Multidrug Resistance Associated Protein 2
by
Matthew John Harris
A thesis submitted for the degree of
Doctor of Philosophy
John Curtin School of Medical Research
Australian National University
Canberra, Australia
THE I OHN CURTIN
SCHOOL OF MEDICAL RESEARCH
This thesis describes the results of research undertaken in the Molecular Genetics
Group, Division of Molecular Medicine, John Curtin School of Medical Research,
Australian National University, Canberra. The research was undertaken between May
1997 and October 2000 while on an Australian National University PhD Scholarship.
The results and analyses presented in this thesis are my own original work
accomplished under the supervision of Professor Philip Board except where otherwise
acknowledged.
Matthew Harris
.
I would like to pay special thanks to Professor Philip Board for his supervision of the
project. His mentorship has been especially encouraging and inspirational, providing
guidance and support that has held me to the end. Thanks also to my Advisor Dr Rohan
Baker for project development and encouragement.
The prestige of The John Curtin School of Medical Research and the enthusiasm from
the staff has been a day to day inspiration. The staff and students of the Molecular
Genetics Group (Division of Molecular Medicine) have been a huge support,
particularly Marjorie Coggan and Dan Liu. My friends and fellow students, are
especially acknowledged. Thankyou Jack Flanagan, Matt Taylor, Simon Prasad, Katie
Sloper and Genevieve Herbert for your friendship and support.
The support from my family has been fantastic. Thankyou Mum and Dad. You always
believed in me, which made me believe in me. Thankyou also to my brother Steve and
extended family of uncles, aunts and cousins who have encouraged me all the way.
Thankyou to all my friends (and Dot) for your individual touches of encouragement and
love.
Without you all I wouldn't have made it.
..
ABC Transporters are a family of membrane proteins characterized by an ATP Binding
Cassette, hence ABC. The proteins are found throughout nature from bacteria to humans
and predominantly function to transport a broad range of substrates out of the cell. This
thesis focuses on one subfamily of mammalian ABC transporters called the multidrug
resistance proteins, a name derived from their ability to create cellular resistance to
cytotoxic 'drugs'. Multi drug resistance is commonly acquired in tumours after the
patient receives chemotherapy . Specifically, the thesis examines the multi drug
resistance protein MRP2 which, at the commencement of these studies, was known to
be a liver specific transporter described generally as the canalicular multispecific
organic anion transporter ( cMOA T) . MRP2 was suspected to be a drug resistance
protein due to its similarity in substrate specificity and protein sequence to MRP 1 but
the expression and function of MRP2 in tumours had not been described.
On attempting to characterise the role of MRP2 in multidrug resistance, the protein was
observed to localise intracellularly in the non-polarised L 1210 cell line. The protein
does not function as an efflux pump under these conditions. This observation led to
investigations into the basic sorting mechanisms for ABC transporters , focusing on
MRP2. Some fundamental questions concerning ' why proteins go where ' in the cell
were answered based on the discovery of the MRP2 apical targeting signal. The motif is
a PDZ interacting domain , more commonly known to be involved in protein
stabilisation in the membrane than as a targeting signal. The PDZ interacting domain
targets MRP2 to the apical membrane in polarised cells and also contributes to the
intracellular accumulation seen in non-polarised cells . Homology modelling of the
C-terminal cytoplasmic domain of MRP2 enabled the spatial position of the motif to be
predicted. The motif presents to the side of the domain and is spatially distinct from the
active sites of the protein with probable access for the proposed protein-protein
associations. The deletion of the motif redirected the mutant (~MRP2) to the basolateral
membrane in polarised cells and allowed the protein to reach the plasma membrane in
non-polarised cells. This enabled the development of a non-polarised cell line to further
characterise the function of MRP2.
Submitted Paper
Harris MJ and Board PG (2000) Identification of the Apical Membrane Targeting
Signal of the Multidrug Resistance Associated Protein 2 (MRP2/cMOAT).
Submitted Patent
Patent Application Entitled: Modified proteins, isolated novel peptides, and uses
therefor.
Inventors: Matthew J. Harris and Professor Philip G. Board
ATP bp cDNA C-terminal cMOAT DJS DNA DNP-GS EHBR EtOH GS-DNP GSH GST GS-X GY/TR-kb kDa LB LTC4 MDCK MDR mRNA MRP N-terminal PCR Pgp SUR TGN ·TMD Adenosine triphosphate Base pairs
Complementary deoxyribonucleic acid
Carboxy terminus
Canalicular multispecific organic anion transporter.
Dubin-Johnson Syndrome
Deoxyribonucleic acid
Dinitropheny 1 gl utathione
Eisai Hyperbilirubinemic rats
Ethanol
2,4-Dinitrophenyl glutathione
Glutathione
Glutathione S-transferase
Glutathione conjugate
Groningen yellow/transport deficient rats
Kilo base pairs
Kilodaltons
Luria broth
Leukotriene C4
Madin-Darby Canine Kidney cell line
Multidrug resistance
Messenger ribonucleic acid
Multidrug resistance associated protein
Amino terminal
Polymerase chain reaction
P-glycoprotein
Sulfonylurea receptor
Trans golgi network
Transmembrane domain
CHAPTER I
INTRODUCTION
1.1 ABC Transporters
1.1. l STRUCTURE OF ABC TRANSPORTERS
1.1.2 STRUCTURE-FUNCTION RELATIONSHIPS
1.1.3 SUBSTRATES OF ABC TRANSPORTERS 1.2 Multidrug Resistance
1.2.1 CHEMOTHERAPY AND CANCER
1.2.2 THE MUL TIDRUG RESISTANCE PROTEIN
