0022-538X/08/$08.00⫹0 doi:10.1128/JVI.01063-08
Copyright © 2008, American Society for Microbiology. All Rights Reserved.
Herpes Simplex Virus Type 1 ICP0 Phosphorylation Mutants Impair
the E3 Ubiquitin Ligase Activity of ICP0 in a Cell
Type-Dependent Manner
䌤
Chris Boutell,
1Roger Everett,
1Joshua Hilliard,
2Priscilla Schaffer,
3Anne Orr,
1and David Davido
2*
MRC Virology Unit, Institute of Virology, Church Street, Glasgow G11 5JR, Scotland, United Kingdom1; Department of
Molecular Biosciences, University of Kansas, Lawrence, Kansas 660452; and Department of Molecular and
Cellular Biology, University of Arizona, Tempe, Arizona 857213
Received 20 May 2008/Accepted 13 August 2008
Herpes simplex virus type 1 (HSV-1) infected cell protein 0 (ICP0) is a 110-kDa nuclear phosphoprotein that is required for both the efficient initiation of lytic infection and the reactivation of quiescent viral genomes from latency. The ability of ICP0 to act as a potent viral transactivator is mediated by its N-terminal zinc-binding RING finger domain. This domain confers E3 ubiquitin ligase activity to ICP0 and is required for the proteasome-dependent degradation of a number of cellular proteins during infection, including the major nuclear domain 10 (ND10) constituent protein promyelocytic leukemia. In previous work we mapped three phosphorylation regions within ICP0, two of which directly affected its transactivation capabilities in transient transfection assays (Davido et al., J. Virol. 79:1232–1243, 2005). Because ICP0 is a phosphoprotein, we initially sought to test the hypothesis that phosphorylation regulates the E3 ubiquitin ligase activity of ICP0. Although none of the mutations affected ICP0 E3 ligase activity in vitro, transient transfection analysis indicated that mutations within one or more of the phosphorylated regions impaired the ability of ICP0 to form foci with colocalizing conjugated ubiquitin and to disrupt ND10. Mutations within one of the regions also affected ICP0 stability, and all of these phenomena occurred in a cell type-dependent manner. In the context of viral infection, only one ICP0 phosphorylation mutant (P1) showed a significant defect in viral replication and enhanced protein stability compared to all the other viruses tested. This study suggests that specific cellular environ-ments and context of expression (transfection versus infection) differentially regulate several activities of ICP0 related to its E3 ubiquitin ligase activity via phosphorylation.
Herpes simplex virus (HSV) has two phases of its life cycle: lytic and latent infections (46). Lytic infection is characterized by the expression of all viral genes in a specific cascade (im-mediate-early, early, and late), leading to the production of infectious virus (34). During latent infection, viral gene expres-sion is limited primarily to the latency-associated transcripts, with no infectious virus produced (50). When latently infected neurons are stressed, neurons become permissive for HSV lytic gene expression, leading to the production of infectious virions and the recurrence of diseases, such as those that cause cold sores, herpes keratitis, and encephalitis. A major player in facilitating the switch between lytic and latent infections in the HSV life cycle is the immediate-early regulatory protein, in-fected cell protein 0 (ICP0).
ICP0 is a multifunctional, phosphorylated nuclear protein that acts as a transactivator of all three classes of HSV genes. This transactivating capability is required to induce efficient lytic gene expression and consequently efficient viral replica-tion (9, 11, 14, 20, 21, 24, 31, 45, 51). Moreover, ICP0 is also required for the efficient reactivation of quiescent viral ge-nomes from latency (8, 32, 33, 35, 51, 55). Taken together, these data indicate that ICP0 plays a critical role in both the initiation of lytic replication and the reactivation of latent viral
genomes. The activation of viral gene expression by ICP0 is closely associated with its capacity to disrupt nuclear domain 10 (ND10) structures (also known as protein promyelocytic leukemia [PML] nuclear bodies) (37, 38). These nuclear sub-structures are involved in modulating many cellular processes including proliferation, differentiation, and innate immunity (reviewed in references 25 and 42). The disruption of ND10 occurs through ICP0-directed degradation of PML (the tumor suppressor protein, which is required for ND10 assembly) and, either directly or indirectly, the isoforms of Sp100 that are modified by the small ubiquitin-like modifier (13, 26, 41). In order for ICP0 to target these and a number of other cellular proteins for proteasome-dependent degradation, it requires its zinc-binding RING finger domain, a motif that confers E3 ubiquitin ligase activity to ICP0 (7). Ubiquitin ligases provide the substrate specificity required to mediate the transfer of ubiquitin from their respective ubiquitin-conjugating enzymes onto the substrate proteins targeted for modification (for a review, see reference 30). The ability of ICP0 to target specific cellular proteins for ubiquitination and proteasome-dependent degradation has given rise to the hypothesis that ICP0 coun-teracts the cellular repression mechanism(s) that either ini-tiates or maintains viral genomes in a state of transcriptional quiescence.
As a number of E3 ubiquitin ligases have been shown to be regulated by phosphorylation, we wanted initially to determine the role ICP0 phosphorylation plays in its function. Specifi-cally, we investigated whether phosphorylation affected its E3
* Corresponding author. Mailing address: University of Kansas, 1200 Sunnyside Avenue, 7047 Haworth Hall, Lawrence, KS 66045. Phone: (785) 864-4022. Fax: (785) 864-5294. E-mail: ddavido@ku.edu.
䌤Published ahead of print on 20 August 2008.
10647
on November 8, 2019 by guest
http://jvi.asm.org/
activity in vitro, in transfected cells and in the absence of additional viral factors, two out of the three phosphorylation site (Phos) mutant forms of ICP0 potentially contribute to its E3 ubiquitin ligase activity in a cell type-dependent manner. However, only one ICP0 Phos mutant virus showed any signif-icant reduction in replication relative to wild-type (wt) HSV in cell culture. Our results suggest that the context of expression of ICP0 and cell type may be important in the requirement for phosphorylation to regulate ICP0’s ubiquitin ligase activity.
