Vol. 61, No. 3
JOURNALOFVIROLOGY,Mar. 1987, p.916-920
0022-538X/87/030916-05$02.00/0
Copyright © 1987, AmericanSocietyforMicrobiology
Humoral Immune Responsiveness in Duck Hepatitis B
Virus-Infected Ducks
MICHAEL S.
HALPERN,'*
WILLIAM S. MASON,2 LAURA COATES,2 ANNA P. O'CONNELL,2 AND JAMESM. ENGLAND3The Wistar Instituteof Anatomy andBiology, Philadelphia, Pennsylvania
191041;
InstituteforCancerResearch, Philadelphia, Pennsylvania191112; andDepartment of Pathology, Hospital ofthe University of Pennsylvania,Philadelphia, Pennsylvania 191043
Received 18September 1986/Accepted 20November 1986
Immunofluorescenceassays with fixed tissuesectionswereused tocharacterize antibody reactivity insera obtained from duckhepatitisBvirus-infected ducks.Under conditionsofexperimental infection, antibody to coreantigenbut nottosurfaceantigenwasdetectable.Amajorityof theducks infected at 8daysafterhatching andaminorityof those infected at 1dayafterhatchingshowedatransient anti-coreantigenhumoralresponse;
thisresponse was stronger in theantibody-positiveducks infected onday 8 than in those infected onday1. Antiviral antibody was not detected in the sera of ducks congenitally infected with duck hepatitis B virus.
Several of the infected ducks, but none of the uninfected ducks, exhibited autoantibody reactivity for a-islet-cell-associated antigen.
Muchofthehepatocellulardamageassociatedwith hepa-titis B virus infection has been ascribed to the immune response to viral antigen (9). Despite much clinical interest in this pathology, none of the several animal models of hepatitisBinfectionhasbeenwidely used forimmunological analysis. We have, therefore, initiated a study of immune responsiveness in Pekin ducks infectedwith duckhepatitisB virus (DHBV).
Unlike other animal models ofhepatitis B infection, the DHBV system may be used for a comparison of immune responsiveness under conditions ofexperimental infection (postnatal virus inoculation [6]) versus
congenital
infection (virus transmission from the mother [7],resulting in ahigh levelofreplication inearly-stage embryos [4, 8]). Implicitin such acomparison is an assessment oftheimmunogenicity aswellasthepotentialtolerogenicity of viralantigen. As an initial step in thisanalysis,this studyfocusedonthehumoral response toDHBVinfection,examinedatthelevel of serum antibodyreactivity.Twenty ducks inoculated intravenously with DHBV at 8 daysafterhatchingand 21uninoculated controls were main-tainedfor 16 weeks and monitored at 2-week intervals for serum antibody reactivity to DHBV antigen-positive cells. All ofthe infected ducks became viremic by 2 to 4 weeks postinoculation, asindicatedby a DNAhybridizationassay forviremia (5). Test sera were screened for antibody in an immunofluorescenceassay(described in the legend to Fig. 1) basedontheuseoffixed tissue sections. This form of assay was chosen because the preservation of tissue architecture facilitates testing of whether reactivity is directed to cell-associated antigen. Assays were carried out with sections preparedfrom pancreas tissue of either 2- to week-old or 3-to 4-month-old congenitally DHBV-infected or uninfected ducks. Previous work has shown that numerous scattered pancreasacinar cells in young, congenitally DHBV-infected ducks and a majority of the a cells localized toa islets in older, congenitally infectedducks are positive for both viral coreand surface antigens (1-3).
* Correspondingauthor.
Antibody reactive to viral-antigen-positive acinar cells was detectablein serafrom 16ofthe20 infected ducks but notinserafrom the uninfectedducks(representativeassays areshowninFig. 1).Reactivityfirstappearedat2to6 weeks postinoculation and generally persisted for 4 to 8 weeks before waning. Those serathatshowed strong reactivity to viral-antigen-positive acinar cells also showed reactivity abovebackgroundleveltobothviral-antigen-positiveot-islet cells(Fig. 2)andviral-antigen-positive hepatocytes (datanot shown). Reactivity to acinar cells or hepatocytes from uninfected ducks was not detected in sera from either infectedoruninfected ducks (datanotshown).