1.2.3 MULTIDRUG RESISTANCE ASSOCIATED PROTEIN
1.3 Canalicular Multispecific Organic Anion Transporter
1.3.1 cMOAT
1.3.2 DUBIN-JOHNSON SYNDROME
1.3.3 FUNCTIONAL CHARACTERIZATION OF CMOAT USING MUTANT RATS
1.3.4 cMOAT CLONING
1.3.5 FUNCTIONAL CHARACTERIZATION OF cMOAT USING THE CLONED cDNA
1.3.6 cMOAT EXPRESSION
1.4 The Multidrug Resistance Associated Protein Subfamily
1.4.l MRP3 1.4.2 MRP4 1.4.3 MRP5
l .4.4 MRP6 AND THE ANTHRACYCLIN RESISTANCE ASSOCIATED PROTEIN 1.5 Endogenous Function: ABC Transporters In The Liver
1.6 Protein Targeting
1.6. l CELL MORPHOLOGY
1.6.2 PROTEIN TRAFFICKING
1.6.3 TARGETING SIGNALS
1.6.4 RECEPTORS TARGETING SIGNALS
1.6.5 POL YTOPIC PROTEIN TARGETING SIGNALS
1.6.6 GL YCOSYLA TION
1.6.7 INVOLVEMENT OF PHOSPHORYLATION IN TARGETING
1.6.8 MEMBRANE SPANNING DOMAINS
1.6.9 PDZ MEDIA TED INTERACTIONS 1.6. l O NEURONAL POLARISATION
1.6.11 CHIMERA EXAMPLE
1.6.12 ABC TRANSPORTERS AND THEIR TARGETING SIGNALS
1.7 OBJECTIVES
CHAPTER2
MATERIALS AND METHODS
2.1 Material And Methods
2.3 DNA Manipulation Methods 30
2.3.1 BACTERIAL CULTURES 30
2.3.2 DNA PREPARATIONS 30
2.3.3 RESTRICTION ENDONUCLEASE DIGESTIONS 30
2.3.4 AGAROSE GEL ELETROPHORESIS 30
2.3.5 BASIC CLONING 31
2.3.6 OLIGONUCLEOTIDE PRIMERS 31
2.3.7 AUTO MA TED SEQUENCING 32
2.3.8 POLYMERASE CHAIN REACTION (PCR) 32
2.3.9 SITE DIRECTED MUTAGENESIS 33
2.4 Cell Culture Methods 34
2.4.1 CELL CULTURE 34
2.4.2 TRANSFECTI0NS 35
2.5 Green Fluorescent Protein Fusion Proteins 35
2.6 Immunofluorescence 36
2.7 Faes Analysis 36
2.7.1 RHODAMINE EFFLUX ASSAY 37
2.7.2 DAUNORUBICIN EFFLUX 37
2.8 2,4-Dinitrophenyl Glutathione Efflux 37
2.9 Microscopy 38
CHAPTER3 39
FUNCTIONAL EXPRESSION OF cMOAT/MRP2 IN 11210 CELLS
3.1 INTRODUCTION 40
3.2 OBJECTIVES 40
3.2 METHODS 41
3.2.1 SEQUENCING THE CMOAT CLONE 41
3.2.2 CLONING THE CMOAT CLONE INTO A RETROVIRAL VECTOR 41
3.2.3 TRANSFECTIONS 41
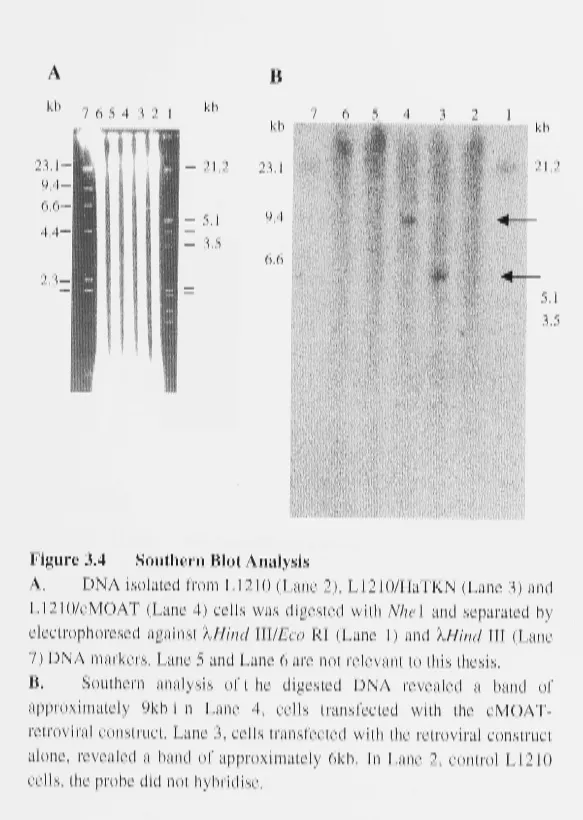
3.2.4 SOUTHERN ANALYSIS 42
3.2.5 NORTHERN ANALYSIS 43
3.2.6 INHIBITOR STUDIES 44
3.2.7 DRUG RESISTANCE ASSAYS 45
3.2.8 MONOCHL0ROBIMANE ASSAY 45
3.3 RESULTS 45
3.3.4 CLONAL SELECTION
3.3.5 GENE EXPRESSION
3.3.6 DINITROPHENYL GLUT A THI ONE TRANSPORT ASSAY 3.3.7 RHODAMINE 123 TRANSPORT ASSAY
3.3.8 INHIBITORS OF RHODAMINE 123 EFFLUX 3.3.9 DRUG RESISTANCE ASSAYS
3.3.10 DAUNORUBICIN EFFLUX
3.3.11 P-GLYCOPROTEIN OVER-EXPRESSION 3.3.12 MONOCHLOROBIMANE ASSAY
3.3.13 lMMUNOFLUORESCENCE MICROSCOPY
3.4 DISCUSSION
CHAPTER4
MOLECULAR TARGETING SIGNALS OF MRP2
4.1 INTRODUCTION
4.2 OBJECTIVES
4.3 MATERIALS AND METHODS
4.3.1 MRPl AND MRP2 GFP CONSTRUCTS
4.3.2 MRP2/MRP1-GFP CHIMERAS
4.3.3 TRUNCATED PROTEINS
4.3.3.1 MRP 1 Truncated Protein 4.3.3.2 MRP2 Truncated Protein
4.3.4 POLARISED MDCK CELLS ON TRANSWELL PLATES
4.4 4.4.1 4.4.2 4.4.3 4.4.4 4.4.5 4.4.6 4.4.7 4.4.8 4.4.9 4.5 RESULTS
MRPl AND MRP2 GFP CONSTRUCTS IN NON-POLARISED MDCK CELLS MRPl AND MRP2 GFP CONSTRUCTS IN POLARISED MDCK CELLS ALIGNMENT OF MRP PROTEINS
MRPl TRUNCATED PROTEIN
MRP2 TRUNCATED PROTEIN
CHIMERIC PROTEINS
OVEREXPRESSION OF GFP FUSION PROTEINS
CFfR TARGETING SIGNAL HOMOLOGY Mv'.1RP2 GFP CONSTRUCT
DISCUSSION
CHAPTERS
CHARACTERIZATION OF ~MRP IN L1210 CELLS
5.3.2 TRANSFECTIONS
5.4 RESULTS
5.4.1
5.4.1.1 5.4.1.2 5.4.1.3
5.4.1.4
TARGETING OFMRP2 IN L1210 CELLS
MRP2 Localisation Using Green Fluorescent Protein MRP2 Localisation Using Immunocytochemistry
11MRP2 Localisation Using Green Fluorescent Protein
11MRP2 Localisation Using Immunocytochemistry
5.4.2 5.4.3 5.4.4 5.4.5 5.4.6 5.5
P-GLYCOPROTEIN IMMUNOLOCALISATION IN L1210 CELLS MRPl LOCALISATION USING GREEN FLUORESCENT PROTEIN. 2,4-DINITROPHENYL GLUT A THI ONE TRANSPORT ASSAY
DAUNORUBICIN TRANSPORT ASSAY DRUG RESISTANCE ASSAYS
DISCUSSION
CHAPTER6
CHARACTERIZATION OF THE MRP2 APICAL TARGETING SIGNAL
6.1 INTRODUCTION
6.2 OBJECTIVES
6.3 METHODS
6.3.1 SITE DIRECTED MUTAGENESIS
6.3.2 MRP2 APICAL TARGETING MOTIF TRANSFER TO MRPl
6.4 RESULTS
6.4.1 ALANINE MUTANTS
6.4.2 MRP2 APICAL TARGETING MOTIF TRANSFER TO MRPl 6.4.3 ALIGNMENT OF ABC TRANSPORTERS
6.4.4 HOMOLOGY MODELLING
6.5 DISCUSSION
CHAPTER 7
GENERAL DISCUSSION
7.1 OVERVIEW
7.2 DISCUSSION
7.2.1 MRP2 EXPRESSION IN DRUG RESIST ANT CELL LINES AND TUMOURS 7.2.2 PDZ INTERACTING DOMAINS IN OTHER MDR PROTEINS
7.2.3 THE PDZ INTERACTING DOMAIN IN POLARISED AND NON-POLARISED CELLS 7.2.4 TRADITIONAL PDZ MEDIA TED ROLES AT THE PLASMA MEMBRANE
7.2.5 DRUG SCREENING APPLICATIONS OF L\.MRP2
Al
A2
Bl
B2
B3
Cl
COMMON REAGENTS AND SUPPLIERS
KITS AND THE SUPPLIERS
MRP2 PRIMERS
MRP2 SPECIFIC MUTAGENESIS PRIMERS
MRPl PRIMERS
ECLUSTALW ALIGNMENT OF ABC TRANSPORTERS
REFERENCES
93
94
95
96
97
98
1.1
ABC Transporters
ABC transporters are a large family of membrane proteins found throughout nature. The
members of the family all have a conserved ATP binding site called the ATP Binding
Cassette designated ABC. This enables the protein to utilise the hydroysis of adenosine
triphosphate (ATP) as an energy source for their transport function. The most common
function of these proteins is to pump compounds out of the cell across the plasma
membrane against a concentration gradient. However, ABC transporters have also been
found to pump compounds into a cell and furthermore have been found in the
membrane of cell organelles. As well as having a conserved site for ATP binding, the
family has conserved cytoplasmic regions and similar membrane topologies. The family
of ABC transporters has been slowly growing since the discovery of the bacterial
histidine transport operon (Higgins et al. 1982), culminating in the "ABC" designation
at the point when thirty homologues had been sequenced (Hyde et al. 1990), to the
present day with hundreds of ABC transporters described and constant endeavors to
discover more.