MATERIALS AND METHODS
Cells and viruses.Vero, HEp-2, U2-OS, and HeLa cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and passaged as described previously (5, 48). Human fetal foreskin fibroblast cells (HFFF-2), obtained from the European Collection of Cell Cultures, were grown in Dulbec-co’s modified Eagle’s medium containing 10% fetal calf serum, 1% glutamine, and antibiotics. L7 cells, a Vero cell line stably transfected with the HSV-1 ICP0 gene, were cultured as described by Samaniego et al. (47) and used to titer all ICP0 mutant viruses described below. The wt HSV-1, strain KOS (passage 11), and 7134, an ICP0 null mutant, were propagated as previously described (11, 48).
Transfection and immunofluorescence assays.Transfection of plasmid DNA was carried out using ExGen500 (Fermentas), following the manufacturer’s instructions. Immunofluorescence was carried out as previously described (6), using the appropriate combinations of monoclonal anti-ICP0 (11060), anti-ubiq-uitin (BioMol [FK2 PW8810]) and rabbit anti-ICP0 (r191), and anti-PML (r8) and anti-Sp100 (SpGH) antibodies.
Immunoprecipitation, in vitro ubiquitin ligase assay, and Western blot anal-ysis.HEp-2 cells (1⫻106cells) were transfected with 500 ng of the appropriate
combination of plasmids expressing ICP0 and ICP0 Phos mutants. Sixteen hours posttransfection, the cells were washed and harvested in phosphate-buffered saline. Cell pellets were resuspended in 5 ml of buffer A (50 mM Tris [pH 8], 500 mM NaCl, 1% NP-40, 0.1% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol) supplemented with protease inhibitors (Roche Applied Sciences) and lysed by gentle bath sonication prior to incubation on ice for 30 min. Extracts were clarified by centrifugation (13,000 rpm for 15 min at 4°C) and precleared by tumbling with 50l (50%, wt/vol) of equilibrated glutathione-S-transferase beads for 45 min at 4°C. ICP0 was immunoprecipitated using 1g of ICP0 monoclonal anti-sera 11060 and 30l (50%, wt/vol) of equilibrated protein G Sepharose for 2 h at 4°C. The beads were washed in buffer A (three times in 1 ml) and in buffer B (50 mM Tris [pH 8], 250 mM NaCl, 5 mM MgCl2, 0.1%
NP-40, 1 mM dithiothreitol) (three times in 1 ml) and resuspended in a final volume of 30l in buffer B. In vitro ubiquitin ligase reactions were carried out as previously described (7) using 15l of immunoprecipitated bead extract for 90 min at 32°C. Reaction mixtures were analyzed by Western blot analysis for the presence of polyubiquitin conjugates using a monoclonal anti-ubiquitin antibody (P4D1; Santa Cruz Biotechnology). Western blot analyses for ICP0 were per-formed as previously described, using anti-ICP0 monoclonal antibody 11060 (6).
Construction of ICP0 Phos mutants.HSV mutants carrying mutations in the major ICP0 phosphorylation sites (in the strain KOS background) were created by marker transfer, into the ICP0 null mutant virus 7134, essentially as described previously (11). One microgram of 7134 infectious viral DNA was cotransfected with 2.5g of each linearized Phos mutant plasmid in Vero cells (16). Three to 7 days posttransfection, cells were harvested, frozen, and lysed, and samples were plated on Vero cells overlaid with Dulbecco’s modified Eagle’s medium
contain-or Phos mutant viral DNA) and salmon sperm DNA and 4.5l of FuGENE 6 (Roche Diagnostic Corporation, Indianapolis, Ind.), which were diluted in Opti-MEM (Invitrogen Life Technologies, Carlsbad, CA). The transfection mixture for each sample was divided in half, added to each well containing 0.5 ml Opti-MEM, and left on cells for 5 h at 37°C. Five hours after the addition of the DNA/FuGENE 6 mixture, cells were placed in fresh medium and harvested 48 h and 72 h posttransfection for Vero and HeLa cells, respectively. Viral titers were determined by plaque assays on Vero cells (for KOS) and L7 cells (for 7134, P1, P2, and P3).
(ii) Infections.Vero, HeLa, and HEp-2 cells were plated for 24 h and infected with KOS, 7134, the Phos mutants, and their MR counterparts at a multiplicity of infection (MOI) of 0.1. After a 1-h incubation, unabsorbed virus was inacti-vated by washing once with an acid-glycine saline solution (10) and twice with phosphate-buffered saline, and cells were harvested 24 h postinfection. Viral titers were determined on Vero cells (KOS and MR viruses) or L7 cells (7134 and Phos mutants).
RESULTS
Characterization of the effects of ICP0 Phos mutations on ubiquitin conjugation in vitro and in cell culture.In a previous study, we identified two regions of ICP0 phosphorylation that were required for its maximal transactivating activity in trans-fection reporter assays (16). Additional studies have indicated that ICP0’s transactivating activity is dependent upon its RING finger E3 ubiquitin ligase activity. Mutations or deletions within the RING finger domain of ICP0 not only compromise its ability to conjugate ubiquitin but also greatly reduce its capability to act as a viral transactivator in reporter assays and significantly impair viral replication (7, 19–23). E3 ubiquitin ligases provide the substrate specificity to catalyze the forma-tion of ubiquitin chains on target proteins, typically directing them to the 26S proteasome for degradation. As ICP0 has previously been shown to induce the colocalization of conju-gated ubiquitin in cell culture, a phenotype that is dependent on its RING finger domain (23), we initially wanted to examine the capacities of ICP0 Phos mutations to form conjugated ubiquitin chains both in cell culture and in vitro.
