0022-538X/79/03-0856/07$02.00/0
Formation of Rous Associated Virus-60:
Origin
of the
Polymerase
Gene
R. C. SAWYER,t* C. W. RETTENMIER, ANDH. HANAFUSA TheRockefeller University, NewYork, New York10021
Received forpublication4August1978
The DNA ofnormal chickenembryos containssequencesrelatedtothe avian
leukosis-sarcoma viruses. RNA-dependent DNA polymerase of these virusesis
encodedbya
genetic
element knownasthepolgene.Thenatureof theendoge-nous virus pol gene in chicken cells was investigated by testing its ability to
participate in genetic recombination. Rous-associated virus-60-type recombinant
viruses isolated after infection of chicken cells with strains tsLA337PR-B or
tsNY21SR-A, both of whichproducea
temperature-sensitive
DNApolymerase,
alsopossessed thetemperature-sensitive
lesion. These resultsareconsistent with thehypothesis that theendogenous viral information used for the generation of Rous-associated virus-60 is deficientin atleastpartof thepolgeneand thatthedefectincludes thatportion represented by the lesionsin NY21 andLA337.The frequency ofpolymerase-negative BH-Roussarcomavirus aformation was not
affected by the levels ofendogenous viral
expression,
which suggeststhat theadefectisnotderived from theendogenous polgene.
Thegag,pol,andenvgenesof avian
leukosis-sarcomaviruses
(ALSV)
direct thesynthesis
ofviral internal structuralproteins,
RNA-depend-ent DNA
polymerase,
andenvelope
glycopro-tein,respectively.
Normal chicken cells containas anintegral partof theirDNA genetic
infor-mation relatedtoALSV (1,2, 7, 36,40).Several lines of chickens spontaneously release avirus, Rous-associated virus-0
(RAV-0),
whichpos-sesses subgroup E glycoprotein specificity (6,
41). Themajority of chicken cells donot
spon-taneously release virus
particles,
but they do produce varyingamounts ofviralRNA,group-specific (gs) antigens,andsubgroupEenvelope glycoprotein (chicken helper factor [chf]) (2,3,
7, 10, 14, 19, 32, 39, 43,44).
Infection of chicken cells with an ALSV
re-sults intheproduction notonly of the progeny
of theexogenousvirus but also ofagroupofnew
subgroupEvirusescalled Rous-associated
virus-60 (RAV-60) (15, 16). The titers of RAV-60
obtainedfrom chicken cells reflect the amounts
ofendogenous viralRNApresent in thesecells.
RAV-60 differs from RAV-0 in its more rapid
growth in avian cells that are susceptible to
subgroup E viruses (13, 27, 34). Nucleic acid
hybridization studies comparing the RNAs of
different RAV-60 isolates withthose of the
ex-ogenous ALSV parent and endex-ogenous viral
se-quences indicated that RAV-60 is a group of
tPresent address: Cancer ResearchLaboratories,
Wood-haven,NY11421.
genetic recombinants (13, 22, 39).
Analysis
ofelectrophoretic
markers in ALSVstructuralpro-teins
suggested
that crossing-over could take place between the exogenous and endogenous viralgaggenesduring the formation ofRAV-60(33).
In thispaper we have examinedthecapacity
of the
endogenous
ALSV-related pol gene toparticipate in genetic recombination. We have attempted to determine the origin of the pol
geneof RAV-60by infectingcells withparental aviansarcomavirusescontaininga
temperature-sensitive(ts) lesionin thepolgeneand examin-ing the polymerase of the recovered
RAV-60.
Two
parental
tspolmutantswereused forthispurpose,tsLA337PR-B (26, 28) and
tsNY21SR-A (Metroka andHanafusa, manuscript in prep-aration), and in all cases the RAV-60 isolates obtainedwerefoundto containthets
polymer-asegeneof the parentalsarcomavirus.
We nexttested thepossibility that a-type
BH-Rous sarcoma virus(BH-RSVa), which does not
synthesize functional polymerase, might be
formedbyrecombination ofpolymerase-positive
BH-RSV(-) with a defective endogenous pol
gene. However, unlike RAV-60production, the
frequency of BH-RSVa formation is
independ-entof thelevelsofendogenousviral gene
expres-sion. The defect inBH-RSVa canapparently be
localized to a portion of the pol gene because
these variants are able to recombine with tsLA337, but not with tsNY21, to generate
vi-ruseshavingwild-type polymerase.
856
on November 10, 2019 by guest
http://jvi.asm.org/
RAV-60 pol GENE 857
MATERIALS AND METHODS
Cells and viruses. Chicken embryo cells were
prepared from fertile eggs (SPAFAS, Inc.,Norwich, Conn.) andwereassayedforgsantigen bycomplement
fixation andforchf by the formation ofBH-RSV(chf) (16, 17). Embryosweredesignatedgs-chf (negative forgsantigen andchf),
gspchf
(positive forgsantigenand chf) or gsjhE (low levels ofgs antigen and
ex-tremely high levels ofchf). Japanese quail embryo
cells wereprepared fromfertileeggs (also from
SPA-FAS)inthesamemannerandwerefirst used for virus
propagation after the third subculture. The media, conditions fortissueculture, and propagation of ALSV have been previously described (17,24).