1.1.1 Structure of ABC Transporters
A typical ABC transporter has two repeating domains consisting of a transmembrane
region, usually made up of six membrane spanning helices, followed by a cytoplasmic
domain (Hyde et al. 1990). A repeat of these two domains then follows. The protein can
be coded by separate genes for each domain, such as the histidine permease transporter
in Salmonella typhimurium, or the protein can be coded by one continuous gene, such as
mdr 1, which codes for the multidrug resistance protein (P-glycoprotein) in humans.
Figure 1.1 illustrates the organisational structure of a number of transporters.
There are additional variations to those structures presented in Figure 1.1. One variation
(pertinent to the proteins studied in this thesis) is an additional transmembrane domain
at the N-terminus of a number of subfamilies. This topology was predicted by analysis
of hydropathy plots of a number of transporters, including the sulfonylurea receptor
(SUR) and MRPl subfamilies from a range of species (Varadi et al. 1998). The basic
IBIKI
Oligopeptide Permease
S.typhimurium
Histidine Permease
S.typhimurium
P-glycoprotein human
(modified from [Hyde, 1990 #39])
Figure 1.1 Organisation and Gene Structure of ABC Transporters
The ABC Transporters depicted in this figure demonstrate the typical organisation of the protein in the membrane, with two transmembrane domains and two cytoplasmic domains. The transporters are only three examples of several alternative gene structures.
1. Oligopeptide perm ease has four genes and each domain is coded by one separate gene, designated B, C, D and F.
2. Histidine Perm ease complex is coded by three genes. One gene for the cytoplasmic domains (HisP) and two for each of the transmembrane domains (HisQ and HisM).
3. P-glycoprotein from humans is coded by one gene, mdr 1.
Extracellular
diagram in Figure 1.2. The structures were defined experimentally by analysis of
glycosylation sites (Bakos et al. 1996; Hipfner et al. 1997).
1.1.2 Structure-Function Relationships
The most common function of an ABC transporter is to move compounds across a
membrane usually against a concentration gradient using energy obtained by the
hydrolysis of ATP. ATP hydrolysis occurs in conserved regions of the cytoplasmic
domains where it binds two specific motifs, the Walker A and B motifs (Walker et al.
1982). There is also a 'signature C' motif whose conservation is equally striking and
participates in nucleotide hydrolysis. Figure 1.3 presents a partial alignment of some
well known ABC transporters from six different species demonstrating the conservation
of the ATP binding cassette ( such alignments have been published previously, (Hyde et
al. 1990; Riordan et al. 1989)).
The transmembrane domains have little sequence homology across and within species
but are a primary determinant of the membrane topology of the protein (Higgins 1992).
The importance of the transmembrane helices has become apparent in their role in
binding chemical compounds, 'flipping' aminolipids and ejecting the drugs directly
from the plasma membrane (Raviv et al. l 990; Higgins 1992). These functions indicate
the direct involvement of specific residues in the membrane spanning regions, which are
determinants of substrate specificity (Higgins 1992). Mutations in the transmembrane
regions of the histidine transporter of S. typhimurium (Payne et al. 1985), the maltose
transporter of E.coli (Reyes et al. 1986), and P-glycoprotein in humans (Gros and
Shustik 1991) all alter substrate specificity.
The organisation of the transmembrane regions in association with the cytoplasmic
domains has not been determined and although the protein is often drawn schematically
in the literature, no data has been produced to determine tertiary structure of the entire
protein or protein complex.
1.1.3 Substrates of ABC Transporters
The substrates for the whole ABC transporter family are broad. They include amino
acids, peptides, protein, metals, vitamins, antibiotics, sugars, ions, and ' drugs' ( chemical
CFTR, Pgp, SURI and MRP subfamilies are shown. The hydrophobic peaks spanning approximately twenty amino acids represent single membrane spanning helices. The position of each transmembrane helix and the cytoplasmic ATP binding domain (NBD), allow a prediction of the topology of the protein. These predictions are then confirmed by experimental data.
B. The multidrug resistance protein (P-glycoprotein) and C FTR
CFTR
Pgp
MRPI
, ~
D
~
M
n.~Ti. f
;~
•.. ;'. .· ')./1~,'!
M. . I,I\.
I!,~
i
IL ~-0•
IA.•
'-/ V 11 - t ~
rv
1<
V
~V
'flV .
"J'( •
~MRP2
~
• JM\Jt tulii
l l ~lt R.
n,
~
iA~
. v'1jr
\JV~7ff
Yw~v'yV
V .
SURI
B
PGP, CFTR
TMD1 TMD2
cn..,vllUICl
001•
N-term inus
B
•
B•
MRPI, MRP2, SURI, YCFI
N-term inus
Pgp human
MRP2 human
CFTR bovin
MRP2 rabit MRP3 rat
mdrl caeel
YCFl yeast HisP salty
---VKILKGLNLKVQS-GQTV ---ATVRDVNLDIMA-GQLV ---TPVLKDISFKIER-GQL ---PTIRNVNLDIMP-GQLV ---PTLHSINIQIPK-GALV ---VPILRGMNLRVNA-GQTV ---YKVALKNINFQAKK-GNLT ---GHEVLKGVSLQARA-GDVI STTVQLMQRLYDPT SSLISAMLGEMENV TSLLLMIMGELEAS - SSLMSAMLGEMENV-SSLVSALLGEMEKL - -- STIISLLLRYYDVL--TALLSCMLGDLFRV- - ---
STFLRCINFLEKPSE----Rbsa ecoli ---KALSGAALNVYP-GR .
STMMKVLTGIYTRDA----Signature C
Pgp human - - - ---- - -EANAYDFIMKLPHK- --- -FDTLVGER- - -- - -GA< ,
MRP2 human ---ACALLPDLEMLPGG---DLAEIGEK---GI
CFTR bovin ---ACQLEEDISKFAEK---DNVVLGEG---GI
MRP2 rabit ---ACALLPDLEILPGG---DLAEIGEK---GI
MRP3 rat - - - -TCALLADLDVLPGG- - - - -DQTEIGEK- - - -GI~ ,
mdrl caeel ---MANAEKFIKTLPNG---YNTLVGDR---GT
YCFl yeast ---ACALTIDLAILMDG---DKTLVGEK---GI HisP salty ---ERALKYLAKVGI---DERAQGKY---P Rbsa ecoli ---YAEADKLLAKLN---LRFKSDKL-- -- -VG
WalkerB
Pgp human
MRP2 human
CFTR bovin
MRP2 rabit MRP3 rat
mdrl caeel
YCFl
yeast---EATSALDTESEAVVQVALDKAR-K---GRTTIVIAHRLS-T DPLSAVDAHVGKHIFNKVLGPNGLLK-GKTRLLVTHSMHF- SPFGYLDVLTEKEIFESCICKL--MA-NKTRILVTSKMEH- DPLSAVDAHVGKHIFNKVLGPNGLLNGKTRLLVTHSLHFDPLSAVDSHVAKHIFDQVIGPEGVLAGKTRVLVTHGISF -EATSALDAESEGIVQQALDKAA-K---GRTTIIIAHRLS-T DPLAAVDEHVARHLIEHVLGPNGLLH-TKTKVLATNKVSA-EPTSALDPELVGEVLRIMQQLAEEG---KTMVVVTHEMGFA EPTDALTDTETESLFRVIRELKSQG---RGIVYISHRMKEI HisP salty
Rbsa ecoli
Figure 1.3 Alignment of ATP Binding Cassettes Across Species
IA
The alignment of the nucleotide binding regions from humans to E.coli *(Walker A and
B, and the ABC protein Signature C) demonstrate the high homology throughout nature. This domain is responsible for ATP hydrolysis, used as an energy source for active transport. Based on this conservation, it is likely that the tertiary structure of this domain has been conserved.