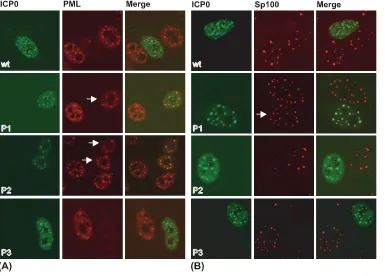
HEp-2 cells (a human larynx carcinoma cell line) were trans-fected with plasmids that expressed wt ICP0 and the mutants P1, P2, and P3 (Fig. 1), and immunofluorescence assays were performed by staining with an antibody that recognizes conju-gated ubiquitin. As shown in Fig. 2A, cells expressing wt ICP0 efficiently induced the formation of colocalizing conjugated ubiquitin. However, such conjugated ubiquitin was not de-tected in P1-expressing cells (Fig. 2A), whereas P2 and P3 induced the conjugation of ubiquitin (Fig. 2A). Thus, one or more residues in the region of P1 are required for ICP0 to polymerize ubiquitin chains efficiently in HEp-2 cells. In order to determine whether residues mutated within P1 indirectly
on November 8, 2019 by guest
http://jvi.asm.org/
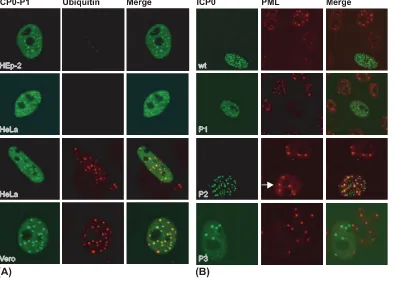
affected the folding of the RING finger domain or its ability to interact with its associated E2 ubiquitin-conjugating en-zyme(s), HEp-2 cells were transfected with wt and mutant ICP0 expression plasmids. ICP0 proteins were immunoprecipi-tated from these cells and assayed for their abilities to catalyze the formation of polyubiquitin chains in vitro. As shown in Fig. 2B, all Phos mutants efficiently catalyzed the formation of polyubiquitin, indicating that these phosphorylation site resi-dues are not required for the intrinsic E3 ubiquitin ligase activity of ICP0 in vitro. These data are consistent with previ-ously published results, which demonstrated that only the first 211 amino acids of ICP0 are required for efficient E3 ligase activity in vitro (7). Thus, P1 has a functional RING domain with similar in vitro activity to that of wt ICP0.
P1 and P2 mutant forms of ICP0 are impaired in their abilities to disperse one or more proteins associated with ND10 structures.Given that at least one of the Phos mutants is altered in its ability to conjugate ubiquitin in cell culture, the next question we asked is whether this result correlates with an inability to disperse two ND10-associated proteins, PML and Sp100, in HEp-2 cells. Transfected cells were monitored for PML and Sp100 staining in immunofluorescence assays. The wt ICP0 induced the dispersal of PML (Fig. 3A); however, PML fluorescence remained colocalized in P1- and P2-express-ing cells (Fig. 3A) but not P3-expressP2-express-ing cells. For Sp100, wt ICP0 and P3 also showed reduced staining (Fig. 3B), whereas P1-expressing cells colocalized with Sp100 (Fig. 3B). In con-trast to PML, P2 was capable of dispersing Sp100, indicating that this mutant form of ICP0 differentially affects the dispersal of these two ND10 constituents in HEp-2 cells. Taken to-gether, these data indicate that two of the three phosphoryla-tion domains of ICP0 are required to dissociate at least one protein from ND10.
The ubiquitin conjugation and PML dispersal activities of P1 are regulated in a cell type-specific manner.In addition to HEp-2 cells, the capacity of ICP0 to form ubiquitin chains was examined in Vero (African green monkey kidney) and HeLa (human cervical carcinoma) cells, two cell types that are
com-monly used to study ND10 structures and HSV replication. Employing the same experimental design described in the leg-end of Fig. 2A, the P1 expression vector was transfected into HEp-2, Vero, and HeLa cells. As shown in Fig. 4A, HEp-2 and a subset of HeLa cells that expressed P1 did not display de-tectable levels of conjugated ubiquitin colocalizing with ICP0. On the other hand, Vero and a subset of HeLa cells exhibited conjugated ubiquitin that colocalized with ICP0 mutant P1. When the Phos mutant forms of ICP0 were examined for PML staining in Vero cells (Fig. 4B), wt ICP0, P1, and P3 were capable of dissociating PML from ND10, and a proportion of cells expressing P2 showed limited colocalization with PML. This latter result had been observed in a previously published study (16). Thus, the E3 ubiquitin ligase activity of the Phos mutants (as measured by the production of colocalizing con-jugated ubiquitin) correlates with their abilities to disperse PML, and these activities of P1 and P2 are modulated by their cellular environment in the absence of other viral proteins. These results also indicate that mutations in P1 and P2 appear not to indirectly alter the structure of ICP0 because their phenotypes in these assays are cell type specific.
[image:3.585.63.259.68.207.2]P1 stability is increased in HEp-2 and HeLa cells. ICP0 RING finger mutants, which are deficient in their abilities to catalyze polyubiquitin formation, disrupt ND10, and transac-tivate HSV-1 gene expression, have an increased protein half-life in cell culture due to their inabilities to induce their own ubiquitination (7, 12, 19, 21–23, 26, 36). Because two of the three Phos mutant proteins were impaired in at least two of
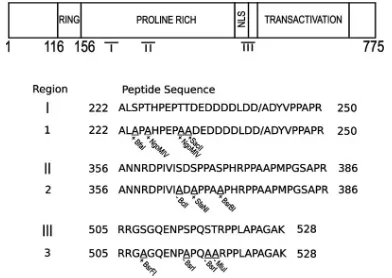
[image:3.585.329.511.70.213.2]FIG. 1. Phos mutations. A schematic diagram indicating the func-tional domains of ICP0, relative regions of phosphorylation (I, II, and III), and amino acid positions and specific amino acid substitutions in each region. The slash mark between amino acids D and A in region I is the boundary between the second and third exons of ICP0. The addition (⫹) or loss (⫺) of a specific restriction enzyme cleavage site is shown below each underlined alanine, which is substituted for a serine or threonine. NLS, nuclear localization signal.
FIG. 2. Mutation of residues within P1 of ICP0 alters its ability to conjugate ubiquitin in HEp-2 cells. HEp-2 cells were transfected with plasmids expressing either wt or Phos mutant forms of ICP0 (P1 to P3). Sixteen hours posttransfection, the cells were either processed for microscopy or harvested for immunoprecipitation. (A) Confocal im-ages showing wt and Phos mutant forms of ICP0 inducing the colo-calization of conjugated ubiquitin in cell culture. ICP0 (shown in green) was detected using rabbit anti-sera r190. Conjugated ubiquitin (shown in red) was detected using the monoclonal ubiquitin antibody FK2. Merged images show the colocalization of ICP0 with conjugated ubiquitin. (B) Western blot analysis of immunoprecipitated ICP0 cat-alyzing the formation of polyubiquitin (Poly-Ub) in vitro. Phos mutant and wt forms of ICP0 were immunoprecipitated from transfected cell extracts and incubated in the presence of a purified ubiquitin conju-gating enzyme mix (Ub mix) containing E1 ubiquitin-activating en-zyme, UbcH5a, and ubiquitin for 90 min at 32°C. Reaction mixtures were subsequently analyzed by Western blotting for the presence of ICP0 and polyubiquitin using rabbit anti-sera r190 and P4D1 antibod-ies, respectively.
