Copyright )1976 American Society for Microbiology Printed inU.S.A.
In Vitro
Synthesis of Turnip Yellow
Mosaic Virus Coat
Protein in
a
Wheat Germ Cell-Free System
C. BENICOURT AND A. L. HAENNI*
Laboratoire de Biochimie duDeveloppement,Institut de BiologieMoleculairedu Centre National de la RechercheScientifique, Universite Paris VII, 75005 Paris, France
Received for publication19April 1976
Turnip Yellow
Mosaic Virus RNA directs the synthesis in vitro ofits coatprotein in
awheat
germcell-free
extract.Optimum
conditions
forsynthesis
havebeen
defined, and the effect of
spermine onspecifically enhancing
coat proteinformation
has been
examined. Identity between the
in vitrosynthesized
coatprotein and authentic
coat proteinof Turnip Yellow
Mosaic Virus wasestab-lished by
analysis
onsodium
dodecyl
sulfate-polyacrylamide gel electrophoresis,
peptide mapping, and immunoprecipitation.
Since the
initial reports
onthe use of a wheat
germ
extractfor
the translation of
mRNA's,
several
plant viral RNAs have been used
suc-cessfully
inthis system
and have directed the
synthesis
ofviral
proteins found
inthe virion
(6, 7, 12, 15, 20, 23, 26-28, 30, 31).
Tumip
Yellow Mosaic Virus (TYMV) RNA (2
x 106 daltons) is large enough to code for
sev-eral
proteins of
averagemolecular weights, and
one
of these
proteins would be expected to be
the viral coat protein (20,000 daltons).
How-ever,
this RNA introduced into various in vitro
protein-synthesizing
systems derived from
pro-caryotes or eupro-caryotes has never been reported
to
lead to the synthesis of viral coat protein (24,
32).
In
this paper, we have used viral RNA in a
wheat
germ extract
and present evidence for
the
invitro
synthesis of TYMV
coatprotein.MATERIALS AND METHODS
Isolation of TYMV RNA and coat protein.
TYMV-infectedChinesecabbageleaveswerekindly
provided by S. Astier-Manifacier and P. Cornuet,
and the viruswas
purified according
to Leberman(17)by using polyethylene glycol and sodium
dex-transulfate.The RNAwasextractedfrom the virus
withwater-saturatedphenolasdescribedby Gierer and Schramm (10) andwasstoredat-80°C.TYMV coatproteinwasisolated from the virionby
precipi-tationof the RNAinthe presence of aceticacid, as indicated by Fraenkel-Conrat (8).
Chemicals. L-['4C]leucine(389mCi/mmol) and
L-[35S]methionine (260 to 500 Ci/mmol) were from
Amersham-Searle. TPCK-trypsin was from
Wor-thington Biochemicals Corp., T, RNase was from
Sankyo, and spermine tetrachloridewasfromSigma
Chemical Co.
Preparation of wheat germ extracts. Commercial wheat germwasagiftof B. Roberts and T. Hall. The extract was prepared essentially as described by
Daviesand Kaesberg (5), withafewmodifications
(T. Hall, personal communication). Two grams of
wheatgermwasground with broken sterilePasteur
pipettesfor1to2min; 8mlofextractionbuffer (100
mM imidazole, 90 mM potassium acetate, 2 mM
calcium acetate, 1 mM magnesium acetate, 1 mM
p8-mercaptoethanol,
and 5 mMdithioerythritol),
broughttopH 7.3with aceticacid,wasadded, and
gentlegrindingwascontinuedfor30 s.The mixture
wascentrifugedfor 10 minat17,500rpm inaSpinco
ultracentrifuge (rotorno. 40). The clearyellow
su-pernatant appearing between the pellet and the
lipid layer was centrifuged again under the same
conditionsafteradjustingto2 mMmagnesium
ace-tate. Theresultingsupernatantwasdialyzed
exten-sively againstasolutioncontaining 10 mMHEPES
(N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic
acid), pH7.5, 90 mMpotassiumacetate, 1mM
mag-nesium acetate, and 1 mM dithioerythritol and
storedat-80°Cinsmall
samples.
Itcontainedabout50mgofproteinsand 4.5 mgof nucleic acidsperml.