LA337subgroupAwasderived by mutagenesis of thePrague strain ofRoussarcoma virus (PR-RSV)
(26, 28). It contains atsdefect in thepolgene.The permissive and nonpermissive temperatures are 35
and 41°C, respectively. NY21 also containsa tspol
gene and was derived by mutagenesis of Schmidt-RuppinRSV-A(SR-RSV-A). The permissive temper-ature is37°Cand the nonpermissive temperatureis 41°C (Metroka and Hanafusa, manuscript in
prepa-ration). Stocks of the two mutants were grown on
gs-chf cellsatthepermissivetemperature.
RAV-60 is formedafter infection of chicken cells
withanALSV(15, 16). Itwasisolated bypassaging
serial 10-fold dilutions of culture fluids from ALSV-infected gs+chf'orgsLhEchicken cells onquailcells
susceptible to subgroupE infection. Thequail cells were subcultured periodically, and virus production was assayed by the appearance of virus-associated
reversetranscriptasein the culture fluids.Theprogeny
virus from thehighestdilutionproducingaproductive
infectionwasagain passagedonquailcellsusingserial 10-fold dilutions.Unlesstheoriginalgs+chfr orgsLhK
stock contained titers ofarecombinantaviansarcoma
virus ofsubgroupEinexcessof the RAV-60titer,the
second passage on quail cells usually yieldedstocks containing only RAV-60, asjudged by the lack of transforming virus or virus infectious for C/E cells. For the isolation of RAV-60 fromNY21,theoriginal chick cellculturefluidsweretreated with antiserum
preparedinchickensagainstSR-RSV-A priorto in-oculation of the quail cells. In addition, the culture
media contained anti-SR-RSV-A serum during the firstpassageinquailcells.
Polymerase assay and gel electrophoresis of
viral proteins. Virus-associated RNA-dependent
DNApolymerase activitywasdeterminedbypelieting the virus in 10 ml of culture fluidinaBeckmantype 40rotor.The reactionmixture in 0.1 ml contained 7.5
.g of(rC).(dG)1218(7:3),0.1% NonidetP-40,5.0,umol ofTris-hydrochloride (pH 8.3),2.0,umolof dithiothre-itol,6.0,imolofNaCl,1.0,umolofmagnesiumacetate, and 500pmolof[3H]dGTP (500cpm/pmol).Thevirus pellet was first suspended in the reaction mixture
without(rC)n(dG)1218and[:H]dGTP.Sampleswere
incubated at 35 and 41°C (LA337) or 37 and 450C (NY21) for 30 min. Thetemplate primer and [tH]-dGTPwerethenadded,and the incubationwas
con-tinued. At theend of 60 min,trichloracetic acid-pre-cipitableradioactivitywasdetermined.
Sodiumdodecyl sulfate-polyacrylamide gel electro-phoresisof viralproteinslabeledwith
L-[3S]methio-nine intissue culture was done as described elsewhere (33).
Cloning ofBH-RSV(-). BH-RSV(-) grown in
both gs-chf- and gs+chf+ cells was cloned in the ab-sence of helper virus by soft-agar colony formation usingUV-treated Sendai virus (17). A sample of 28 x 106 normal cells (gs+chf,gs-chf, orgsLhE)was incu-bated withDEAE-dextran, UV-treated Sendai virus, and1 x106 to5x106focus-forming units of BH-RSV. Thecells were divided into six plates and cultured in
soft-agarmedium. After 12to 17days (17 to21days
forgsLhE cells), individual colonies were picked and
incubated in0.1 ml of trypsin-EDTA solution for 10 to 15min at room temperature(trypsin-EDTA: 0.05% trypsin; 0.02% EDTA; 0.025 MTris-hydrochloride, pH 7.4; 0.14 MNaCl; 0.0005 M KCI; 0.3 mM Na2HPO4; and 6 mMglucose). The cells were plated with 0.4 x 106normal chicken cells on 35-mm culture plates and overlaid with soft agar medium 24 h later.
BH-RSVa(-) is defined as a virus particle that lacks
reverse transcriptase activity (12). Transformed cul-tures that did notproduce infectioussarcomavirus and didnotcontainpolymerase activityinthe culture fluids, but which did produce infectious sarcoma virus aftersuperinfectionwith RAV-1, were classed as
BH-RSVa(-)-transformedcultures. These
BH-RSVa(-)-transformed cells produce physical particles, lacking
polymerase, whichcanbe assayed by [3H]uridine
la-beling,and thelevelof the production of noninfectious
particlesgenerallycorrelated with the level of the titer
ofBH-RSV(RAV-1)obtained upon rescue with RAV-1.
Cultures ofBH-RSVa(polymerase negative)-trans-formed chickcells were grown from individual soft-agarcoloniesusing BHRSVa(RAV-1) aspreviously described (37).
Crosses between BH-RSVa and LA337 and NY21. Cultures of BH-RSVa-transformed chicken cellsweresuperinfected with either LA337 or NY21 at 35 or 37°C, respectively, and maintained at these temperatures. Culture fluids were changed daily. Vi-rus-associated RNA-dependentDNA polymerase ac-tivitywasassayedonculturefluids harvested4days
postinfection. Progenyvirus inculture fluidsharvested
5 days postinfection were then cloned by soft-agar
colonyformation. Soft-agarcolonies of cells infected
with LA337orNY21werealso prepared as controls.