on November 8, 2019 by guest
http://jvi.asm.org/
these activities in certain transfected cell types, we next asked whether the stabilities of the Phos mutant forms of ICP0 were altered relative to wt ICP0. The Phos mutant proteins and wt ICP0 were expressed in HEp-2, HeLa, and Vero cells in du-plicate cultures. Sixteen hours posttransfection, the cells were either harvested directly or treated with the protein synthesis inhibitor cycloheximide (CHX) for an additional 6 h prior to harvesting. Examples of ICP0 Western blots from these exper-iments are shown in Fig. 5A. Protein levels were quantified by densitometry, and differences in protein levels of the ICP0 mutants in the three cell types are shown as bar graphs in Fig. 5B. Mutant P1 had greater stability than wt ICP0, P2, and P3 in HEp-2 cells, consistent with the decreased ubiquitin conju-gation activity seen within HEp-2 cells (Fig. 2A). A similar trend was noted with P1 in HeLa cells, although wt ICP0 stability was reduced in this cell type compared to that in HEp-2 cells. In contrast to these observations, wt ICP0 and the Phos mutants showed the same degree of stability in Vero cells following CHX treatment, indicating that the half-life of ICP0 is greater in Vero cells than in HeLa or HEp-2 cells. These tests indicate that P1 is more stable than wt ICP0, P2, and P3 in the two cell types that show a reduction in its E3 ubiquitin ligase activity and that ICP0 stability is dependent upon the cell type in which it is examined.
Construction of viruses expressing ICP0 Phos mutants.In order to determine the role that ICP0 phosphorylation plays during lytic infection, the Phos mutations in each of the three regions of ICP0 (Fig. 1A and B) were introduced into the
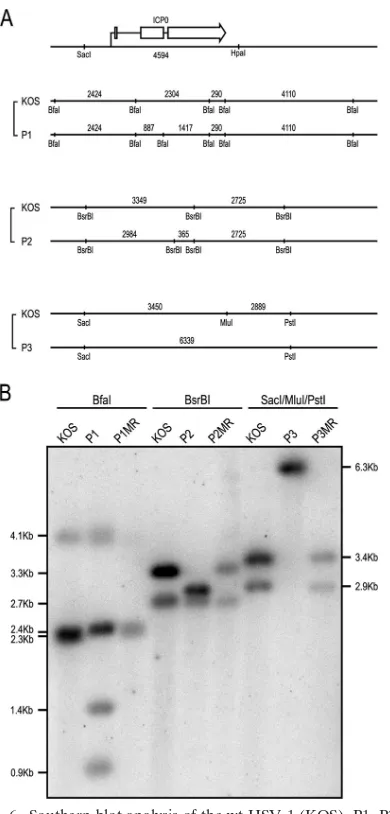
HSV-1 (strain KOS) genome by marker transfer, using the ICP0 null mutant virus 7134 as the parent. This mutant con-tains copies of theEscherichia coli lacZgene in place of both copies of ICP0 coding sequence, under the control of the ICP0 promoter. Phos mutant viruses were identified as clear plaques in the presence of the-galactosidase substrate, X-Gal. The resulting mutant viruses were designated P1, P2, and P3 for phosphorylation regions I, II, and III. Corresponding MR vi-ruses of these mutants, termed P1MR, P2MR, and P3MR, were generated to ensure that any phenotypes observed with these mutants were not due to secondary mutations at other sites in the viral genome. Restriction enzyme digestions and Southern blot analyses were performed with wt HSV-1 (strain KOS), the Phos mutants, and MR virus DNAs (Fig. 6) to confirm mutation and repair of the ICP0 gene. The resulting viruses were then tested in several cell types to ascertain the impact of the Phos mutations on viral replication.
Replication of Phos mutants. (i) De novo virus synthesis from infectious viral DNA.HSV genomic DNA is infectious when transfected into many cell types, and previous studies have demonstrated that ICP0 significantly enhances the pro-duction of new viral particles as part of this process. To deter-mine the extent to which the Phos mutations altered de novo viral particle synthesis relative to the other activities examined for the Phos mutants, viral DNAs from wt HSV-1 (strain KOS), ICP0 null mutant (7134), and the Phos mutants were transfected in Vero and HeLa cells. It was not possible to use HEp-2 cells in these experiments because of their poor
trans-FIG. 3. Mutation of residues within P1 and P2 prevents the dissociation of PML and/or Sp100 from ND10 in HEp-2 cells. HEp-2 cells were transfected with plasmids expressing either wt or Phos mutant (P1 to P3) forms of ICP0. Sixteen hours posttransfection, the cells were processed for confocal microscopy and stained with the ICP0 monoclonal antibody 11060 (shown in green) and rabbit anti-sera against either PML (r8) (A) or Sp100 (SpGH) (B) (both shown in red). Merged images show the colocalization of ICP0 with either PML (A) or Sp100 (B), respectively. White arrows highlight cells that express mutant ICP0 that failed to dissociate either PML or Sp100.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:4.585.100.485.68.344.2]fection efficiency with viral genomic DNA. Vero and HeLa cells were harvested 2 and 3 days posttransfection, respectively, and plaque assays were performed to determine viral titers. Table 1 shows the results obtained from these studies. Trans-fection of wt KOS DNA gave virus yields of titer⬎106PFU/ml
in Vero cells and between 103and 104PFU/ml in HeLa cells.
In contrast to KOS, the yield of 7134 was reduced as much as 4 orders of magnitude in Vero cells and⬃2 orders of magni-tude in HeLa cells. P1 titers were reduced 363-fold in Vero cells and 154-fold in HeLa cells. Interestingly, the yields of P1 were comparable to those of the ICP0-null mutant 7134 in HeLa cells. P2 and P3 titers were marginally reduced relative to those of KOS in both cell types. The replication of P1 was not complemented in Vero cells by cotransfecting a wt ICP0-expressing plasmid, which suggests that P1 may act as a dom-inant-negative mutant on wt ICP0 in this cell type (data not shown). Thus, mutations in P1 affected de novo viral replica-tion in two cell lines, with the most deleterious effect observed in HeLa cells.