Invitroproteinsynthesis.The incubations for in vitro proteinsynthesiswereperformedfor1 h
(un-less otherwise stated)at30°C inconditions similar
tothose of DaviesandKaesberg (5)andcontained20
mMHEPES, pH7.5,2 mMATP,0.2mMGTP,8mM
phosphoenolpyruvate, 20,uMeach of19aminoacids,
the 20th amino acid being either [14C]leucine or
[35S]methionine, respectively,
at 12.5 ,uMor0.5 to1.7
,AM.
Theconditionswereoptimized for Mg2+, K+, and TYMV RNA. A15-,ul
portion of wheat germ extract wasaddedtostartthe reaction(total incuba-tionvolume,50,ul). To controlthe amino acid incor-poration, samples were removed and spotted on Whatman 3MM diskspreviously soakedin10% tri-chloroacetic acid. The diskswereboiledfor10minin 5% trichloroacetic acid and washed successively withethanol,ethanol-ether, and ether. The radioac-tivity retained on the disks was determined with toluene-based scintillator.SDS-polyacrylamide gel electrophoresis of
pro-teins. Toanalyze theinvitrosynthesizedproducts
on sodium dodecyl sulfate (SDS)-polyacrylamide
gels,proteinsynthesiswasallowedtoproceed,after
196
on November 10, 2019 by guest
http://jvi.asm.org/
SYNTHESIS OF TYMV COAT PROTEIN 197
which10mM EDTA and 2 U ofT1RNase per 5 ,ug of
TYMV RNA were added, and incubation followed
for 20 min at 30°C. The reaction was stopped by boiling the
50-Al
samples for 10minwith 25 ,ul of sample buffer (0.19 M Tris-hydrochloride, pH 6.8,30%glycerol, 15%f3-mercaptoethanol, 6% SDS, and
0.03%bromophenolblue). A portion of the samples was layered over 0.1% SDS-15% acrylamide gels
prepared and run according to Laemmli (16). The
gelswerethenstainedwith a Coomassie blue solu-tion (2 g of Coomassie blue, 500 ml of methanol, 100 ml of acetic acid, 500 ml of water) for 20 min and
destained using amethanol-acetic acid-water
solu-tion (30:7.5:62.5, vol/vol/vol). Finally, the gels were
driedandautoradiographedonKodirexX-rayfilms.
Forthe purpose of further characterization, the main radioactive band corresponding to protein syn-thesized in vitro and comigrating on
SDS-polyacryl-amidegels with authentic coat protein purified from
the virion was isolated. To this end, the gel was dried without previous staining and autoradio-graphed. The radioactive band presumably
corre-spondingtolabeledcoatprotein was eluted at
370C
for24h in 0.1% SDS. TYMV coat protein wasthen
added as carrier, and SDS was eliminated by
re-peated precipitation with 20% cold trichloroacetic
acid andsolubilizationwith 0.2 N NaOH. The final
trichloroaceticacid pellet was washed with
ethanol-ether, followed by ether.
Tryptic peptide analysis. Performic acid
oxida-tionand tryptic digestion were carried out essentially
as described by Crawford and Gesteland (4).
['4C]leucine-
(notshown) or[35S]methionine-labeledproteins comigrating with authentic coat protein
and isolated as described above were dried after
removal of trichloroacetic acid. The pellets were
dissolvedinperformic acid to a final concentration
of 1 mg of protein per ml and incubated for 1 h at
0°C. The proteins werelyophilized and redissolved
to 1 mg/mlin 50 mM ammonium bicarbonate, pH
8.6. Theywereincubated for 4 h at 37°C with 1/100
(by weight) of TPCK (tolylsulfonyl phenylalanyl
chloromethyl ketone)-trypsinadded at time zero and
the same amountof enzyme added after 1 h. The
productswerethen lyophilizedandsuspended in 100
,ul of electrophoresis buffer.
The two-dimensional peptide analyses were
car-ried out on Whatman 3MM
according
to Kerr and Martin (14). The first dimension consisted ofelectrophoresisin apyridine-aceticacidbuffer
(pyri-dine-acetic acid-water, 25:1:474, pH 6.5) at 4,000 V
for30min.The second dimension consisted of
chro-matography inn-butanol-pyridine-acetic
acid-wa-ter (90:60:18:72). After stainingwith a
ninhydrin-cadmiumacetate solution(2),the radioactive spots
were locatedbyautoradiography, using Kodirex X-rayfilms.