Absorptionandcolonygrowth weredoneat410C,the
nonpermissivetemperaturefor LA337 and NY21. Po-lymeraseactivityfrom culturesderivedfrom
individ-ualsoft-agar colonieswasassayedasdescribed.
RESULTS
pol
gene of RAV-60. RAV-60 has beenshown to be a recombinant virus containing
genomic RNAsequences fromboththe
endog-enous provirus and exogenous infecting virus
(13, 22, 38). With theexceptionof
RAV-0-pro-ducing cells, normaluninfected chicken cells do notproduceavirus-relatedreversetranscriptase
(12,23, 29,46). Wewereinterestedin
determin-ing whether
theendogenous pol
gene couldres-cueknown mutations in thepolgenes of
exoge-nous viruses during the formation ofRAV-60.
on November 10, 2019 by guest
http://jvi.asm.org/
Two such mutants, LA337 and NY21, were grown ongs+chf+andgsLhE chicken cells.
RAV-60 was isolated from these stocks by repeated
passageatend-point dilutionsonQ/B Japanese
quail cells. After RAV-60 isolates were
deter-minedtobe free of parentalsarcomavirus,they
were tested for the temperature sensitivity of
thepolgene.The datapresentedinTable 1are
expressed as the ratio of counts per minute
incorporated atthe nonpermissive temperature
tothatatthe permissive temperature.The first
column for each virus details thedegreeof
tem-perature sensitivity of the different stocks of
parentalsarcomavirus from which eachisolate
ofRAV-60wasderived. (RAV-60 titersareabout
10-'
of that of sarcoma virus in culture fluidsfromgs+chf+ and gsLhE chicken cells fully
trans-formed by RSV and, thus, do not contribute
measurably to these data.) In each case the
parental LA337 and NY21 incorporate only 10
to20%asmuch[3H]dGMPatthenonpermissive
temperature as at the permissive temperature
using (rC). (dG)1218 asthe template primer in
the polymerase assay. Wild-type Prague RSV
and SR-RSVincorporated slightlymore
radio-activityatthehigher temperature. Insome
po-lymeraseassays, control SR-RSVorRAV-2
in-corporatedslightly fewer countsper minuteat
45thanat37°C (45/370C =0.85to1.0).
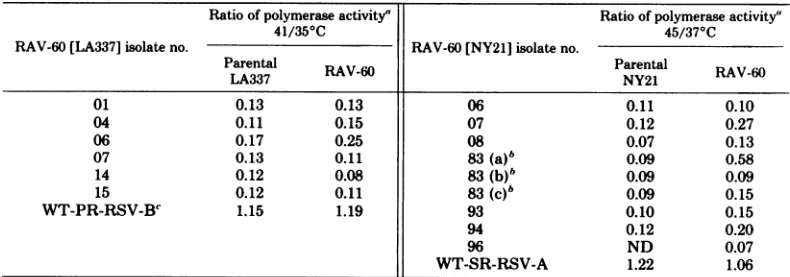
RAV-60 isolated fromwild-typePrague
RSV-Band SR-RSV-A containedawild-type
polym-erasegene. Inaddition, RAV-60 prepared from
RAV-1,RAV-2, andBH-RSV(-)alsocontained
awild-type polymerase gene (data notshown).
However, when a temperature-sensitive virus
was used as the exogenous parent, all of the
RAV-60sisolatedproducedatspolymerase that
wastemperaturesensitivetothesamedegreeas
the parental virus. We notedonlyoneexception,
RAV-60[NY21],isolate 83a. This isolate, while
stilltemperaturesensitive,wassignificantlyless
so than theNY21 stock from which it was
iso-lated (45/370C of 0.58 versus 0.16). However,
twoother isolates of RAV-60(83b and83c)were
purified from the same virus stock obtainedas
culture fluid of NY21-infectedgs+chf+ cellsand
used for the isolation of 83a. Both 83b and 83c
were as temperaturesensitive as the NY21 of
theoriginal stock. With the exception of
RAV-60 [NY21] 07 and 08, all isolateswere derived
from gs+chf+-type cells. The use ofgsLhE-type
cellsdidnotaffecttherecoveryoftsRAV-60as
[image:3.503.66.461.455.594.2]demonstrated by isolates 07 and 08 for RAV-60 [NY21].
Origin of RAV-60gaggene.We haveshown
thatelectrophoretic variants of ALSV structural
proteins canbe used to determine the genetic
origin of RAV-60gaggene products (33). The
mostconvenient marker for thispurposeis the
major structural protein p27. The p27 encoded
by RAV-0 has alowerelectrophoretic mobility
in sodium dodecyl sulfate gels than the p27 of
exogenousALSV.One RAV-60isolatewas
pre-viously showntohaveap19markerspecific for
the exogenousvirus used initsproduction and
ap27 that comigrated with that ofRAV-0 (33).