In tests of a-cell reactivity with sera from uninfected ducks,thelarge majority ofcells in
viral-antigen-positive
a islets showed no fluorescence above the background level observed with the section as a whole (Fig. 2C and D). However, aminority of cells, comprising from5 to20% of thetotal numberofcells in individuala islets, was fluores-cent tolevelsconsiderablyabovebackground(Fig.2C;asin all the assaysof duck serafrom uninfected ducks, fluores-cence was scored with a fluorescein-specific barrier filter because oftheuseofafluorescein-labeledgoatanti-chicken immunoglobulinserum). Asimilar percentage of fluorescent islet-associated cells was observed in assays ofsera from uninfected ducks as tested with pancreas sections from age-matched uninfectedducks(datanotshown). Most,ifnot all, ofthese cellsweredoublyfluorescent, scoring positively with either a rhodamine- or fluorescein-specific filter. Be-causeislet tissue in pancreas sections that were unreacted with any sera showed a minority of autofluorescent cells as scored with either filter, the use of the rhodamine-specific filter served to enumerate autofluorescent cells in tissue reactedwith fluorescein-labeled antisera. Since virtually all positivecells detected in pancreas tissue assayed with sera fromuninfectedducks and with the fluorescein-labeled anti-immunoglobulin serum were doubly fluorescent, it seems likelythat in these assays thefluorescence scored with the fluorescein-specific filterwasdue to autofluorescence rather thantoantibody reactivity.Thedoubleimmunofluorescence assays(Fig. 1 and 2) did notin themselvesdistinguish between duck antibody recog-916
on November 10, 2019 by guest
http://jvi.asm.org/
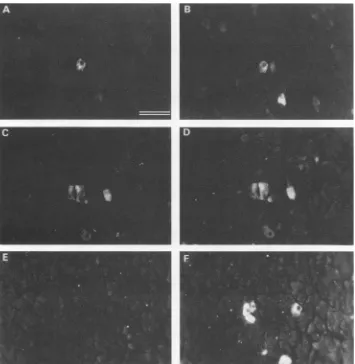
FIG. 1. Serum antibody reactivity forviral-antigen-positive pancreas acinar cells. The two panels in each row show double immunoflu-orescenceassays of the same field ofexocrine pancreas cells from a 3-week-old congenitally DHBV-infected duck. (A and C) Serum from a10-week-oldDHBV-infected duck (infection at 8 days after hatching). (B and F) Rabbit anti-surface antigen serum (2). (D) Rabbit anti-core antigenserum(3). (E) Serum from a10-week-old uninfected duck. Tissue sections were prepared as previously described (2). The following order was used for the addition of each reagent (30 to 40 ,ul) to an individual section: (i) the test duck serum (diluted 1:3 with phosphate-buffered saline), (ii) rabbit antivirus serum (diluted 1:40 with serum from an uninfected duck and further diluted to 1:200 with phosphate-buffered saline), (iii) fluorescein-labeled goat antibody directed to chicken immunoglobulin (the affinity-purified goat antibody preparation was purchased from Kirkegaard & Perry, Gaithersburg, Md., and used at a concentration of 200 ,ug/ml; its reactivity in the immunofluorescence assaywasinhibitedbyacompeting preparation of duck immunoglobin), and (iv) arhodamine-labeled immunoglobin fraction of agoat anti-rabbitimmunoglobin serum (diluted 1:320 withphosphate-buffered saline). Bar equals 20 ,um.
nition of viral antigen or recognition of nonviral antigen induced as a consequence of infection. Nevertheless, in termsoftherelative levels of fluorescence associated with individual acinar cells (Fig. 1),thepattern of staining medi-atedbythe duckantibody paralleled that mediated bycore
antigen-reactive
but not by surface antigen-reactive rabbit antibody.These assaystherefore suggested that much, ifnot all, of the reactivity in thepositive duckserawasdirectedto viral core antigen.To test this inference, preparations of DHBV surface
antigen particles
orimmature viralcores werepurified
from serum orliver,respectively, asdescribedpreviously
(3),and wereassayed fortheircapacitytocompetewith the reactiv-ity of the positive duck sera. Whereas competition with surfaceantigen particles hadno discernibleeffect,competi-tion with immaturecoresreduced the levelof cell-associated fluorescence to that ofthe
background
(representative
as-sayswithviral-antigen-positive
acinar cellsareshown inFig.