Immunodiffusion experiment. Immunodiffusion testswereperformedin 0.8%agaroseplates contain-ing 30mMveronal, pH 8.3, and 0.02% sodium azide.
The [35S]methionine-labeled material comigrating
with authentic coat protein and isolated from the
polyacrylamidegels,asdescribedabove,was
redis-solvedin150mMNaCl and loaded into the central well. Twoexternal wells wereloaded,respectively,
with anti-TYMV rabbitserumkindly provided byJ. M. Bove and normal rabbit serum. After
immuno-precipitation, the plates were washed for several
days in 10 mMTris-hydrochloride, pH 7.4, 150 mM NaCl. They weredried, stained for 20 min withan amido black solution (1 g of amido black, 0.42 M aceticacid, 0.42 M sodium acetate, 15%glycerol) and destained with methanol-acetic acid-water. The agarosepellicle wasautoradiographedon aKodirex X-ray film.
RESULTS
Optimal
conditions for
protein
synthesis.
The
optimum
Mg2+
and K+
concentrations for
protein synthesis determined with TYMV RNA
asmessenger are
3.3
and 140
mM,
respectively
(1).
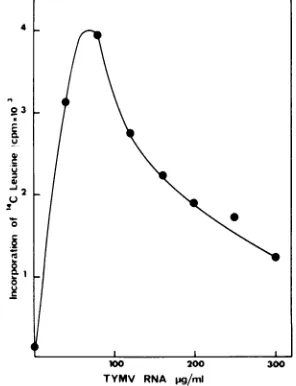
The effect of
TYMV RNA concentration
onprotein
synthesis showed
that the level of
incor-poration
increased withincreasing
RNAcon-centrations
upto80
to100,ug/ml,
beyond which
protein synthesis
wasinhibited
(Fig. 1). This
might reflect interaction
ofMg2+
withthe
mRNA, which would result in
adecrease
offree
Mg2+ in
the incubationmixture.
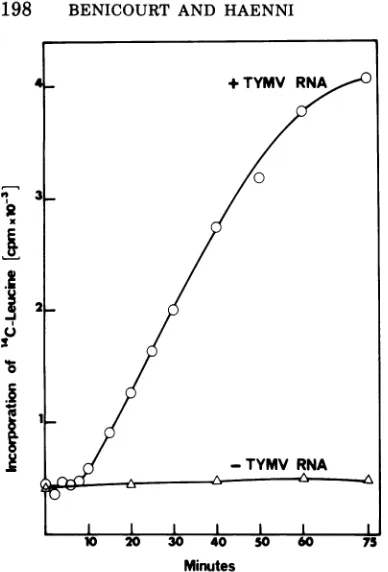
Kinetic experiments indicated that in the
presence
of TYMV
RNA incorporation of
[I4C]leucine
into
proteins
was linear for about60 min
at300C (Fig. 2); there
wasvirtually
noincorporation in the absence of added mRNA
(see also Fig. 3). The products synthesized at
different times
wereanalyzed by
SDS-poly-acrylamide gel
electrophoresis
andautoradiog-raphy:
the
results showed that the
profile
of the
4
53
0
.00 200 300
TYMV RNA pg/mi
FIG. 1. Dependence on TYMV RNA
concentra-tion.The reactionswereperformedwithoptimal con-centrationsof Mg2+(3.3mM)and K+(140 mM)and variousTYMV RNA concentrations.Samples (2
pl)
wereremoved, spottedonWhatman 3MM
disks,
andcounted.
20,
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.508.280.428.418.611.2]FiI
react
TYAI
vario.nan
in vi
betv,
resu
prec
toyi at tk
SI
yses gels
ing 20%
(Fig
stror
mole
cons cal s Tr TYI cule
argii
dues thior two
mole yielc
bele(
to stained
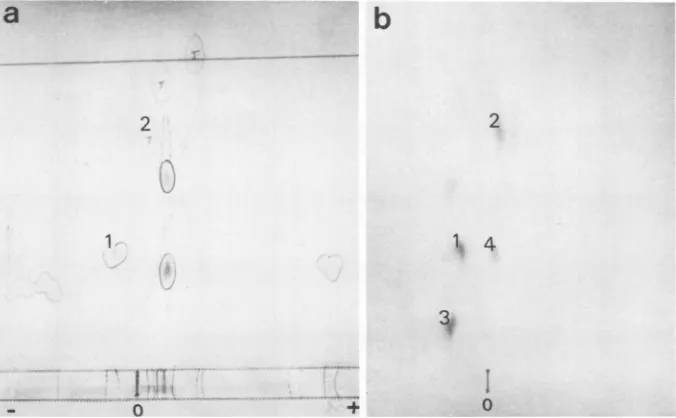
peptides.