From this we concluded that the endogenous
viralgag gene encodesa p27closely relatedto
that of RAV-0. Wehave examined the
electro-phoretic mobilities of the structuralproteins of
RAV-60sobtained in thepresentstudy. For this
analysis, Prague RSV-B, SR-RSV-A, RAV-0,
and the RAV-60swerelabeled with
[:35S]methi-onine, and the viral proteinswereseparated by
TABLE 1. Thermallability of RA V-60polymerase derived from two ts polymerase mutants Ratio ofpolymeraseactivity" Ratio ofpolymeraseactivity"
41/35°C 45/37-C
RAV-60[LA337] isolateno. RAV-60 [NY21] isolate no.
Parental RAV-60 Parental RAV-60
LA337 NY21
01 0.13 0.13 06 0.11 0.10
04 0.11 0.15 07 0.12 0.27
06 0.17 0.25 08 0.07 0.13
07 0.13 0.11 83(a)b 0.09 0.58
14 0.12 0.08 83 (b)b 0.09 0.09
15 0.12 0.11 83 (c)b 0.09 0.15
WT-PR-RSV-B' 1.15 1.19 93 0.10 0.15
94 0.12 0.20
96 ND 0.07
WT-SR-RSV-A 1.22 1.06
aSampleswerepreincubatedatthe indicated temperature in the absence of template-primer and
deoxytri-phosphate for 30 min. [3H]dGTP and (rC).-(dG)1218 were added, and incubations were continued at the respective temperature for60 min.Trichloroacetic acid-precipitable radioactivity was then determined. ND, Notdone.
bThree separate isolations of RAV-60 from the same original virus stock.
'WT,Wild-type; PR-RSV-B, Prague RSV subgroup B; SR-RSV-A, Schmidt-Ruppin RSV subgroup A.
on November 10, 2019 by guest
http://jvi.asm.org/
RAV-60pol GENE 859
electrophoresis in 12% polyacrylamideslab gels
containing 0.1% sodium dodecylsulfate. The p27
proteinsof all RAV-60 isolates listedinTable 1
comigrated with the p27's of theexogenous pa-rental viruses.
Formationof BH-RSVa.BH-RSV(-)
spon-taneously segregates a polymerase-minus
mu-tant, BH-RSVa, thatisunableto direct
synthe-sis ofanypolymerase-relatedprotein (11, 12, 35). It is possible that BH-RSVa is generated in a
manneranalogous to theformation of RAV-60,
in which recombination occurs between an
ex-ogenous infecting virus and the endogenous
se-quences. During RAV-60 formation the
polym-erasegene is derived from the exogenous virus.
Thereciprocal recombinant, ifit occurs,would
presumably contain an endogenously supplied polymerase gene. The a form of BH-RSV(-) might represent this reciprocal recombinant. Since the amount of RAV-60 produced in chickencells isproportionaltothe level of
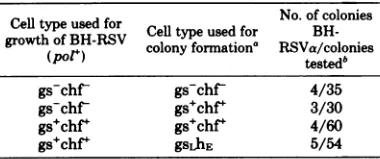
en-dogenous viralexpression (15, 16,45), wetested thehypothesis that BH-RSVa might be formed inasimilarmannerby measuring thefrequency
of aformation from stocks ofBH-RSV(-)grown
and subsequently cloned in chicken cells with varying levels of endogenousgeneexpression.If the hypothesis were true, the percentage ofa
clonesshould be highestinthosecells with the
highest levels of endogenous gene expression. Theresultsarepresented in Table2.They show that,regardless of thecell typeused for
cloning
the virus, 5 to 10% of theresulting
cloneswereof the a type. Unlike RAV-60 production, BH-RSVa titers do notappeartobe
dependent
onthe level of
expression
of theendogenous
viralgenes. Therefore, the result seems to suggest
thatthe formation ofBH-RSVa
probably
doesnotrepresent recovery intovirus ofadefective
endogenous
polymerase
gene linkedtogsanti-TABLE 2. Effectofcellphenotypeonthegeneration
ofpolymerase-negativeBH-RSVa
Celltp
sd typeused forCl No. of coloniesgrowth ofgrowthofBH-
BHoRSV
colonyCel
typeformation'
used for RSVa/coloniesBH-(pol') testedb
gs-chf gs-chf 4/35
gs-chf gs+chf+ 3/30
gs+chf+ gs+chf+ 4/60
gs chf' gsLhE 5/54
aVirus wasclonedby soft-agar colony formation.
bIndividual coloniesweretestedfor infectious virus
(for those colonies grown on chf+ or hE cells) and
polymerase activity in culture fluids. Colonies
sus-pected of being BH-RSVa were further tested for positiverescuebyavian leukosisvirusRAV-1,and for lack ofrescueby reticuloendotheliosisvirusand
pheas-antvirus(43).
genand chf. Further,extensiveeffortsto isolate
a
pot
mutantfrom SR-RSVhave consistentlybeen unsuccessful (unpublished data). For this
reason we did not attempt to isolate a
pot
LA337 or NY21.