• mdrl caeel = multidrug resistance protein from C.elegans; HisP salty= Histidine permease from
specific substrates such as STE6 in yeast which transports the a-factor mating peptide
but does not transport any other known peptides or proteins (Kuchler et al. 1989).
Comparatively, P-glycoprotein in humans can transport a broad range of hydrophobic
drugs and can also act as a chloride channel, though the two functions are quite distinct
(Gill et al. 1992).
The ABC transporters relevant to this thesis are those ABC transporters involved in
drug resistance. 'Drugs' refers to a broad range of primarily exogenous compounds such
as chemotherapeutic and cytotoxic agents. These ABC transporters ' pump ' the drug out
of the cell, which decreases its intracellular accumulation, evading the toxic effects of
the agents. Figure 1.4 illustrates the proposed modes of transport of ' drugs ' from the
cell by the ABC transporter, P-glycoprotein ((Raviv et al. 1990) ; reviewed in
(Gottesman and Pastan 1993)). This phenomenon is often found in cancer cells where
specific ABC transporters are over-expressed or up-regulated and cause broad resistance
to anticancer agents, termed multidrug resistance (MDR). The ABC transporters
involved in this process are referred to as multidrug resistance proteins. MDR is an
extension of a system functioning under normal situations to rid the organism of toxic
compounds, generally termed detoxification. Detoxification involves a pathway of
enzymes, which can include cytochrome P-450 enzymes (Phase I) and the glutathione
transferase enzymes (Phase II). Phase III involves the elimination of the compounds
from the cell by the ABC transporters. Figure 1.5 is a schematic diagram showing the
possible sequences in the process. This pathway, when active in cancer cells, leads to
clinical resistance to treatment by chemotherapeutic agents in cancer patients.
1.2
Multidrug Resistance
1.2.1 Chemotherapy and Cancer
Chemotherapy is a common form of treatment for cancers in humans. Alternatives to ( or
in conjunction with) chemotherapy include radiotherapy or surgery that are only
possible on solid tumours, usually in the benign form. Some benign tumours such as
testicular cancer respond well to chemotherapy (sometimes without the need for
surgery) but for metastatic tumours and systemic cancers such as leukemia,
chemotherapy is commonly the sole resource. However, treatment of cancers with many
Figure 1.4
,~
(
~\.---,
OUT
CELL
MEMBRANE
IN
Figure from [Gottesman, 1993 #47]
Modes of Transport Function by ABC Transporters
be pumped directly from the cell without further metabolism, usually by P-glycoprotein
(as seen in Figure 1.4). Alternatively, a drug X1 enters the cytoplasm and is oxidised by
D r ~ g - ~ ~ - - - -
X
Phase I metabolism
P-glycoprotein
I I
X1
cyt-P450I
X'
Phase
I
Oxidation
Phase II metabolism
X1
GST
GS-X
~
Phase
II
Coniuaation
Phase 1111Elimination
Phase
I and 11 metabolism
X1
cyt-P450X'
GST
GS-X - - - ,
~
Phase
I
Oxidation
drugs. There are two forms of resistance, intrinsic and acquired. Typically,
adenocarcinomas of the colon and kidney are unresponsive to anticancer agents on first
exposure to the drugs, thus they have an 'intrinsic' resistance. In contrast, leukemias and
neuroblastomas, for example, initially respond well to chemotherapy. However, after a
varied remission period, the cancer cells that remain in the system regenerate and have
acquired resistance to the anticancer agents. In intrinsic and acquired resistance, the
resistance is not only to the initial drugs but also extends to a range of chemotherapeutic
agents that are only similar in size and hydrophobicity, hence 'multidrug' resistance.
The seriousness of this phenomena has led to a huge amount of research into tumour
physiology over the past forty years.
The first step was to generate models of MDR from human carcinomas (Kessel et al.
1968; Beck et al. 1979; Shen et al. 1986). Drug resistant clones of normal cells were
also generated by exposure to particular chemotherapeutic compounds which produced
varying levels of MDR (Ling and Thompson 1974). These two systems 12roduced the
initial observations of reduced accumulation of chemotherapeutic drugs in the
cytoplasm of cells (Kessel et al. 1968). Reduced accumulation was due to reduced
permeability of the membrane to these drugs (Ling and Thompson 197 4) and increased
efflux (Dano 1973). Stemming from this work, Chinese hamster ovary cells resistant to
colchicine were used to first identify a 1 70kDa protein in the membrane of highly
resistant clones (Juliano and Ling 1976). The protein was shown to be glycosylated and
the reduced Qermeability of the cells led to the name P-glycoprotein (Pgp) also referred
to as the multidrug resistant protein.
1.2.2 The Multidrug Resistance Protein
Understanding the functionality and genetics of P-glycoprotein was of critical
importance to understanding multidrug resistance in cancer patients. This is highlighted
by the large volume of research in the 1980s in this area. First, the putative gene for
multidrug resistance (mdr 1) was determined (Roninson et al. 1984), (Gros et al. 1986),
(Roninson et al. 1986), (Shen et al. 1986) and subsequently correlated to P-glycoprotein
(Ueda et al. 1986), (Riordan et al. 1985). Then, the forced over-expression of the mdr 1
· cDNA for Pgp was shown to confer MDR in previously drug sensitive cells (Gros et al.
1986) and was found to be over-expressed in MDR cell lines (Kartner et al. 1985). The
there was more thari one gene in each, designated mdr 1, mdr 2 and mdr 3 depending on
the species (reviewed in (Gottesman and Pastan 1993)). However, there is only one
homologue that confers MDR (mdr 1 in humans) while the other homologue (mdr3 in
humans) had no known function at the time but was subsequently shown to be a
phosphotidylcholine flippase (Smith et al. 1994) and biliary lipid transporter (Smit et al.
1993). The sequence of Pgp was shown to have homology to bacterial peripheral
membrane components (Chen et al. 1986) and it was not until the number of ABC
proteins had grown significantly to over thirty proteins that Pgp was shown to be a
member of the ABC transporter family (Hyde et al. 1990).
P-glycoprotein is localised to the excretory/secretory membrane of a number of organs
(Thiebaut et al. 1987), (Fojo et al. 1987), (Cordon-Cardo et al. 1989):
• canalicular membrane of the liver
• proximal tubules of the kidneys
• adrenal medulla and cortex
• pancreas
• the epithelial cells of the jejunum and colon
• blood-brain barrier
This expression facilitates its normal function as an efflux pump, clearing the body of
endogenous and exogenous substrates.
P-glycoprotein is over-expressed in cancerous tissue of the above organs and mdr 1 is
also 'switched on' and expressed at high levels in neuroblastomas, leukemias and
lymphomas (Goldstein et al. 1989). This increased expression has been directly
correlated with the MDR phenotype in these tissues. However, Pgp was not found in
some MDR cancer tissues, such as many types of lung cancer. This led to the search for
other MD R proteins.