Four main radioactivespots
reproducibly
appear, twoof which
corre-Z_
+ TYMV
RNA
spond to ninhydrin spots (spots 1
and 2) (Fig.
4);
one
of
the
twoother
spots(spot 3
or4)
presum-ably
corresponds
tothe
NH2
terminal peptide,
the other
spotcorresponding
tothe incomplete
hydrolysis of
alysyl-glutamic
bond which
is
3_ / trypsinresistant (R. Peter,
Ph.D.
thesis, Univ.
of
Strasbourg, Strasbourg, France, 1972) and
located
near theNH2
terminus: itleads
to adodecapeptide
thatdoes
not reactwith
ninhy-drin.
When,
instead
of[35S]methionine,
[14C]leu-2-_
f5cine
isused, the
in vitrosynthesized protein
comigrating
with TYMV
coatproteinalso gives
rise to
peptides
thatcorrespond
topeptides
derived from authentic
coatprotein (notshown
here).
_ /
Immunodiffusion
experiment. The TYMV
RNA-directed
protein
synthesized
invitro
and
TYMV RNA
comigrating
with
authentic
coatprotein
wasalso characterized by immunoprecipitation.
The results
areshown
inFig.
5.With
anti-TYMV
rabbit
serum aspecific
precipitation
oc-1
20 30 40 so 60 75curs with both authentic coat protein and
withMinutes
the corresponding in vitro synthesized protein,
2. Kinetics ofin vitroproteinsynthesis. The as ascertained
by
the coincidence ofstaining
G.
*T 7 andautoradiography.
tion was
performed
inoptimized conditions forfVRNA (where required), Mg2+, and K+. At From these results and from the tryptic
pep-)us times,
2-pl
samples were applied to What-tide analysis,
weconclude that the product
syn-3MM disks and counted.
thesized in vitro is the TYMV coat
protein.
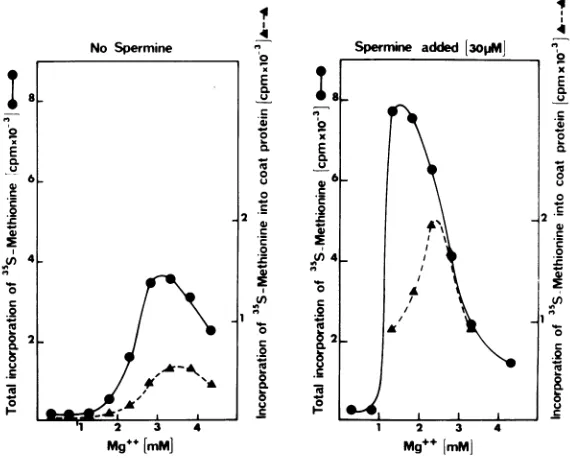
Effect of
spermine
onin
vitro
translation.
Spermine
has
been
reported
tohave
astimula-itro
synthesized polypeptides did
not vary tory effect on translation in thewheat
germveen 2
and
75 min(data
notshown). These
cell-free
systemand
has
therefore been
used
for
ilts speak against the synthesis of
alarge
the
translation of several mRNA's
(19, 22,30).
ursor protein
that
would undergo cleavage Sincepolyamines can replace Mg2+ and interactield
the
mature coat protein,but
we cannot with RNAmolecules
(9, 29), wechecked the
uis
stagerule
outsuch
apossibility.
optimal Mg2+ concentration for polymerization)S-polyacrylamide
gel analysis. The anal-
inthe absence
orin the presenceof
spermine.of the
autoradiographs
ofpolyacrylamide
At
30,uM,
spermine stimulates total
protein
show that
synthesis of
aprotein comigrat-
synthesis twofold and the optimal
Mg2+
concen-with TYMV
coatprotein
represents 15 to tration islowered
from
3.3 to1.5mM
(Fig.
6).of the
total
invitro
synthesized products
To
define whether
or notthe
stimulation
by
3).