Recombinationbetween BH-RSVa and a
ts mutant.Infectiouspseudotypes of BH-RSVa
arereadily formed by superinfectingwith a leu-kosis virus such as RAV-1. Upon subsequent
cloning of such pseudotypedstocks, some clones
will be polymerase-positive BH-RSV(-), of
which atleastaportion of the polymerase gene
ispresumably derived fromthehelpervirus. To
determine whetherBH-RSVa contained a
par-tialpolymerase gene capable of genetic recom-bination,weanalyzedcrossesbetweenit and two mutants, LA337 and NY21, containing different
temperature-sensitive defectsinthepolymerase
gene. The progeny of these crosses were
screened for wild-type recombinantsby cloning atthenonpermissivetemperature
(410C).
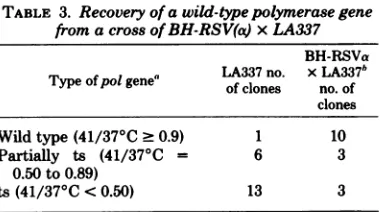
Clon-ing in thismannerpreferentially selects forwild-typevirusif they exist in the stock. The results (Table 3) show that BH-RSVa
readily
recom-bines with LA337 to yield wild-type virus. By
this selection technique more than 90% of the
BH-RSVa x LA337 progeny that are able to
initiatecolony formationat
410C
contain eitherawild-typeoronlypartially temperature-sensi-tivepolymerase. On the other hand, only 1 out
of11 BH-RSVax NY21progenycloned in this
manner waswildtypeforpolymerase (datanot
shown). Both crosses utilized the same
BH-RSVa stock. Thus BH-BH-RSVa contains at least the part of the
polymerase
gene encompassing thelesion in LA337 butnotNY21 and iscapable of genetic recombination with LA337 but notNY21 toformwild-type polymerase.
DISCUSSION
The expression of theendogenous retrovirus-relatedgagandenvgenesofchicken cellsin the
form ofgsantigens and chf is well documented (2, 3, 7, 10, 32, 39, 44).
However,
no functionalproduct
of theendogenous
pol
gene has been detected (12, 34, 39, 46). That the endogenous viral genes do contain at least somepol
genesequences can be inferred from the
following
lines of evidence.
Hybridization
datausing
acomplementary DNA
probe
representing
theALSVpol gene revealed that some, butnotall,
ALSVpol gene sequencesarerepresentedin the
polyadenylic acid-containing
RNA ofgs+chf+
chicken cells (43). Additionalsequences
appar-ently lacking in gs+chf+ cells are found in the RNA ofgsLhE-type cells. The
gs+chf+
cells alsocontain a
protein
of molecularweight
120,000which contains some of the
tryptic
peptides
ofVOL. 29, 1979
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.503.52.242.501.581.2]860 SAWYER, RETTENMIER, AND HANAFUSA
TABLE 3. Recoveryof a wild-type polymerase gene from a crossofBH-RSV(a)xLA337
BH-RSVa LA337no. xLA337h
Typeofpolgene' of clones no.of clones Wild type(41/370C >0.9) 1 10
Partially ts (41/370C = 6 3
0.50 to 0.89)
ts(41/370C<0.50) 13 3
a Anexogenouspolymeraseassaymeasured the in-corporation of[3H]dGMP using (rC).(dG)1218 as a templateprimer. Assignment of gene typewasbased onthe ratio ofcountsper minuteincorporatedat410C
tothatat350C.Wild-type polhada41/35°C ratio of
- 0.90;41/350Cof0.50 to0.89=partiallyts;41/350C
of <0.50=ts.
'Stocks of LA337ortheprogenyofamixed
infec-tion ofBH-RSVa and LA337wereclonedby soft-agar
colony formation at410C.The samestock of LA337 was used forcloning and thecrosseswithBH-RSVa.
reverse transcriptase (8). Finally, Panet et al.
(31) demonstrated that uninfected cells
con-tainedsmall amounts ofmaterialantigenically related to reverse transcriptase. These results areconsistentwith the hypothesis
that
theen-dogenous viral genes contain a defective pol
gene. The defectiveness of this gene isfurther
supported by the observations that BH-RSVa
grown in chicken cells doesnot spontaneously
revert to the polymerase-positive f8 type, and
RAV-60 cannot be obtained from BH-RSVa-infected cells(12).
As part of our attempts to deternine the
origin ofthe various genes ofRAV-60, we
ex-amined thenatureof theendogenouspol gene.
Westudied whether it couldcorrectthe ts defect
in thepolgene oftwo different aviansarcoma
virusesby testingfor therecovery of wild-type
RAV-60. In
addition,
weexamined whether the polymerase-negative defect of BH-RSVamightbe derived from the endogenous pol gene by comparing thefrequency ofa-typeformationby
polymerase-positive BH-RSV(-) in chicken
cells with varying degrees of endogenous gene
expression. That the BH-RSVa pol gene was
different from theendogenousgene was further
confirmedbydemonstratingthat theBH-RSVa
could recombinewith LA337 to yield wild-type
polymerase.
Inevery RAV-60 isolate we tested, the
polym-erase wascharacteristic of the exogenous parent.