(ii) Replication and plating efficiencies of Phos mutants.
Because of the difficulty in performing infectious viral DNA and plaque assays in HEp-2 cells to assess the role of ICP0 phosphorylation in lytic infection, we performed replication assays in Vero, HEp-2, and HeLa cells. Cells were infected at an MOI of 0.1 with KOS, 7134, the Phos mutants, and their
FIG. 4. P1 and P2 inhibit the ability of ICP0 to induce the colocalization of conjugated ubiquitin and/or dissociate PML in a cell type-dependent manner. (A) P1 induces the colocalization of conjugated ubiquitin in a cell type-dependent manner. HEp-2, HeLa, and Vero cells were transfected with a plasmid expressing ICP0 Phos mutant 1 (ICP0-P1). Sixteen hours posttransfection, cells were processed for confocal microscopy and stained with the ICP0 rabbit anti-sera r190 (shown in green) and the monoclonal ubiquitin antibody FK2 (shown in red). (B) Dissociation of PML by P1 is cell type dependent. Vero cells were transfected with plasmids expressing either wt or Phos mutant (P1 to P3) forms of ICP0. Sixteen hours posttransfection, the cells were processed for confocal microscopy using the ICP0 monoclonal antibody 11060 (shown in green) and PML rabbit anti-sera r8 (shown in red). Merged images show the colocalization of ICP0 with either conjugated ubiquitin (A) or PML (B), respectively. The white arrow highlights a cell that expresses P2 that has failed to induce the dissociation of PML.
FIG. 5. P1 increases the relative stability of ICP0 in HEp-2 and HeLa cells. (A) HEp-2, HeLa, and Vero cells were transfected with plasmids expressing either wt or Phos mutant (P1 to P3) forms of ICP0. Sixteen hours posttransfection, either cells were harvested or duplicate samples were treated with CHX (final concentration, 100g/ml) for an additional 6 h. ICP0 protein levels were determined by Western blot analysis and quantified by densitometry. (B) Histograms depict percent change in the relative stability of each CHX treatment sample com-pared to that of the untreated control sample. Bars represent the mean average of the results from three independent experiments.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:5.585.91.237.480.632.2]MR virus counterparts, and samples were harvested 24 h later. As shown in Table 2, KOS replicated to high levels in all three cell types. Relative to titers of KOS, titers of the 7134 mutant were impaired 2.4 logs in Vero cells and⬃1 log in HeLa cells but were similar to those of KOS in HEp-2 cells. Of the Phos mutants, P1 was the only virus to show a slight growth defi-ciency (⬃1.2 logs; 16-fold) in Vero and HeLa cells, with a less-marked reduction (⬃1 log) in HEp-2 cells. Viral produc-tion for P2, P3, and the marker rescuants were comparable to or marginally reduced (ⱕ1 log) relative to KOS in the three cell lines examined.
Plaque formation of the Phos mutants was subsequently tested in U2-OS cells, a cell line in which ICP0 is not required for efficient HSV replication (53), and HFFF-2 cells, a primary nontransformed human cell line in which the requirement of ICP0 to stimulate virus replication is critical (28). Results from this study are shown in Table 3. The efficiencies of plating of P1MR, P2, P2MR, P3, and P3MR between U2-OS and HFFF-2 cells were similar to KOS, ranging from 1.6- to 4.4-fold. Notably, plaque formation by P1 was reduced 20-fold in HFFF-2 compared to that in U2-OS cells, which is an approx-imately fivefold further decrease compared to KOS. Thus, one or more of the putative phosphorylated residues in phosphor-ylation region I are important for fully efficient plaque forma-tion of HSV-1 in HFFF-2 cells.
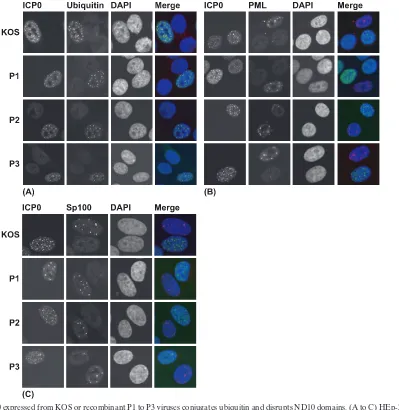
[image:6.585.66.261.65.472.2]Ubiquitin-conjugating and ND10-disrupting activities of the Phos mutants in HEp-2 cells.The Phos mutant viruses were then examined for ICP0’s E3 ubiquitin ligase-associated activ-ities in HEp-2 cells, given the results obtained in our transient assays with this cell line (Fig. 3 to 5). HEp-2 cells were infected with KOS or P1-P3 mutant viruses for 7 h, and the infected cells were assayed for their abilities to conjugate ubiquitin, disrupt the ND10-associated proteins PML and Sp100, and decrease PML levels. In contrast to the results in transfected cells presented earlier, wt ICP0 and the P1, P2, and P3 mutant forms all colocalized with conjugated ubiquitin (Fig. 7A) and
FIG. 6. Southern blot analysis of the wt HSV-1 (KOS), P1, P2, P3, and their MR viruses. (A) A schematic of the ICP0 gene and surround-ing sequences with restriction enzyme sites. (Top) The arrow indicates the initiation and direction of ICP0 transcription. The exons of ICP0 are shown as open boxes. (Bottom) Relevant restriction enzyme sites used in Southern blot analysis and fragment lengths (shown in base pairs) for wt and mutant virus pairings. Fragment lengths are not to scale. (B) A Southern blot of separated digests was probed with a
32P-labeled, 4.6-kb fragment containing the ICP0 gene from SacI to
HpaI (see panel A). Molecular masses of expected DNA fragments (in kilobases) are indicated to the left and right of the figure.
bViral titers for KOS were determined on Vero cells, and viral titers for 7134 P1, P2, and P3 were determined on L7 cells. Similar viral titers were obtained from a second independent viral DNA preparation of P1 in these experiments. Additional transfection studies in U2-OS cells, an ICP0-complementing cell line, showed that P1 viral DNA was of comparable quality to KOS and 7134 viral DNAs (data not shown).