Among the
manyweak
bands,
three spermine wasspecific,
wecompared the
incor-nger ones,
corresponding
topolypeptides of porationof
[35S]methionine
intototal protein
hcular
weights of
45,000,25,000,and
13,000, and into coat protein in the presence orabsence
tantly appeared; their
possible physiologi- of spermine at various Mg2+concentrations.
To,ignificance will be discussed
later. this effect, the synthesized products wereana-'yptic
peptide analysis. The sequence of lyzed bySDS-polyacrylamide
gelelectrophore-4V coat protein is
known
(21).This
mole- sis asindicated
inMaterials
andMethods.
Incontains 189 amino
acids,
ofwhich
10 are eachcase,
theradioactive
bandcorresponding
nyl
plus lysyl
and four are methionyl resi- to TYMV coatprotein was cut out and itsradio-Since the
molecule carries an acetylme- activitywas compared to the total radioactivitynyl residue
atits NH2 terminus, and since found forthat sample. The results are shown inof
the other
methionyl groups within the Fig. 6. In the absence of spermine, the Mg2+-cule
areadjacent,
tryptic digestion should concentration yielding maximum synthesis ofI ten
ninhydrin-stained
and three 35S-la- coat protein also yields maximumincorpora-d
peptides, of
which two should correspond tion ofmethionine into total protein, whereasIs
4
a
I
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.508.64.258.61.347.2]SYNTHESIS OF TYMV COAT PROTEIN 199
a
1 2 3 4
000
-ci>
000
000
--
d000
-n
23.500
--->
1
1.700
-c0
FIG. 3. Analysis by SDS-polyacrylamide gel electrophoresis of in vitro synthesized products. Protein
synthesiswasperformedatoptimal concentrations of Mg2+ and K+ using[35S]methionineaslabeled amino
acid.Eachslotwasloadedwith30 lofsample containing10
pi
ofsamplebuffer and, from lefttoright:(1) 20pg eachof the following protein markers: phosphorylaseA(molwt, 94,000),bovineserumalbumin (molwt,
68,000), ovalbumin (mol wt, 43,000), reduced gamma globulin (mol wt, 50,000 and 23,000), and cytochrome c (mol wt, 11,700); (2) 20
pl
ofawheatgermcell-freeincubation mixture performed intheabsence ofTYMVRNA (10,000 cpm); (3) 20
pg
ofTYMV coat protein (mol wt, 20,000); (4) 20 piof a wheatgermcell-freeincubation mixture carried out in the presence of TYMV RNA at0.1mg/ml(825,000cpm). Aftermigration,
the gelwas (a)stainedwithaCoomassiebluesolution,dried, and (b)autoradiographed.
a
2
1
0 0
FIG. 4. Fingerprint analysis ofTYMV coatprotein added to in vitro synthesized material comigrating withcoatproteinanddigested by trypsin.Electrophoresisandchromatographywereperformedasdescribed
inMaterials and Methods.Asample (200 p)containing700pgofTYMVcoatproteinand110,000cpmof [35S]methionine-labeled material comigrating with coat protein was spotted on Whatman 3MM after
performicacidtreatmentandtrypsinhydrolysis. Thepeptides were(a)stained withaninhydrin-cadmium
acetatemixture and(b)autoradiographed.
in the presence of spermine the optimal Mg2+
concentrations for coat protein and for total
protein synthesis are,respectively, 2.5 and 1.3
mM. Moreover, in the absence of spermine
about 15% of the methionine is incorporated
intocoatproteinovertherangeofMg2+
concen-trations tested. Whenspermineis added to the
incubationmixture,onedistinguishestwo
situ-M.W.
94
68
50
43
b
2 4b
2
1 4
VOL.
20,
1976I
f-i
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.508.56.451.74.258.2] [image:4.508.83.421.365.574.2]a
b
anti TYMAV
cow,-- *
prot e in
[image:5.508.70.463.74.276.2]norma serum
FIG. 5. Immunodiffusiontest. The reactionwas
performed
asdescribed in Materials and Methods. Thewells wereloaded with200 pgofTYMVcoatproteinand 70,000cpm
of
[35S]methionine-labeled
product
comigratingwithauthentic coat protein onSDS-polyacrylamide gel and isolated from the gel (see Materials and Methods), or 30
pi
ofanti-TYMVrabbit serum (63mg/ml),or 30PIu
of normal rabbit serum (58mg/ml).(a)Stained with amido black; (b) autoradiographed.
