigen (HBeAg)derived from hepatitis Bcore
antigen (HBcAg):theiruseincharacterizationanddetectionof
HBeAg.J. Gen.Virol. 65:899-908.
4. Ganem,D.,and H. Varmus.1987. The molecularbiologyof the
hepatitis-Bviruses. Annu. Rev. Biochem.56:651-693.
5. Garcia, P.,J.Ou,W.Rutter,and P.Walter.1988.Targetingof thehepatitisBvirus precoreproteintotheendoplasmic reticu-lummembrane: aftersignal peptide cleavagetranslocationcan be aborted and theproductreleased intothecytoplasm.J.Cell Biol. 106:1093-2004.
6. Hoofnagle, J., D.Shafritz,andH. Popper. 1987. Chronic type B hepatitis and the healthy carrier state. Hepatology
7:758-763.
7. Imai, M., M.Nomura,T. Gotanda,T.Sano,K.Tachibana,H.
Miyamoto, K. Takahashi, S. Toyama, Y. Miyakawa, and M.
Mayumi. 1982. Demonstration oftwo distinctantigenic deter-minantsonhepatitis Beantigen bymonoclonalantibodies. J.
Immunol. 128:69-72.
8. Junker, M., P. Galle, and H. Schaller. 1987. Expression and
replication of the hepatitisBvirus genome underforeign pro-motercontrol. Nucleic Acids Res. 15:10117-10132.
9. Ou,J.,0. Laub, andW.Rutter.1986. HepatitisBvirus gene function: the precoreregiontargets thecoreantigentocellular membranes and causes the secretion of the e antigen. Proc.
on November 9, 2019 by guest
http://jvi.asm.org/
HBe PROTEIN BIOSYNTHESIS AND ANTIGENICITY 1321
Natl. Acad. Sci. USA 83:1578-1582.
10. Salfeld, J., E. Pfaff,M.Noah, and H. Schaller. 1989.Antigenic determinants and functional domains in core antigen and e
antigen from hepatitisBvirus. J. Virol. 63:798-808.
11. Sallberg, M., U. Ruden,L.0.Magnius, H.P.Harthus,M.Noah,
andB.Wahren. 1991. Characterisation ofalinearbindingsite
foramonoclonalantibodytohepatitisBcoreantigen. J. Med. Virol.33:248-252.
12. Sallberg, M., U. Ruden, B. Wahren, M. Noah, and L. 0.
Magnius. 1991. Human and murine B-cells recognize the HBeAg/beta (or HBe2) epitope as a linear determinant. Mol.
Immunol. 28:719-726.
13. Schlicht, H.-J., and H. Schaller. 1989. The secretory core
protein of human hepatitis B virus is expressed on the cell
surface.J.Virol. 63:5399-5404.
14. Schlicht, H.-J., andG. Wasenauer. 1991. Thequaternary struc-ture, antigenicity, and aggregationalbehavior of the secretory
coreprotein of human hepatitis B virusare determined by its
signalsequence.J. Virol.65:6817-6825.
15. Standring, D., J. Ou, F. Masiarz,and W. Rutter.1988.Asignal peptide encoded within theprecoreregion ofhepatitis B virus directs the secretion ofa heterogeneous population ofe
anti-gensinXenopusoocytes.Proc.Natl. Acad. Sci. USA 85:8405-8409.
16. Takahashi, K., A.Machida,G. Funatsu, M.Nomura,S. Usuda,S. Aoyagi, K. Tachibana,H. Miyamoto, M. Imai, T. Nakamura, Y. Miyakawa, andM.Mayumi. 1983. Immunochemicalstructureof hepatitis B e-antigen in theserum.J. Immunol. 130:2903-2907. 17. Wasenauer, G., J. Kock, and H.-J. Schlicht. 1992. A cysteine
and ahydrophobic sequencein the noncleavedportion of the pre-C leader peptide determine the biophysical properties of the secretorycoreprotein (HBe protein) of human hepatitis B virus.
J.Virol. 66:5338-5346. VOL. 67, 1993