TABLE 2. Replication of wt, ICP0 null mutant, Phos mutants, and their MR viruses in Vero, HeLa, and HEp-2 cellsa
Virus Vero log PFU/ml HeLa log PFU/ml HEp-2 log PFU/ml
KOS 6.04 (⫾0.08) 2.95 (⫾0.13) 3.90 (⫾0.14) 7134 3.60 (⫾0.12) 1.85 (⫾0.11) 4.04 (⫾0.08) P1 4.88 (⫾0.15) 1.78 (⫾0.18) 2.85 (⫾0.04) P1MR 6.11 (⫾0.01) 2.55 (⫾0.03) 3.60 (⫾0.07) P2 5.88 (⫾0.13) 2.65 (⫾0.05) 3.52 (⫾0.11) P2MR 6.09 (⫾0.01) 2.40 (⫾0.08) 3.61 (⫾0.03) P3 5.84 (⫾0.13) 2.65 (⫾0.13) 2.90 (⫾0.14) P3MR 6.21 (⫾0.04) 2.93 (⫾0.11) 3.51 (⫾0.08) aCells were infected at an MOI of 0.1 for 1 h. Twenty-four hours postinfec-tion, cells were harvested. Viral titers for KOS and MR viruses were determined on Vero cells and viral titers for 7134, P1, P2, and P3 were determined on L7 cells. Values in parentheses are the standard errors of the means.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:6.585.301.542.90.174.2] [image:6.585.301.541.599.692.2]dispersed PML (Fig. 7B) and Sp100 (Fig. 7C) from ND10. PML degradation was also observed in Hep-2 cells infected with KOS, P1, P2, P3, and their MR derivatives (data not shown). These results indicate that the phosphorylation sites in regions I, II, and III (Fig. 1) appear not to be required for these activities of ICP0 during lytic viral replication in HEp-2 cells.
[image:7.585.44.284.89.196.2]P1 has greater stability in HFFF-2 cells during viral repli-cation. ICP0 mutants with increased protein stabilities have been shown to be impaired for viral replication (5, 12, 36). Because P1 showed greater stability in transient transfection assays and replicated at reduced levels in primary HFFF-2 cells, we then wanted to examine the stabilities of the Phos mutant forms of ICP0 relative to those of wt ICP0 in HFFF-2 during viral infection. For these experiments, cells were in-fected with 2 PFU of wt, mutant, and MR viruses per cell. Four hours postinfection, CHX was added to cells to block de novo
[image:7.585.97.496.277.687.2]FIG. 7. ICP0 expressed from KOS or recombinant P1 to P3 viruses conjugates ubiquitin and disrupts ND10 domains. (A to C) HEp-2 cells were infected independently in triplicate and infected with 1 PFU wt HSV-1 (KOS) or P1 to P3 recombinant HSV-1 viruses per cell. The cells were fixed 7 h postinfection and stained for ICP0 and conjugated ubiquitin (A), ND10-associated proteins PML (B) or Sp100 (C), and with DAPI (4⬘,6-diamidino-2-phenylindole).
TABLE 3. Relative efficiencies of plating of ICP0 mutant viruses in U2-OS and HFFF-2 cellsa
Virus
U2-OS HFFF-2 EOP (U2-OS/
HFFF-2)
Expt 1 Expt 2 Expt 1 Expt 2 Expt 1 Expt 2
KOS 1 1 0.29 0.21 3.5 4.8
P1 1 1 0.05 0.05 20.0 20.0
P1MR 1 1 0.62 0.31 1.6 3.2
P2 1 1 0.49 0.32 2.0 3.1
P2MR 1 1 0.28 0.62 3.6 1.6
P3 1 1 0.23 0.72 4.4 1.4
P3MR 1 1 0.29 0.37 3.5 2.7
a
Viruses were titrated in U2-OS and HFFF-2 cells. Titers for each virus determined in U2-OS cells were given the relative value of 1, and titers on HFFF-2 cells were calculated as the ratios to those in U2-OS cells. The efficiency of plating (EOP) for each virus was determined by dividing the U2-OS viral titers by HFFF-2 viral titers. Values shown are from two independent experiments.
on November 8, 2019 by guest
http://jvi.asm.org/
protein synthesis, and cells were analyzed at hourly intervals thereafter. A comparison of the stabilities is shown in Fig. 8. P1 was considerably more stable than wt protein (expressed from wt or MR viruses), P2, and P3, indicating that a decrease in protein turnover for P1 correlates with a decrease in viral replication in HFFF-2 cells.
DISCUSSION
The ability of ICP0 to target specific cellular proteins for ubiquitination and proteasome-dependent degradation is thought to be crucial for promoting high levels of viral gene expression, replication, and reactivation. While it has long been established that ICP0 undergoes phosphorylation during infection (2, 3, 15, 52), little is known as to how these phos-phorylation events specifically regulate the biological functions of ICP0 (3, 4, 16, 17, 43). In this study we initially sought to determine whether ICP0 phosphorylation modulated its E3 ubiquitin ligase activity and its associated ability to induce the dispersal and degradation of PML and Sp100 from ND10. Our results bring to light new complexities in trying to understand the biological phenotypes of this important regulatory protein. Although the Phos mutations did not affect ICP0’s E3 ubiq-uitin ligase activity in vitro, cell-based transfection assays in the absence of other viral factors showed that mutation of two out of the three phosphorylated regions inhibited the ability of ICP0 to form conjugated ubiquitin chains (Fig. 2, row 2) and to dissociate PML (Fig. 3, rows 2 and 3) and Sp100 (Fig. 4B, row 2) from ND10 structures. Moreover, the reduced ubiquitin conjugation activity of P1 in transfected HEp-2 and a portion of HeLa cells directly correlates with an increase in its protein stability compared to either wt ICP0 or P2 and P3 mutants in the two cell types. These results are consistent with previous data that demonstrate that ICP0 mutants with reduced E3 ligase function have increased stabilities due to decreased auto-ubiquitination activity and therefore increased half-life (5, 12). Thus, this study is the first direct evidence to suggest a potential mechanism as to how phosphorylation may regulate this activity of ICP0. In direct contrast to HEp-2 and HeLa cells, however, expression of P1 in Vero cells promoted ubiq-uitin conjugation and dissociation of PML from ND10 struc-tures. This apparent difference in ICP0 E3 ubiquitin ligase activity between cell types does not correlate with a decrease in protein stability. In actuality, expression of wt ICP0 in Vero
results observed in HeLa and HEp-2 cells in transient expres-sion assays. In contrast to our infectious viral DNA studies, P1 mutations in the context of an infectious virus had only a slight impact on productive replication in Vero, HeLa, or HEp-2 cells. This minor effect of P1 on viral replication is likely com-pensated by higher levels of ICP0 expression and/or the pres-ence of other viral transactivators, for example, VP16 and ICP4, aiding the virus to initiate lytic replication in these three cell types (1, 40, 49). Analysis of the P1 mutant in HFFF-2 cells, a primary human fibroblast cell line, demonstrated a small but reproducible impairment in viral replication, which correlated with a decrease in P1 turnover (Fig. 8). Taken together, this study suggests that specific cellular environments and context of expression (transfection versus infection) may differentially affect the influence of phosphorylation on several activities related to ICP0-directed ubiquitination.