m
0
E
C
.2
0
C
.2
EU
0
0.2
0
._
-W
4w
No Spermine 4x0
E
0._ a U
-C
0 0.
a m 0
U 0 C C
.0
C~ 0 0. 0
1CL
Mg++[mM]
I 4
0 x
E
a
._0
-r w
EU
U
4._0
CL
Mg++[mM]
FIG. 6. Effect of spermineoncoatproteinsynthesis. Reactionswerecarriedout atoptimum K+ and TYMV RNA concentrations, varyingMg2+ concentrations, and in the absenceorpresenceof30
MM
spermine. The samples were treated as describedin Materialsand Methods,and25pl
ofeach was layeredon an SDS-polyacrylamide gel. After electrophoresis, thegel wasstained, dried,andautoradiographed to detectcoatprotein.It wasthen sliced, and the sliceswereincubatedat roomtemperaturefor24hinthe presenceof1 mlof
Soluene350(Packard)and10mlofTriton-Fluorpriortocounting. Theamountofradioactivityinthe slices containingthe in vitrosynthesizedcoatproteinwascomparedtothe totalradioactivity.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.508.125.410.354.583.2]SYNTHESIS OF TYMV COAT PROTEIN 201
ations: at 1.3 mM Mg2+ 15%
of the methionine
gives rise to coat protein, whereas at 2.5 mM
more than 30% of the methionine is
incorpo-rated into coat protein. This could mean that
spermine has an overall stimulatory effect on
total protein synthesis in optimized
Mg2+
conditions but has a
specific
stimulatory effect
on
coat protein synthesis at 2.5 mM
Mg2+.
DISCUSSION
Translation of TYMV RNA in a wheat germ
cell-free
system gives rise to coat protein
syn-thesis,
as
demonstrated by gel electrophoresis,
tryptic peptide analysis, and
immunoprecipita-tion.
The specific effect of spermine on TYMV coat
protein
synthesis cannot be explained entirely
on
the
basis of different
Mg2+
requirements for
the translation of various cistrons contained in
the genome. In another system (T4 mRNA and
an
Escherichia coli cell-free system) it has been
reported that the optimum
Mg2+
concentration
for a specific protein does not follow that for the
synthesis
of total protein (25). Further, it is
noteworthy that the optimum ionic
concentra-tions
for protein synthesis depend on the
mRNA
used (31), probably reflecting
differ-ences in
the structures of these
mRNA's.
Apart
from coat protein, three other main
polypeptides
are
synthesized with
TYMV RNA
(Fig.
4).
They appear not to be precursors or
fragments
of TYMV coat protein because their
ratios
remain constant throughout incubation
and
even
after protein synthesis has reached
the
plateau (not presented here).
Experiments are in progress to define the
nature
and possible biological activity of these
proteins. One of them could correspond to a
component
of the
replicating system of the
vi-rus.
The
Q,8
replicase is composed of one
phage-coded
subunit and three host
cell-coded
sub-units:
EF-Tu,
EF-Ts, and the ribosomal protein
Si
(3, 13). If
the
TYMV replicase
shows similar
properties, interesting experiments could be
de-signed
to
try to
elucidate
the amino acid
recep-torfunction
of the 3' terminal part of TYMV
and of other
plant viral
RNA genomes in
con-nection
with the observation that they form
complexes
with
the elongation factors and GTP
(11, 18).
It is known
(C. W. A.
Pleij,
Ph.D.
thesis,
State Univ. of
Leiden, Leiden, The
Nether-lands, 1973) that TYMV RNA when isolated
from the virion contains, besides
molecular
spe-cies
of
21to23S
thought to represent the intact
genome,
large amounts (up to 50%) of shorter
RNA
molecules
(between 8 and 16S), which can
be
separated
from the 23S molecules after a
heat
treatment
and have
been
considered
to
be
degradation products of the 23S molecules.
Throughout
this
work,
unfractionated
TYMV
RNA was used for incubations, and
con-sequently the
experimental results
do
notshow
whether
the coat
protein
formed
is
atranslation
product of the intact RNA molecule.
Very
re-cently, experiments
by
Pleij
etal.