1.2.3 Multidrug Resistance Associated Protein
In 1992 a second multidrug resistance gene, the multidrug resistance associated protein
(MRP), was discovered (Cole et al. 1992). This discovery stemmed from the
observation that P-glycoprotein was not found to any substantial levels in nearly all
forms of cancerous lung (Lai et al. 1989). To study this phenomena, a cell line model
(SCLC makes up 25% of all lung cancers). A multidrug resistant clone of H69, H69AR,
did not express Pgp commensurate to the level of drug resistance (Mirski et al. 1987)
and also was not sensitised to chemotherapeutic drugs by Pgp functional inhibitors
(Cole et al. 1989). Differential screening of H69 and H69AR mRNA with a partial
cDNA library from H69AR isolated a clone expressed 200 fold greater in H69AR. This
enabled the cloning of the full length cDNA of the putative protein, designated MRP
(Cole et al. 1992). Other studies at the same time demonstrated that a 190kDa
glycosylated membrane protein was responsible for MDR in non Pgp expressing MDR
cell lines (Barrand et al. 1993; McGrath et al. 1989). This was later confirmed to be
MRP (Barrand et al. 1994; Almquist et al. 1995). Since the publication by Cole et al.
(1989), subsequent studies demonstrated the correlation of MRP to MDR in cell lines
(Zaman et al. 1993; Slovak et al. 1993; Schneider et al. 1994; Thomas et al. 1994;
Barrand et al. 1994); and tumours (Thomas et al. 1994). MRP over-expression in drug
sensitive cells was shown to subsequently confer multidrug drug resistance (Grant et al.
1994; Cole et al. 1994; Zaman et al. 1994) and was also importantly shown to act as an
efflux pump, decreasing the intracellular accumulation of these drugs (Zaman et al.
1994).
Alternate to the typical drug resistance studies, (Jedlitschky et al. 1994; Leier et al.
1994; Muller et al. 1994) demonstrated that MRP is a glutathione conjugate (GS-X)
efflux pump. The GS-X pump has been extensively described based on ATP dependent
transport of glutathione conjugates and reduced glutathione from cells (Ishikawa 1992).
That MRP was shown to transport glutathione conjugated leukotriene C4 (L TC4) and
similar derivatives, 2,4-dinitrophenyl glutathione (DNP-GS), and reduced glutathione
(GSSG), confirmed MRP as a GS-X pump (reviewed by (Ishikawa et al. 1997)). These
studies were the first to link the active glutathione conjugate transporter, first described
by (Board 1981), with a known MDR protein.
MRP knockout mice (mrpl -/-) are viable and fertile and have been used to characterize
the function of the protein further. The mice display hypersensitivity to the cytotoxic
drug etoposide (Lori co et al. 1997; Wijnholds et al. 1997). Isolated cells from the mice
display no transport of glutathione conjugates such as 2,4-dinitrophenyl glutathione and
L TC4 (Wijnholds et al. 1997). The mice show an impaired inflammatory response to
inhibiting the role that L TC4 plays in mediating inflammation (Wijnholds et al. 1997).
MRP was shown to have a distinct regulatory role in GSH metabolism since all tissues
from the mrpl-1- mice previously known to express MRP had elevated GSH levels
(Lorico et al. 1997).
In contrast to P-glycoprotein, which is primarily expressed in the gastrointestinal tract
and liver, MRP is normally distributed throughout the body in tissues including the
lungs, haematopoietic system and most organs (Kruh et al. 1995; Abbaszadegan et al.
1994). To investigate the cellular localisation of MRP, MRP was expressed in a pig
kidney epithelial cell line grown as a monolayer on a porous membrane to form
polarised cells (Evers et al. 1996). Immunocytochemistry in conjunction with confocal
and electron microscopy showed that MRP was surprisingly localised basolaterally, the
opposite membrane to P-glycoprotein. This difference in normal macro- (tissue
distribution) and micro- ( cellular localisation) localisation is a key issue in this thesis
and will become apparent in later chapters.
The number of reports characterizing MRP substrates is constantly increasing in the
literature. The primary characteristics of the substrates are amphiphilic, anionic and
range in size. Typical examples of these compounds in comparison to P-glycoprotein
substrates are shown in Figure 6.
1.3
Canalicular Multispecific Organic Anion Transporter
1.3.1 cMOAT
The possibility of a third multidrug resistance protein was proposed after sequencing the
cDNA gene for the canalicular multispecific organic anion transporter, cMOAT
(Buchler et al. 1996; Paulusma et al. 1996; Taniguchi et al. 1996; Ho et al. 1997). This
protein is the focus of this thesis. The presence of a protein responsible for canalicular
transport of a range of organic anions was apparent for many years due to analysis of
bile products. That a distinct protein ( cMOA T) responsible for the transport of organic
anions existed, only became apparent due to a human hereditary condition, a mutant
0
OH
... ,,
... ,,l
O 0JL
~~
\)'><NH---... I
H HO
Leukotriene C4
o~u
OH tf-,,,,•· "'tlH2
0
I ~n3
H N ~ C O O
-0 2 N ~ r u N H ~ 0 0
-~ S 0
N02
S-(2, 4-dini trophen y I) gl u tathi one
fl
~H'~oo-o H N ~
/'-... II
/ s ~ ~ '.,..coo--ooc
~~s
II
....,
~ N H 0
-OOC ~H-
II
NH3 0 Glutathione disulphide (GSSG)
OH
I I
MoO O OH 0
Daunorubicin
~
Mo
HO
[image:28.1200.46.1178.66.698.2]NH 2
Figure 1.6 P-glycoprotein and MRPl Substrates
I HCl
Me
OH
vinblastine
N
CH3o·
MRP substrates are commonly, amphiphilic, anionic and conjugated with glutathione. P-glycoprotein substrates are often hydrophobic and unconjugated. Substrates for MRP and P-glycoprotein can be of similar size.
OMe
Colchicine
0
\N~
anions from the liver. The lack of function in these disorders was the basis for naming
the protein:
• canalicular (localisation in the apical membrane of the liver)
• multispecific (range of substrates)
• organic anion ( all the known substrates were anions)
• transporter (products were transported into bile)
It was not until the sequence for this putative protein was determined that it was realised
that MRP was its closest homologue. Due to the similarity of protein sequence and
substrates, the primary question asked about cMOAT, is whether cMOAT is a multidrug
resistance protein, i.e. confers multidrug resistance in tumours .
1.3.2 Dubin-Johnson Syndrome
The rare human hereditary condition, Dubin-Johnson Syndrome (DJS), has the
symptoms of mild chronic hyperbilirubinemia and the phenotype of dark pigment in the
liver, giving rise to the reference "Black liver" (Dubin 1954). The patients have
impaired transport of organic anions into bile via the canalicular membrane (Arias
1961). Figure 7 illustrates basic hepatocyte anatomy and highlights the bile (canalicular)
membrane.
The first animal model with similar excretion deficiencies as DJS patients was a
Corriedale sheep (Alpert et al. 1969). The discovery of a mutant rat with the same
symptoms made available a more suitable model for laboratory experiments and has
been used for many studies on canalicular transport of organic anions. The transport
mechanism of organic anions was shown to be distinct from the two other forms of bile
products, organic cations and bile salts (reviewed in (Oude Elferink et al. 1995)).
1.3.3 Functional Characterization of cMOAT using Mutant Rats
Bilirubin is an organic anion produced from the metabolism of heme-proteins. Dubin
Johnson Syndrome is characterized by jaundice, caused by the accumulation of bilirubin
in blood serum (hyperbilirubinemia). Two rat models of hereditary hyperbilirubinemia
have been described, Groningen yellow/Transport deficient Wistar rats (GY/TR-)
(Jansen et al. 1985) and Eisai hyperbilirubinemic rats (EHBR) (Hosokawa et al. 1992).
In the liver, bilirubin is conjugated to glucuronides and excreted into bile in this form ..
Lateral membrane
Figure 1.7
Modified from (Bloom and Fawcett 1975, page 704.)
Hepatocyte Anatomy
The polarised hepatocyte has three membranes.
1. Cell to cell membrane includes tight junctions and is referred to as
the lateral membrane.