Usingtwodifferent ts pol mutants asexogenous
parents, we were unable to recoverRAV-60 that
was not temperature sensitive for polymerase
from either gs+chf+ or
gsLhF
chicken cells.Al-thoughthetotal number of RAV-60examined is notverylarge, ifoneconsiders that all of them
contain theenv gene whichspecified
subgroup
E glycoprotein and thatpoland env areoftengenetically linked (5, 9, 28, 47), the failure to
isolate a RAV-60 with wild-type reverse
tran-scriptase is clearly significant. Although more
RAV-60isolates fromgsLhEcellsmust bestudied
before itcanbe concluded that thepolgene of
these cells cannot
recombine,
the results fromthe two embryos tested are identical to those
from gs+chfr embryos. Furthermore, we dem-onstrated that
wild-type
ALSV yielded wild-type RAV-60. Theseresultsare consistent withthose of Wangetal. (43), who have shown that the endogenous viral RNA of
gs+chf7 (but
notgsLhE) cells contains a deletion in the pol
se-quences.
Apparently
the additionalsequences inthe larger
endogenous
viral RNAtranscript
found ingsLhE-type
cells is not sufficient toenable it to correct the ts defects ofLA337 or
NY21. One RAV-60 isolate (RAV-60
[NY21]
83a)waslesstemperaturesensitive
(the
45/370C
ratio forpolymeraseassay was0.58,versusthat for theoriginal NY21, 0.16). Our
experience
withNY21 isthatuponcloningweobserve about 10%
of clonesthat areconsiderably lesstemperature
sensitive than the
45/370C
ratio of0.1 to 0.2usuallyobtained (datanot
shown).
We believe that if RAV-60wasrepeatedly
cloned fromany onestock of NY21 grown ings+chf+
cells,5 to10% of theisolateswouldprobably be only
par-tiallytemperaturesensitive.
Thegag geneproteinsareinitiallysynthesized
as a singleprecursor polypeptidein which p19 has beenmappedattheamino-terminal end and p15atthe
carboxy-terminal
end(42). Eisenmanetal. (8) failedtodetectthePr76 gag precursor
protein in gs+chf+ cells, but did observe a
120,000-daltonpolypeptide containing the
tryp-tic
peptides
ofp27, p19, and p12 but not p15. Thus thelack ofp15 and viral reverse transcrip-taseinnormalcells canbemosteasily explained
bypostulating
a deletion in the gs+chf+tran-scripts ofsome 1,900 nucleotidesencompassing the3'(p15)region of thegag geneandextending
substantially
into thepol gene. In a previousstudy in which the appropriate gs protein
markers were used, the p15 of RAV-60 was
always supplied bytheexogenous virus (33).By asimilaranalysis, every RAV-60isolate in this
study wasfound to have the p27 of the
exoge-nousparental virus. We have observed very few
clonesof RAV-60 in which the entire gag gene
was notsupplied by the exogenous virus. The
relativescarcity ofRAV-60containing any
en-dogenousgag information isprobablydue to the
fact that an additional recombination event is
requiredtopick up a portion of the endogenous
gaggene.
Analysisof the "c" region of three isolates of
on November 10, 2019 by guest
http://jvi.asm.org/
RAV-60polGENE 861
RAV-60,
derived
from RAV-1, RAV-2,andBH-RSV(-),
respectively,suggested that this regionwasofexogenous origin inallthreeisolates (W.
Hayward, personal communication). Taken
to-gether, the data suggest that the majority of
RAV-60 areformedby adouble crossover
mech-anism with the crossovers occurring at or near
the5'and 3' ends of the env gene.
If theendogenous pol gene does not
recom-bine with the ts mutants LA337 and NY21 to
restore wild-type polymerase, it might still be
recovered into virus as a defective polymerase
gene.One candidate for sucharecombinantwas
BH-RSVa, since thismutant does not contain anydetectable polymerase protein.BH-RSVa is
spontaneously generated byBH-RSV (12). We
examined the frequency of BH-RSVaformation
onchickencells with differentlevels of endoge-nous virus gene expression. We reasonedthat,
justasthe titers of RAV-60areproportional to
thelevel of endogenousgene expression, so too
should the titers of BH-RSVa be proportionalif
the pol gene of BH-RSVa is derived from a
defectiveendogenous polgene. Infact, regard-less of the celltypeusedtogrow BH-RSV stocks
or toclone them, 5 to 10%of theprogeny were
BH-RSVa. Thus, thelevel of endogenous viral
gene
expression
does not seem to affect the frequency of a-type formation, leading us toconclude that BH-RSVa doesnot represent
re-coveryof the endogenouspolymerasegeneinto
virus. Itseemslikely that this defect, thoughtto
beadeletion,isgenerated insomeothermanner.
If, however, recombination between exogenous
andendogenous viralgenetic information is
un-related to the extent of the expression ofthe
endogenous gene (e.g., direct recombinationat
the DNA
level),
the above experiment wouldnotresolve this
question.
The ability of BH-RSVa to recombine with LA337(butnot
NY21)
furthersubstantiates that the defect in BH-RSVa isdifferent from that in theendogenous
polymerase
gene.In these stud-ies cultures thatproduced
virus-associatedpo-lymerase in the culture fluid but no sarcoma
virus infectiousfor
C/E
chicken cellswerepre-sumedto havebeen initiated
by
infection with BH-RSVa that had recombined with LA337 tobecome
BH-RSV(-). By
thesecriteria,
slightly
less than half the cultures were derived from
thistype of recombinant.