Role of P1 sites in ICP0 function.The RING finger of ICP0 and regions adjacent to it have been shown to be important for ICP0 to induce the conjugation of ubiquitin and activate viral gene expression (7, 20, 21). Because mutations in P1 impair the E3 ubiquitin ligase activity (in cultured cells if not in vitro) and lie near the RING finger of ICP0, our data provide further support of the ancillary function these adjoining domains have on the E3 ubiquitin activity of ICP0. Furthermore, these ob-servations suggest that phosphorylation can regulate this pro-cess. Because phosphorylation has been reported to control the auto-ubiquitination and/or degradation of the E3 ubiquitin ligases MDM2, Cbl, and COP1 (18, 29, 39, 54), our results with P1 suggest that this may also be the case for ICP0. The de-creased ability of P1 to catalyze the formation of ubiquitin chains and the associated increase in protein stability suggest that in restrictive cells, inhibition of ICP0 phosphorylation in region I potentially hinders its interactions with cofactors re-quired for its E3 ubiquitin ligase activity, auto-ubiquitination, and consequently its ability to be targeted for proteolysis. Al-ternatively, the mutations made within the P1 motif might compromise the E3 ubiquitin ligase activity of ICP0 in intra-cellular contexts, with the same consequences.
One such interaction that may be affected is that of ICP0’s interaction with USP7. This protein, a ubiquitin-specific pro-tease, removes ubiquitin from target proteins, including E3 ubiquitin ligases, promoting their stabilization. A complicating issue is that wt ICP0 mediates the degradation of USP7 during HSV infection in Vero, HEp-2, and HeLa cells (5). Viruses that do not contain the RING finger domain or cannot bind to USP7 are unable to degrade USP7 (5, 12). If inhibition of ICP0-USP7 binding leads to reduced ICP0 protein stability, it
p.t., posttreatment.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:8.585.92.229.67.150.2]is possible that enhanced ICP0-USP7 interactions (through increased USP7 stability and/or binding) upon inhibition of ICP0 phosphorylation could diminish its turnover. In support of this model, a link between the phosphorylation state of E3 ubiquitin ligases and USP7 binding has been made, as phos-phorylation of MDM2 E3 ubiquitin ligase family members has been shown to reduce their affinity for USP7, destabilizing their protein levels (39). Lastly, the activity of USP7 has been described to be cell type dependent, which may also contribute to the cell-specific phenotypes observed with P1 in our studies (44).
P2 differentially affects the localization of two ND10-associ-ated proteins.One striking result from our experiments is the modulation of ICP0 activities by mutation of the P2 region. Our transient assays in HEp-2 cells showed that P2 cannot dissociate PML from ND10 but is capable of dissociating Sp100. This mutant form of ICP0 can form polyubiquitin chains in HEp-2 cells, demonstrating that its E3 ubiquitin ligase activity appears to be intact, at least at this level of analysis. Notably, this is the first mutant form of ICP0 de-scribed that differentially affects the staining of two ND10-associated proteins. Our results indicate that in the absence of other viral factors in HEp-2 cells, the phosphoacceptor sites in region II of ICP0 play a role in dispersing or degrading PML but are not required for dispersal of Sp100. While initial results on the consequences of ICP0 expression on Sp100 expression and localization favored a model involving a direct effect of ICP0 on Sp100, more recent data suggest that depletion of PML either by ICP0 or by RNA interference leads to changes in Sp100 that are indistinguishable from those that occur dur-ing HSV infection. Thus, the differential effects of P2 on PML and Sp100 do not necessarily imply alterations in the substrate specificity of ICP0 by loss of potential phosphorylation (27). It is equally possible that in HEp-2 cells the P2 mutant is unable to disperse/degrade PML, but nonetheless is able to affect PML in such a way that Sp100 is no longer stably associated with ND10.
The potential impact that the P2 mutations might have on HSV replication is not apparent in this study, as these lesions do not appreciably alter HSV growth or plaque formation in the cell types tested. The distinct phenotypes associated with P2 may only become evident when assaying for other activities of ICP0 or in another context (e.g., in vivo infection and reac-tivation experiments). Future work with the Phos mutants will ascertain which specific phosphorylation sites on ICP0 are re-quired for selected activities of this multifunctional protein that is crucial for efficient HSV replication and reactivation.
ACKNOWLEDGMENTS
This work was supported in part by the Medical Research Council (in Glasgow), NIH grant number P20 RR016475 from the INBRE Program of the National Center for Research Resources (D.D.), Pub-lic Health Service grant RO1AI72357 from the National Institute of Allergy and Infectious Diseases (D.D.), and Public Health Service grant RO1CA20260 from the National Cancer Institute (P.S.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Ben Combs and Adam Bayless for technical assistance and members of the Azuma and Davido laboratories for helpful com-ments and suggestions.
REFERENCES
1.Ace, C. I., T. A. McKee, J. M. Ryan, J. M. Cameron, and C. M. Preston.1989. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J. Virol.63:2260– 2269.
2.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman.1984. Character-ization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol.52:108–118.
3.Advani, S. J., R. Hagglund, R. R. Weichselbaum, and B. Roizman.2001. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol.75:7904–7912.
4.Advani, S. J., R. R. Weichselbaum, and B. Roizman.2000. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. USA
97:10996–11001.
5.Boutell, C., M. Canning, A. Orr, and R. D. Everett.2005. Reciprocal activ-ities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol.79:12342–12354. 6.Boutell, C., A. Orr, and R. D. Everett.2003. PML residue lysine 160 is
required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol.77:8686–8694.