2. The sinusoidal (basolateral) membrane faces the Space of Disse, leading to the hepatic sinusoid surrounding the blood vessels.
isolated canalicular membrane vesicles or perfused livers from the mutant rats were
compared to normal rats. The transport of the organic anions was firstly determined to
be an ATP dependent system 'pumping' against the concentration gradient that exist
across the canalicular membrane (Kobayashi et al. 1990; Kobayashi et al. 1991). The
mutant rats have enabled characterization of the cMOAT mediated transport of organic
anions (Jansen et al. 1987; Kitamura et al. 1990) including bilirubin glucoronides
(Nishida et al. 1992; Jedlitschky et al. 1997), bromosulphthalein (Sathirakul et al.
1993), reduced glutathione (Fernandez-Checa et al. 1992), cysteinyl leukotrienes and
2,4-dinitrophenyl glutathione (Ishikawa et al. 1990; Jedlitschky et al. 1997).
A perceptive study that initiated the search for the putative gene for DJS, compared the
transport mechanisms in erythrocytes from DJS patients and TR- rats (Board et al.
1992). This study demonstrated that GS-DNP transport from erythrocytes is not
impaired in the DJS patients and TR- rats, indicating that the erythrocyte and canalicular
ATP-dependent transporters were "potentially genetically distinct". The genetic
distinction became apparent after cloning the MRP gene in 1992 and subsequent cloning
of the cMOAT gene in 1996.
1.3.4 cMOAT Cloning
The first report of the cMOAT cDNA sequence was derived from a rat liver cDNA
library. The rat sequence had 53% identity to human MRP at the cDNA level and 47%
identity at amino acid level (Paulusma et al. 1996; Ito et al. 1997; Buchler et al. 1996).
Tissue expression in the rat was observed in the liver, kidney, duodenum and jejunum.
A putative mutation was found in the cDNA of the EHBR rats leading to a premature
stop codon (Ito et al. 1997). The mutation in TR- rats also causes a premature stop
codon but is at a different position to EHBR (Paulusma et al. 1996). The human
cMOAT sequence was first reported after screening a cisplatin resistant cell line with
conserved fragments of other ABC transporters (Taniguchi et al. 1996). Human
cMOAT was shown to have 46% identity to human MRP. A human cDNA clone was
also reported by another group who found a mutation causing a premature stop codon in
DJS patients (Paulusma et al. 1997). With the aim of finding a CFTR homologue, the
· rabbit cMOAT was cloned and called the epithelial basolateral chloride conductance
1 1
Several mutations that cause DJS have been described (Paulusma et al. 1996; Paulusma
et al. 1997; Kajihara et al. 1998) and indicate that the protein is acutely truncated as a
result of point mutations.
1.3.5 Functional Characterization of cMOAT Using the Cloned cDNA
The use of the hereditary disorders for functional characterization was informative to
the extent that experiments only allowed a possible answer of 'plus-minus' transport. To
further characterize the protein, the cDNA of the gene was necessarily required to be
expressed in a cell line(s). This was the primary objective for this thesis. The work for
this thesis began in mid 1997 and at that time no studies were reported characterizing
cMOAT based on the expression of the cDNA. The importance of this research is
highlighted by the many studies reported in the literature since 1997 by several
laboratories around the world.
The first study that expressed the cMOAT cDNA, used the rat clone and transiently
expressed the cDNA in COS-7 cells and in Xenopus oocytes (Madon et al. 1997). Using
the COS-7 cells, cMOAT was shown to transport GS-DNP. The oocytes (using cRNA)
demonstrated increased transport of LTC4, both ATP dependant. There was no increase
in ~ taurocholate (bile salt) transport. Membrane vesicles isolated from NIH3T3 cells
transfected with rat cMOA T cDNA also demonstrated ATP dependent transport of
GS-DNP and L TC4 (Ito et al. 1998).
The first report to transfect human cMOAT cDNA used Madin-Darby Canine Kidney
(MDCK) cells which are polarised when grown as a monolayer on a porous membrane
(Evers et al. 1998). Cells expressing cMOAT were shown to transport GS-DNP , GSH
conjugated prostoglandin A1 (PGA1), GSH conjugated ethacrynic acid, and the
chemotherapeutic agent vinblastine but not daunorubicin. A study using the rat cDNA,
similarly expressed the protein in the apical membrane of MDCK cells and
demonstrated transport of the fluorescent organic anion, glutathione bimane (Kinoshita
et al. 1998).
1.3.6 cMOAT Expression
As the name suggests, cMOA T is normally localised to the canalicular membrane of the
the proximal tubule epithelia of the kidney (Schaub et al. 1997), duodenum and nerve
(Kool et al. 1997). However, due to its similarity in amino acid sequence and substrate
specificity to MRP, whether cMOAT is actually an MDR protein needed to be
determined. To answer this question, many studies have tried to detect levels of
cMOAT expression in cancer cells.
cMOA.T expression was investigated in various human cancer cells using RT-PCR and
immunoblotting to detect m.RNA and protein in human lung, gastric and colorectal
cancer cells and MDR cell lines (Narasaki et al. 1997). The highest level of expression
,vas detected in a human hepatoma cell line HepG2 cells. A cisplatin resistant cell line
sho,ved cMOAT expression, as did the non drug selected lung, gastric and colorectal
cancer cells. All these cancer cells expressed m.RNA at levels lower than I 0% of that of
HepG? cells. In another study, cMOA_T R.J.""\A levels and protein levels were extensively
analysed in a large cohort of drug selected resistant cell lines derived from many tissues
and cancers (Kool et al. 1997) . Cell lines resistant to cisplatin (a glutathione conjugated
chemotherapeutic agent) had varying levels of cMOAT R._1\TA and protein with increases
in some cell lines derived from ovarian, colon and epidermoid carcinomas. Doxorubicin
resistant cell lines had increased cMO_A.. T R_NA levels in some cell lines derived from
the lung.
Further characterization of cMOAT has been reported since these studies and v.rill be
co\~ered in context in the results chapters.
1.4
The ~tlultidrug Resistance Associated Protein Subfamily
The first indication that there ,;vere a number of ~ R proteins of the P-glycoprotein
lineage and the ~fRP lineage came from analy sis of the ~-CBI expressed sequence tag
·sn
database (_..\1Iik;1Jets er al. 1996). This paper high1ighted an excellent method forfinding-~ ne'-',- _-\BC trc.ns-noners bv searching: the database regularI ->y ~ ~ v with conserved
reQJ.o::is dis1inct to the familY. - r This method "'va.s also used bv (Kool ~ et al. I991J to sneci-9cally :.dentify ~fRP homologues. They reponed three ne,::-.T homologs ot bot
~fRP anci c~fQ_-\_T ~~ ~~ 0m,c. ~ -L1-, a ~ a \ ,m n .)..., \/D n ...
~ V J V _1-.[\_I ~ - ~ l nd ~vfRP5. Due to these
0 :0 21.:e-s.
-
c~fQ_-\_T iscMOAT=MRP2
Although some groups have adopted the newer name, many research groups still use cMOAT; no consensus has been reached. The thesis will now refer to the gene and protein as MRP2 in the majority of chapters. Also due to the MRP homologs, MRP was
renamed MRPl. Therefore, from this point on in the thesis:
MRP=MRPl
The normal tissue distribution of each of the three new homologs was examined using
RNA expression levels (Kool et al. 1997). The RNA expression levels of the MRP
homologues were compared and are presented in Figure 1.8.
1.4.1 MRP3
MRP3 was first reported in 1997 (Kool et al. 1997) and since then there has been many
reports by a number of research groups to determine its function, tissue distribution, membrane localisation and an interesting inducible characteristic different to MRP 1 and cMOAT.