(submitted
for
publication),
likewise
using
the wheat germ
translation system,
have
demonstrated
that the
isolated 23S RNA
molecules do
notdirect the
synthesis of the
coatprotein,
but that the
sedi-mentation constant
of
the molecules that
aretranslated
into
coatprotein
is of the order of
8S
(250,000
daltons).
In
agreement
with the
results
reported here,
these
authors have also observed
that
unfractionated
TYMV
RNA leads
tothe
synthesis of
coat
protein.
It remains to
be
determined whether these 85
RNA
molecules
aredegradation
products
is-sued
from the
processing
of the viral
RNA,
orwhether
they represent
preferential replication
products
that
would
be
encapsidated
during
vir-ion
formation.
ACKNOWLEDGMENTS
We are indebtedtoF. Chapeville, inwhoselaboratory this workwascarriedout,for hisenthusiasticinterestand support, andtoL. Bosch forallowingustorefertowork performedinhislaboratory(C.W. A.Pleijetal.) priortoits publication.WeareverygratefultoB.E.Robertsand R. C. Mulligan, whoinformedusofexperimentstheyhad per-formed withTYMVRNAasmRNA, andtoA. Delfourfor hisadvice concerning thepeptideanalyses. WethankM. Garafoli forpurifying theTYMV, andG. Beaud;A. Pro-chiantz, and S. Teixeira for useful discussions.
This workwassupported bygrantsfrom NATO(no. 769) and from theATPDiff6renciation Cellulaire (no. 1.394),
Centre National de la Recherche Scientifique.
LITERATURE CITED
1. Benicourt, C.,and A. L. Haenni. 1975. Translation of TYMV RNA in awheatgerm cell-freesystem, p. 189-196. In A. L. Haenni and G. Beaud (ed.),In vitro transcription and translation of viralgenomes, vol. 47.INSERM, Paris.
2. Blackburn,S.1965.Thedetermination ofaminoacids by high-voltagepaperelectrophoresis,p. 1-45. In D. Glick(ed.), Methods ofbiochemical analysis,vol.13. IntersciencePublishers,NewYork.
3. Blumenthal, T., T. A. Landers, and K.Weber. 1972. BacteriophageQ,8replicasecontainstheprotein bio-synthesis elongationfactorsEFTuandEFTs. Proc. Natl. Acad. Sci.U.S.A. 69:1313-1317.
4. Crawford,L.V.,and R. F.Gesteland.1973.Synthesis ofpolyoma proteininvitro. J.Mol. Biol. 74:627-634. 5. Davies,J. W., and P.Kaesberg. 1973. Translation of
virusmRNA:synthesisofbacteriophageQ/3proteins in a cell-free extract from wheatembryo.J. Virol. 12:1434-1441.
6. Davies, J. W., and P. Kaeberg. 1974. Translation of virusmRNA: protein synthesis directedby several virusRNAsinacell-freeextractfrom wheat germ. J. Gen.Virol. 25:11-20.
7. Efron, D., and A. Marcus. 1973.Translation of
TMV-VOL. 20, 1976
on November 10, 2019 by guest
http://jvi.asm.org/
202
RNA in a cell-freewheatembryo system. Virology 53:343-348.
8. Fraenkel-Conrat, H. 1957. Degradation of TMV with aceticacid. Virology 4:1-4.
9. Fukuma, I., and S. S. Cohen. 1975. Polyamines in bacteriophage R17anditsRNA. J. Virol. 16:222-227. 10. Gierer,A.,and G. Schramm.1956.Infectivityof
ribonu-cleic acid fromTMV. Nature(London)177:702-703. 11. Haenni,A.L., A.Prochiantz, and P. Yot. 1974. tRNA
structures inviralgenomes, p. 264-276. In D.Richter (ed.),Lipmannsymposium:energy,biosynthesis and regulationinmolecular biology. deGruyter and Co., Berlin.
12. Hunter, T. R., T.Hunt,J. Knowland, and D.Zimmern. 1976. MessengerRNA forthe coat proteinof tobacco mosaic virus.Nature(London)260:759-764. 13. Kamen, R., M. Kondo, W. Romer, and C. Weissmann.
1972. Reconstitutionof Q,B replicase lacking subunit awithproteinsynthesis-interferencefactor i. Eur. J. Biochem.31:44-51.
14. Kerr, I. M., and E. M. Martin. 1971. Virus protein synthesisinanimal cell-freesystems: nature of the productssynthesizedinresponse to RNA of encepha-lomyocarditisvirus. J.Virol. 7:438-447.