7.Boutell, C., S. Sadis, and R. D. Everett.2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol.76:841–850.
8.Cai, W., T. L. Astor, L. M. Liptak, C. Cho, D. M. Coen, and P. A. Schaffer.
1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol.
67:7501–7512.
9.Cai, W., and P. A. Schaffer.1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol.66:2904–2915.
10.Cai, W. Z., S. Person, C. DebRoy, and B. H. Gu.1988. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1. An analysis of linker insertion mutants. J. Mol. Biol.201:575–588.
11.Cai, W. Z., and P. A. Schaffer.1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfec-tion of viral DNA. J. Virol.63:4579–4589.
12.Canning, M., C. Boutell, J. Parkinson, and R. D. Everett.2004. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem.
279:38160–38168.
13.Chelbi-Alix, M. K., and H. de The´.1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene18:935–941.
14.Chen, J., C. Panagiotidis, and S. Silverstein.1992. Multimerization of ICP0, a herpes simplex virus immediate-early protein. J. Virol.66:5598–5602. 15.Davido, D. J., D. A. Leib, and P. A. Schaffer.2002. The cyclin-dependent
kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol.
76:1077–1088.
16.Davido, D. J., W. F. von Zagorski, W. S. Lane, and P. A. Schaffer.2005. Phosphorylation site mutations affect herpes simplex virus type 1 ICP0 func-tion. J. Virol.79:1232–1243.
17.Davido, D. J., W. F. Von Zagorski, G. G. Maul, and P. A. Schaffer.2003. The differential requirement for cyclin-dependent kinase activities distinguishes two functions of herpes simplex virus type 1 ICP0. J. Virol.77:12603–12616. 18.Dornan, D., H. Shimizu, A. Mah, T. Dudhela, M. Eby, K. O’Rourke, S. Seshagiri, and V. M. Dixit.2006. ATM engages autodegradation of the E3 ubiquitin ligase COP1 after DNA damage. Science313:1122–1126. 19.Everett, R., P. O’Hare, D. O’Rourke, P. Barlow, and A. Orr.1995. Point
mutations in the herpes simplex virus type 1 Vmw110 RING finger helix affect activation of gene expression, viral growth, and interaction with PML-containing nuclear structures. J. Virol.69:7339–7344.
20.Everett, R. D.1987. A detailed mutational analysis of Vmw110, a trans-acting transcriptional activator encoded by herpes simplex virus type 1. EMBO J.
6:2069–2076.
21.Everett, R. D.1988. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J. Mol. Biol.202:87–96. 22.Everett, R. D.1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol.70:1185–1202.
23.Everett, R. D.2000. ICP0 induces the accumulation of colocalizing conju-gated ubiquitin. J. Virol.74:9994–10005.
24.Everett, R. D.2006. The roles of ICP0 during HSV-1 infection, p. 39–64.In R. M. Sandri-Goldin (ed.), Alpha herpesviruses: molecular and cellular biology. Caister Academic Press, Wymondham, United Kingdom. 25.Everett, R. D., and M. K. Chelbi-Alix.2007. PML and PML nuclear bodies:
implications in antiviral defence. Biochimie89:819–830.
26.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson.1998. The disruption of ND10 during herpes simplex virus
on November 8, 2019 by guest
http://jvi.asm.org/
quest of the host cell by herpes simplex virus 1. J. Virol.78:2169–2178. 32.Halford, W. P., C. D. Kemp, J. A. Isler, D. J. Davido, and P. A. Schaffer.2001.
ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol.75:6143–6153.
33.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol.
75:3240–3249.
34.Honess, R. W., and B. Roizman.1974. Regulation of herpesvirus macromo-lecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol.14:8–19.
35.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer.1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol.63:759–768.
36.Lium, E. K., and S. Silverstein.1997. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4zinc ring finger reveals a requirement for
ICP0 in the expression of the essential␣27 gene. J. Virol.71:8602–8614. 37.Maul, G. G., H. H. Guldner, and J. G. Spivack.1993. Modification of
discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol.74:2679–2690.
38.Maul, G. G., A. M. Ishov, and R. D. Everett.1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology217:67–75.
39.Meulmeester, E., Y. Pereg, Y. Shiloh, and A. G. Jochemsen.2005. ATM-mediated phosphorylations inhibit Mdmx/Mdm2 stabilization by HAUSP in favor of p53 activation. Cell Cycle4:1166–1170.
40.Mossman, K. L., and J. R. Smiley.1999. Truncation of the C-terminal acidic transcriptional activation domain of herpes simplex virus VP16 renders ex-pression of the immediate-early genes almost entirely dependent on ICP0. J. Virol.73:9726–9733.
41.Mu¨ller, S., and A. Dejean.1999. Viral immediate-early proteins abrogate the
2. Lippincott Williams & Wilkins, New York, NY.
47.Samaniego, L. A., N. Wu, and N. A. DeLuca.1997. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J. Virol.71:
4614–4625.
48.Schaffer, P. A., G. M. Aron, N. Biswal, and M. Benyesh-Melnick.1973. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology52:57–71. 49.Steiner, I., J. G. Spivack, S. L. Deshmane, C. I. Ace, C. M. Preston, and N. W.
Fraser.1990. A herpes simplex virus type 1 mutant containing a nontransin-ducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J. Virol.64:1630–1638.
50.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman.
1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science235:1056–1059.
51.Stow, N. D., and E. C. Stow.1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol.67:2571–2585. 52.Wilcox, K. W., A. Kohn, E. Sklyanskaya, and B. Roizman.1980. Herpes
simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J. Virol.33:167–182. 53.Yao, F., and P. A. Schaffer.1995. An activity specified by the osteosarcoma
line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol.69:6249–6258.
54.Yokouchi, M., T. Kondo, A. Sanjay, A. Houghton, A. Yoshimura, S. Komiya, H. Zhang, and R. Baron.2001. Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J. Biol. Chem.276:
35185–35193.
55.Zhu, X. X., J. X. Chen, C. S. Young, and S. Silverstein.1990. Reactivation of latent herpes simplex virus by adenovirus recombinants encoding mutant IE-0 gene products. J. Virol.64:4489–4498.