Figure 8 illustrates that MRP3 was expressed similarly to MRP2 with moderate to high
levels in the liver, duodenum, colon and adrenal gland. Kool et al. analysed the
expression levels and correlation to resistance in doxorubicin and cisplatin resistant
cancer cell lines for the three new homolog~es. MRP3 ( and MRP5) was expressed in some of the cell lines but the levels of expression did not correlate to the resistance
observed.
MRP3 was reported as cMOAT2/MRP3 based on protein sequence similarity, 56% to MRPl and 45% to MRP2 (Uchiumi et al. 1998). mRNA was found to be expressed in
the liver, colon, small intestine and prostate, similar to the results presented in Figure 8.
MRP3 was also reported as MRP-like protein 2 (MLP2) (Hirohashi et al. 1998). In this
study, the full length rat cDNA for MRP3 was cloned. The tissue distribution was determined by Northern analysis and was similar to other reports except that no RN A was found in normal rat liver. However, MRP3 /MLP2 RNA was found in EHBR liver
••e•
•
•
..
Kid1h:y
...
•
•
•
..
HlaJJ1..•r
••••
' :•
•
..
Spk1..·11
••••
: .... ••
: .. :..
\ l.1111111;.iry gland ND ,, . , ND .'-:D
Sali\;1ry ~b11J '.'JI) -.
..
Thn11id
••••
.... , ~)•
'
f'1..·:-.1is
••••
()', .. '
..
:-.;,:n1..·
•
•
tJ..
Stomach
...
•
. _: -..
Lin:r ' . )
••••
....
; )•
Ciall hladder
...
ND '.'iD•
..
D11odc11um
..
..
...
ND NDColon
...
;=j...
\ )..
. ·\drcnal gland
••••
( =)...
; -)•
Sl,;cktal mu sck
..
, _ _ 1 ....'-°=-'
....
•kart
•
' ·--'.
_,..
Brain
•
(j 1) ~)...
Pl;iccnla
..
•.) ~ (°j•
Ovary
..
::~i•
P,.incre:is
•
•
....•
• ... •
Tl1nsil ND ._, -
•
•
..
·' ND. not dctl.'rmincJ: 'J. no cxprc:-.:-.ion : • • • • •· h1w to high 1..·xprc~sion.
From Kool et al. (1997)
Figure 1.8 RNA Expression of the MRP Subfamily
Levels of RNA transcripts of rvIRPl, rvIRP2, MRP3, MRP4, and MRP5 in human tissues.
14
normal liver. Although the expression of MRP3 in the liver was marked, whether MRP3
was involved in biliary excretion (i.e. expressed in the canalicular membrane) was not
determined directly until MRP3 protein was detected in the basolateral surface of the
hepatocyte using a specific antibody (Konig et al. 1999). Therefore, MRP3 is localised
in the sinusoidal membrane of the hepatocyte, alongside MRPl and is not involved in
secretion of products into the bile.
A third study characterizing MRP3 demonstrates that MRP3 is expressed in lung and
intestine of rats and was found in the liver of GY/TR- rats and EHBR rats which are
deficient in cMOAT (Ortiz et al. 1999). However, MRP3 was reported to localise to the
canalicular domain of the liver in these rats. Personal communication with Professsor
Irwin Arias (Ortiz et al. 1999) suggested the inconsistency might have been because of
the antibody used for the detection of MRP3. They also report that 24 and 72 hours after
bile duct ligation, MRP3 mRNA in the liver is increased several fold. This is an
important observation and suggests that the build up of bile products in the liver is
responsible for the increase in mRNA and presumably protein.
The transport properties of MRP3 are different to MRPl and MRP2. The glutathione
conjugates GS-DNP and L TC4 were poor substrates for MRP3 compared to the
glucoronide conjugates which are good substrates for all three transporters (Hirohashi et
al. 1999). MRP3 was also shown to transport bile salts, unlike MRP2. The endogenous
bile salts taurocholate and glycocholate and their two sulfated forms, were all substrates
for MRP3 but only the sulfated forms are substrates for MRP2 (Hirohashi et al. 2000).
In another study, MRP3 transfected HEK293 cells were able to confer resistance to
etoposide ( 4 fold resistance) and vincristine ( 1. 7 fold resistance) but not to a range of
other typical anticancer agents (Zeng et al. 1999).
MRP3 will no doubt be investigated further to determine its role in multidrug resistance.
The current literature suggests a role in excretion of organic anions and bile salts
through the sinusoidal membrane, to a low extent under normal conditions but to a
much higher extent during cholestasis and biliary obstruction such as in DJS. In tissues
15
1.4.2 MRP4
The first report of MRP4 was the partial sequence by Kool et al. (1997) and
concurrently by Lee et al. (1998) who called the protein MOAT-Band reported the full
cDNA sequence. Its sequence identity is 39% to MRPl and 38% to cMOAT at the
amino acid level and is shorter than the other MRP family members, lacking the
N-terminal transmembrane domain. Figure 8 shows that MRP4 is expressed at low levels
in only a few tissues but Lee et al. reported low RNA levels in the heart, skeletal
muscle, pancreas, spleen, thymus, gonads and intestine and to a high level in prostate.
Multidrug resistance was not found to be mediated by MRP4 in lung cancer cell lines
(Young et al. 1999) and although MRP4 was expressed in most of the cancer cells lines
tested by (Kool et al. 1997) no associated resistance was found.
A human T-lymphoid cell line CEM-rl was found to have intrinsic resistance to
antiviral agents and was therefore investigated for ABC transporter activity (Schuetz et
al. 1999). An 800-1000% amplification of MRP4 mRNA and corresponding elevated
protein was found in the resistant cells. This finding has an impact on the treatment of
patients with antiviral agents such as AZT, an anti-HIV drug. A decrease in intracellular
accumulation of these drugs enables two events to occur. First, it allows the host cell to
survive. Secondly, drug levels are low enough to allow the virus to replicate and
continue infection. The unique role of MRP4 in these lymphoid cells will no doubt be
investigated further as it may render some tissues more susceptible to viral replication
than those that do not express MRP4. This study also prompts the question as to the
involvement of the other MRP homologs in generating this phenotype.
1.4.3 MRP5
MRP5 expression was found in a range of cell types, particularly skeletal muscle, as
shown in Figure 8. When the MRP5 cDNA is expressed in polarised MDCK cells, the
protein localises in the basolateral membrane (Wijnholds et al. 2000), the same as
MRPl and MRP3. MRP5 has been characterized recently as an organic anion
transporter with substrates including 2,4-dintrophenyl glutathione, anticancer drugs
including 6-mercaptopurine and, like MRP4, anti-retroviral nucleoside analogs used for
HIV infection (Wijnholds et al. 2000). Lung cancer patients exposed to platinum drugs
had increased expression of MRP5 in both normal and tum10ctr lung tissue (Oguri et al.
16
were not correlated to drug resistance. A study aimed at determining a correlation
between expression levels and drug resistance did not find MRP5 involvement in drug
resistance despite finding mRNA expression in all cell lines and patient samples (Young
et al. 1999)
The ability of MRP5 to confer drug resistance has been demonstrated, plus its
expression in tumours has been shown but further investigation into the impact of
MRP5 on clinical multidrug resistance is required.
1.4.4 MRP6 and the Anthracyclin Resistance Associated Protein
The anthracyclin resistance associated gene (ARA) was reported to encode a novel ABC
transporter protein amplified in a MDR leukemia cell line (Longhurst et al. 1996). The
protein had one predicted ATP binding site and appeared only half the size of typical
ABC transporters. The gene was expressed in drug resistant cell lines along with MRPl.
MRP6 is located on chromosome 16 immediately adjacent to MRP 1 in the reverse
direction (Kool et al. 1999). The 3' end of the MRP6 gene was identified as the ARA
gene which, at the cDNA level, appears to be alternatively spliced and contain 5'
portions of MRPl (Kool et al. 1999), (Belinsky and Kruh 1999). MRP6 mRNA is found
predominantly in the liver and kidney. In drug resistant cell lines, MRP6 was only
over-expressed in a lung cancer cell line. However, in this situation, the MRPl gene was
co-amplified so the contribution to drug resistance may not be directly related to MRP6.