15. Klein,W.H., C. Nolan, J. M. Lazar, and J. M. Clark. 1972.Translation ofSatellite Tobacco Necrosis Virus RNA.I. Characterization ofinvitro procaryoticand eucaryotic translation products. Biochemistry 11:2009-2014.
16. Laemmli, U. K. 1970. Cleavage ofstructural proteins during the assembly of thehead of bacteriophage T4. Nature(London)227:680-685.
17. Leberman, R. 1966. The isolation of plant viruses by means of"simple" coacervates. Virology 30:341-347. 18. Litvak, S., A. Tarrago, L. Tarrago-Litvak, and J. E. Allende. 1973. Elongation factor-viral genome inter-action dependent on the aminoacylation of TYMV and TMVRNAs. Nature(London) New Biol. 241:88-90.
19. Marcu,K., and B. Dudock. 1974. Characterization of a highlyefficientprotein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1:1385-1397.
20. Marcus, A. 1970. TMV RNA-dependent amino acid in-corporation in a wheat embryo system in vitro. J. Biol.Chem. 245:955-961.
21. Peter, R.,D.Stehelin,J.Reinbolt, D. Collot, and H. Duranton. 1972. Primary structure of TYMV coat
protein.Virology 49:615-617.
22. Roberts, B. E., M. Gorecki, R. C. Mulligan, K. J. Danna, S. Rozenblatt, and A. Rich. 1975. SV40 di-rectsthe synthesisof authenticviralpolypeptidesin a linked transcription-translation cell-free system. Proc. Natl.Acad. Sci. U.S.A. 72:1922-1926. 23. Roberts, B. E., and B. M. Paterson. 1973. Efficient
translation of TMV RNAand rabbit globin 9S RNAin acell-freesystemfromcommercialwheat germ. Proc. Natl.Acad. Sci. U.S.A. 702:2330-2334.
24. Rolleston, F. S.,I. G.Wool, andT. E. Martin. 1970. Binding and translation of Turnip-Yellow-Mosaic-Virusribonucleicacidby skeletal-muscleribosomes from normal and diabeticrats. Biochem.J. 117:899-905.
25. Schweiger, M., and L. M. Gold. 1969.BacteriophageT4 DNA-dependent in vitrosynthesisof lysozome.Proc. Natl. Acad. Sci.U.S.A.63:1351-1358.
26. Schwinghaner, M. W., and R. H.Symons.1975. Frac-tionation of CucumberMosaic Virus RNA andits translation in a wheat embryocell-freesystem. Virol-ogy 63:252-262.
27. Shih, D. A., and P. Kaesberg. 1973. Translation of Brome Mosaic Virus RNA in acell-free system de-rived from wheat embryo. Proc. Natl. Acad. Sci. U.S.A.70:1799-1803.
28. Shih,D.S., and P.Kaesberg.1976. Translation of the RNAsof theBrome Mosaic Virus:themonocistronic natureofRNA1 and RNA2.J.Mol. Biol. 104:77-88. 29. Takeda,Y., and T.Ohnishi. 1975.Binding of tRNAto
polyaminesinpreferencetoMg++.Biochem.Biophys. Res. Commun. 63:611-617.
30. Thang,M.N., L.Dondon,D.C.Thang,E.Mohier,L. Hirth, M. A.Le Meur,and P.Gerlinger.1975. Trans-lationof Alfalfa Mosaic VirusRNAsinplant cell and mammalian cell extracts, p. 225-232. In A. L. Haenni andG. Beaud(ed.),In vitrotranscriptionand transla-tionofviral genomes,vol.47.INSERM,Paris. 31. VanVlotenDoting, L.,T. Rutgers,L.Neeleman,and
L.Bosch. 1975. In vitro translation of the RNAs of AlfalfaMosaicVirus, p. 233-242. In A. L. Haenni and G.Beaud(ed.),In vitrotranscription andtranslation of viralgenomes,vol.47.INSERM,Paris.
32. Voorma,H.O., P. W.Gout,J.vanDuin,B. W. Hoo-gendam,and L.Bosch. 1965.Theread-out ofTurnip Yellow Mosaic Virus ribonucleic acid as a polycis-tronic messenger incell-freesystemsof Escherichia coli. Biochim. Biophys.Acta 95:446-460.