We conclude from these data and those of
Wanget al. (43) thatnormalchickencells
con-tain as part of the
endogenous
virus genes adefective virus-related reverse
transcriptase
gene. There is
evidence,
however,
that normalchicken cellscontain genetic information
capa-ble of
recombining
with LA337 toyield
wild-type virus. LA337-transformed chicken cells
yield a significantly higherpercentage of wild-typevirions
after
treatmentof the cells with the DNAof normal chicken
cellsby
DNA transfec-tion techniques (4). In this study, DNA fromJapanese quail cells was also effective. Since
quail cells appear to contain little or no DNA
sequences homologous to ALSV RNA (W. S.
Hayward, personal communication), these
re-sults suggest that the LA337pol gene may be
capable of recombining withanon-ALSV cellu-larDNApolymerasegenein this system.
ACKNOWLEDGMENTS
We thankC. M. Metroka for supplying NY21. In addition, wethank Lynn Baldassari for excellent technical assistance and Randie Davidson for help in preparing the manuscript. Thiswork was supported byPublic Health Service research grant CA-14935 from theNational Cancer Institute and by grantVC-225 fromthe American Cancer Society. R.C.S. was afellowof the Jane Coffin ChildsMemorial Fund for Medical Research.
LIERATURE CITED
1.Baluda, M. A.1972.Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc. Natl. Acad. Sci. U.S.A. 69:576-580.
2. Chen,J. H., and H. Hanafusa. 1974. Detection of a protein of avian leukoviruses in uninfected chick cells byradioimmunoassay.J. Virol.13:340-346.
3. Chen,J.H.,W.S.Hayward,andH. Hanafusa.1974. Avian tumor virus proteins and RNA in uninfected
chickenembryo cells. J. Virol. 14:1419-1429. 4. Cooper, G. 1978. Markerrescueofendogenouscellular
genetic information relatedtothe avian leukosis virus genecoding RNA-directed DNApolymerase. J. Virol.
25:788-796.
5. Cooper, G. M., and S. G. Castellot. 1977. Assay of noninfectiousfragments of DNA of avian leukosis virus-infected cellsby markerrescue.J.Virol. 22:300-307. 6.Crittenden, L. B.,J. V.Motta,and E. J. Smith.1977.
Genetic control of RAV-0productioninchickens.
Vi-rology76:90-97.
7. Dougherty,R.M., and H. S.DiStefano.1966.Lack of relationship between infection and avian leukosis virus and the presence of COFALantigen in chickembryos.
Virology29:586-595.
8. Eisenman, R., R.Shaikh, and W. S. Mason. 1978. Identification ofanavian oncoviruspolyproteinin un-infectedchickcells. Cell14:89-104.
9.Friis, R.R., W. S. Mason, V. C. Chen, and M. S.
Halpern.1975.Areplicationdefectivemutantof Rous sarcomavirus which failstomakeafunctionalreverse
transcriptase.Virology64:49-62.
10.Hanafusa, H.,T.Aoki,S.Kawai,T.Miyamoto,and R.E.Wilsnack.1973.Presence ofantigencommon to avian tumor viral envelope antigen in normal chick
embryocells.Virology56:22-32.
11.Hanafusa, H.,and T.Hanafusa.1968.Further studies on RSV productionfrom transformed cells. Virology 34:630-636.
12.Hanafusa, H., and T. Hanafusa. 1971. Noninfectious RSV deficientin DNA polymerase. Virology 43:313-316.
13.Hanafusa, H., W. S. Hayward, J. H.Chen, and T. Hanafusa.1974.Controlofexpressionof tumor virus genesinuninfected chicken cells. ColdSpringHarbor Symp.Quant. Biol.39:1139-1144.
14.Hanafusa,H.,T.Miyamoto,andT.Hanafusa.1970.A cellassociated factor essential for formationofan infec-VOL. 29, 1979
on November 10, 2019 by guest
http://jvi.asm.org/
SAWYER, HANAFUSA
tiousform of Rous sarcoma virus. Proc. Natl. Acad. Sci. U.S.A. 68:314-321.
15. Hanafusa, T.,H.Hanafusa,and T.Miyamoto. 1970. Recovery of a new virus fromapparently normal chick cells by infection with avian tumor viruses. Proc. Natl. Acad.Sci. U.S.A. 67:1797-1803.
16. Hanafusa, T., H. Hanafusa, T. Miyamoto, and E. Fleissner. 1972. Existence and expression of tumor virusgenes in chick embryocells. Virology 47:475-482. 17. Hanafusa, T.,T.Miyamoto,and H. Hanafusa. 1970. A type ofchick embryo cell that fails to supportfonnation
of infectious RSV.Virology 40:55-64.
18. Hayward,W.S.1977.Sizeandgeneticcontentofviral RNAs in avianoncornavirus-infected cells. J. Virol. 24: 47-63.
19. Hayward,W.S., andH. Hanafusa. 1973. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J. Virol. 11:157-167.
20.Hayward,W.S., andH.Hanafusa. 1975. Recombina-tionbetweenendogenousand exogenous RNA tumor virusgenesasanalyzed by nucleic acidhybridization.J. Virol.15:1367-1377.