The co-amplification could be explained by their adjacent position on the chromosome
but the genes are in opposite directions. Investigations into the promoters for each gene
would be informative to this observation. The ARA protein may confer MDR as
reported by Longhurst et al. ( 1996) but in fact, the resistance may be due to MRP6
expression and/or MRPI. Also, the typical ABC transporter functions as a dimer
consisting of two repeating transmembrane domain and a nucleotide binding domain
(Figure 1.1 ). If ARA were to function as a transporter then it is likely to form a dimer.
No evidence for ARA dimer formation has been reported.
· The MRP subfamily is further expanding with the mention of a seventh member in a
very recent review (Borst et al. 2000). The naming of these proteins as multidrug
ability to confer MDR, however the other members of the family do not compare to
MRPI in their ability to confer MDR and tend to have more defined endogenous roles.
The specific roles of each of these family members in multidrug resistance in cancer
cells and their correlation to clinical multidrug resistance is yet to be rigorously
determined.
1.5
Endogenous Function: ABC Transporters in the Liver
The liver is one tissue with specific expression of a number of multidrug resistance
proteins. However, under normal conditions the ABC proteins function simply as
transporters of endogenous and exogenous substrates. The liver expresses a number of
transporters involved in the production of bile. Bile production is controlled by
regulating the proteins involved in the pathway, primarily the sinusoidal and canalicular
transporters. For example MRP 1 and MRP3 are expressed at low levels in the
sinusoidal membrane of the hepatocyte and therefore the transport of organic anions
back into the blood is minimised. Conversely, cMOA T /MRP2 and P-glycoprotein are
expressed at much higher levels and therefore the organic anions are transported into
bile. This creates a seemingly vectorial passage of organic anions from blood through
the hepatocyte into bile.
One fundamental question arises from this observation. What are the cellular
determinants that sort these proteins to their specific localisation in distinct membranes?
This leads to the field of protein targeting, a major component of this thesis.
· 1.6
Protein Targeting
1.6.1 Cell Morphology
Many organs in the body have two compartments or 'sides' that are separated by a
physical barrier of epithelial cells. Typical examples of this division are the stomach,
which separates the gut contents and the blood, the liver, which separates the blood
from bile, and the kidney, which separates blood from urine. The epithelial cells that
form the barrier are ref erred to as polarised cells as they have two distinct membranes
polarised cell is a neuron, which has two membrane domains, one consisting of the dendrites and the other comprising the axon. The two membranes of a polarised cell have specific protein and lipid composition, which are kept distinct by the unyielding
tight junctions. The distinct proteins in each membrane are responsible for the vectorial
transport of compounds. For example, nutrients from the stomach are transport into the cell, metabolised, then transported into the bloodstream. Conversely in the hepatocyte,
waste or excess compounds move out of the bloodstream, are metabolised, then
transported into the bile duct for excretion. In contrast, a non-polarised cell does not have specific membrane domains. The plasma membrane has a homogenous protein and lipid composition and does not separate two compartments. Examples of non-polarised cells are the haematopoeitc cells or fibroblasts. Figure 1.9 illustrates the distinction
between polarised and non-polarised cells.
1.6.2 Protein Trafficking
In order to create and maintain the specific membrane domains in polarised cells,
trafficking pathways regulate the targeting of the plasma membrane proteins. Proteins
are assembled in the endoplasmic reticulum (ER) after mRNA translation and are subsequently targeted to the Golgi Apparatus and then the Trans-golgi network (TGN)
for sorting and post-translational modification. Proteins destined for the plasma
membrane in polarised cells are targeted from the TGN to the basolateral and/or the apical membranes by three known mechanisms; directly, indirectly and randomly.
These mechanisms are represented in Figure 1.10.
Hepatocyte canalicular proteins were originally reported to transcytose from the basolateral to the canalicular membrane (Matter and Mellman 1994) but since then
some hepatocyte proteins have been discovered to be directly targeted to the apical
membrane. Such proteins include the ABC transporters MDRl and MDR2 from rat (Kipp and Arias 2000).
Matter and Mellman (1994) propose "a common unified theory" of protein sorting
which states that since many different cell types recognise the same molecular targeting
From UCSF Dept. of Anatomy, From Department of Cell Biology, Histology Image Internet database, Urinary image 5
Http:/ !anatomy .ucsf.edu/Pages/ Anatomy 115/ur.html
Figure 1.9 The Polarised and Non-Polarised Cell
Vanderbilt University Medical Center Website:
http://www.mc.vanderbiltedu/histo/blood/lymphocytes.html
A. Histology of epithelial cells from a kidney section focusing on a distal convolution of the
distal tubule. The basolateral 1ne1nbrane (BL) faces the blood or adjacent cells and the apical 1ne1nbrane (AP) faces the lumen (L).
B. Histology of blood cells. The 1najority of cells in this i1nage are red blood cells, with a
c::J
~~•
o
ER
GA
TGNb
2
•
o
oe
GA
TGN
\
J'
c::J
~ER
3
~
•
i ;llER
GA
•
n .. ·"•
L:_j
TGN
r ' t
Endocytosis
a
~//~>·
~- > ~ ~.
Degraded
~! b
ER= endoplasmic reticulum; GA= golgi apparatus; TGN= Trans golgi network; a = apical; b = basolateral
T ranscytos1s .a
Recycling b
[image:42.1200.82.761.55.891.2]From Mostov and Cardone (1995)
Figure 1.10 Mechanisms of Protein Sorting
The diagram represents the three mechanisms of protein targeting.
1. Direct targeting from the TGN to the apical or basolateral membrane.
2. Indirect targeting where the protein is trancytosed to the correct membrane after first arriving at the incorrect membrane.
axonal targeting in the neuron - similarly basolateral signals are recognised as dendritic
targeting signals.
1.6.3 Targeting Signals
There are an increasing number of molecular targeting signals being reported that are intrinsic to a particular protein. These signals target the proteins to domains such as the ER, mitochondria, peroxisome, endosomal compartments and, pertinent to this thesis, the plasma membrane domains. This thesis is only concerned with the molecular
targeting signals that help create the polarised cell by sorting proteins to the apical or basolateral membranes. Proteins expressed in the plasma membrane of a non-polarised cell are generally considered to have a default pathway, as there are no other options. In comparison, the polarised cell has to traffic proteins to the basolateral and apical
membranes and therefore presents more specific signal based targeting systems. The literature presents different signals for membrane proteins that only span the plasma membrane once, such as receptors, compared to multiple membrane spanning proteins
(polytopic proteins), such as ABC transporters. The following sections highlight the range of possible membrane signals.
1.6.4 · Receptors Targeting Signals
A typical membrane receptor has an extracellular domain, a single membrane spanning
domain and a cytoplasmic tail ( commonly referred to as Type 1 membrane protein).
Basolateral targeting signals are commonly found in the cytoplasmic tail. This is usually
determined by deleting the cytoplasmic tail, resulting in unpolarised or apical membrane
targeting. For example, the LDL receptor is a single transmembrane protein normally
targeted to the basolateral membrane. By constructing truncated mutants Y okode et al. (1992) demonstrate that the cytoplasmic tail was responsible for basolateral targeting as the truncated mutants localised apically. Within this stretch there are four amino acids spaced at intervals of three, which was speculated to be the face of a helix responsible
for the targeting. Furin is also a Type 1 membrane protein and in a similarly designed
study to the LDL receptor, a number of sorting signals were characterized (Simmen et al. 1999). Furin has four defined sorting motifs in the cytoplasmic domain of the protein. The basolateral targeting signal is initially dominant over: the transcytosis