21. Hunter, E.,andP. K.Vogt. 1976. Temperature sensitive mutantsof avian sarcoma viruses:genetic recombina-tion with wild typesarcoma virus and physiological
analysis ofmultiplemutants.Virology69:23-34. 22. Kang, C.Y.,andH.M.Temin.1972.Endogenous
RNA-directed DNA polymerase activity in uninfected chicken embryos.Proc.Natl. Acad. Sci.U.S.A. 69:1550-1554.
23. Kang,C.Y., and H. M. Temin.1973.Lack of sequence
homologyamongRNAs of avianleukosis-sarcoma vi-ruses,reticuloendotheliosis viruses, and chicken endog-enousRNA-directedDNApolymeraseacitivity. J. Vi-rol. 12:1314-1324.
24. Kawai, S., and H. Hanafusa. 1972. Plaque assayfor somestrains of avian leukosis virus.Virology 48:126-135.
25. Kawai, S.,and H.Hanafusa.1973.Isolation of defective mutant of aviansarcomavirus.Proc.Natl. Acad. Sci. U.S.A. 70:3493-3497.
26. Linial, M., andW.S.Mason. 1973. Characterization of twoconditionalearlymutantsofRous sarcoma virus. Virology 53:258-273.
27. Linial, M.,and P. E.Neiman. 1976.Infection of chick cellsbysubgroupE viruses.Virology73:508-520. 28. Mason,W.S.,R.Friis,M.Linial,and P. K.Vogt.1974.
Determination of the defective function in two mutants of Roussarcomavirus.Virology61:559-574.
29. Mizutani, S.,andH. M. Temin.1973.Lack ofserological relationship among DNApolymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken cells. J.Virol. 12:440-448.
30. Neiman,P. 1973.Measurement ofendogenousleukosis virusnucleotide sequences in the DNA of normal avian embryosbyRNA-DNAhybridization.Virology 53:196-204.
31. Panet, A.,D.Baltimore, and T. Hanafusa. 1975. Quan-titationofavian RNA tumor virusreversetranscriptase byradioimmunoassay. J. Virol. 16:146-152.
32. Payne,L. N., and R. C. Chubb. 1968.Studieson the nature and geneticcontrol of an antigen innormalchick embryos which reacts in theCOFALtest.J.Gen. Virol. 3:379-391.
33. Rettenmier,C., and H.Hanafusa.1977.Structural pro-tein markers in the avian oncoviruses. J. Virol. 24:850-864.
34. Robinson, H. L. 1976. Intracellular restriction on the growth ofinducedsubgroup E avian type C viruses in chicken cells. J. Virol. 18:856-866.
35. Robinson, W. S., and H. L Robinson. 1971. DNA polymerase in defective Roussarcomavirus.Virology
44:456-465.
36. Rosenthal,P.N.,H. LRobinson,W.S.Robinson,T.
Hanafusa, and H. Hanafusa. 1971. DNA in
unin-fectedand virus-infected cellscomplementarytoavian tumor virus RNA. Proc. Natl. Acad. Sci. U.S.A. 68:
2336-2340.
37. Sawyer, R., and H.Hanafusa.1977.Formation of
retic-uloendotheliosis virus pseudotypes of Rous sarcoma virus. J. Virol.22:634-639.
38. Shoyab, M.,and M. A. Baluda.1975.Homologybetween avianoncornavirusRNAs and DNA fromseveral avian species. J.Virol. 16:1492-1502.
39. Smith,E.J., J. R.Stephenson,L. B.Crittenden,and S. A.Aaronson. 1976. Avian leukosis-sarcoma virus geneexpression.Noncoordinate control of group-spe-cificantigens invirus-negative avian cells. Virology 70: 493-501.
40. Varmus,H.E.,R. A.Weiss,R. R.Friis,W.Levinson, and J. M. Bishop. 1972. Detection of avian tumor virus-specificnucleotide sequences in avian cell DNAs. Proc. Natl. Acad. Sci. U.S.A. 69:20-24.
41.Vogt, P.K., and R. R. Friis. 1971. An avian leukosis virusrelated to RSV(0): properties and evidence for helper activity. Virology 43:223-234.
42. Vogt,V.M.,R.Eisenman,and H.Diggelmann. 1975. Generation of avianmyeloblastosisvirusstructural pro-teins byproteolyticcleavage of a precursor polypeptide. J.Mol. Biol. 96:471-493.
43. Wang,S.Y.,W.S.Hayward,and H.Hanafusa. 1977. Genetic variation in the RNA transcripts ofendogenous viral genes in uninfected chicken cells. J. Virol. 24:64-73.
44. Weiss,R. A.1969.The host range ofBryan strain Rous sarcoma virus synthesized in the absence of helper virus.J. Gen. Virol. 5:511-528.
45.Weiss, R.A., W. S. Mason, and P. K. Vogt. 1973. Genetic recombination and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology 52:535-552.
46. Weissbach,A., A. Bolden, R. Muller, H. Hanafusa, and T.Hanafusa. 1972. Deoxyribonucleic acid polym-erase activities in normal and leukovirus-infected chickenembryocells. J. Virol. 10:321-327.
47.Wyke, J. A., J. G. Bell, and J. A. Beamand. 1974. Genetic recombination among temperature sensitive mutantsof Roussarcoma virus. ColdSpring Harbor Symp. Quant. Biol. 39:897.