3;dataarenotshownforassayswithviral-antigen-positive a cells or hepatocytes). We therefore concluded that, as scored in the immunofluorescence assay, the specificity of theduckantibodywas directedto viral coreantigen. How-ever, the
possibility
is not excluded that the absence of detectable antibody to viral surface antigen reflects the in vivocomplexing of antibodyratherthanthelackofimmune response.Analysis was then extended toducks experimentally in-fected with DHBV at 1 day after hatching or congenitally infected with DHBV. Astested withseraobtained upto 16 weeks afterhatching,one or morebloodsamplesfrom 13of 30 ducks infected on day 1 exhibited
reactivity
for viral-antigen-positivepancreasacinar cells.Bycontrast,noblood samples from sevencongenitally
infected ducks exhibited suchreactivity (datanotshown),aresultconsistentwith the premise of immune tolerance.For 12oftheducks infectedon
day
1whose serashowedon November 10, 2019 by guest
http://jvi.asm.org/
918 NOTES
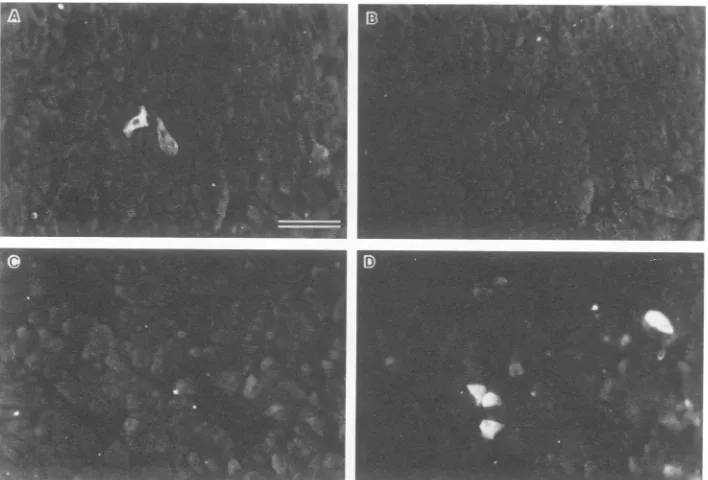
FIG. 2. Serumantibody reactivityforviral-antigen-positivea cells. Thetwopanels in each row show double immunofluorescence assays
ofthe samefield ofpancreas froma4-month-oldcongenitallyDHBV-infected duck. (A) Serum froma 10-week-old DHBV-infectedduck
(infectionat 8 daysafter hatching).(BandD)Rabbit anti-surfaceantigen serum.(C) Serum froma10-week-old uninfected duck. The large structures resolvedin panels B and Dcorrespondtoaislets,asshownbythereactivityofantiglucagonserumfor thesestructuresinadjacent
sections. Barequals 20 p.m.
C I
FIG. 3. Effectof competition with purified viral antigen on serum antibody reactivity for viral-antigen-positive pancreas acinar cells. The twopanels ineach row show doubleimmunofluorescence assays of the same field of exocrine pancreas cells from a 3-week-old congenitally
DHBV-infected duck. (A) Serum from a10-week-old DHBV-infected duck (infection at 8 days after hatching) in competition with purified
surface antigen particles(40
K.g/ml).
(B)Rabbit anti-surface antigen serum in competition with purified surface antigen particles. (C) Same serumasin panel A in competition withpurified immature viral cores (20 ,ug/ml). (D) Rabbit anti-surface antigen serum in competition withpurified immature viral cores. Bar equals 20p.m.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.135.487.71.314.2] [image:3.612.136.490.426.666.2]A"*
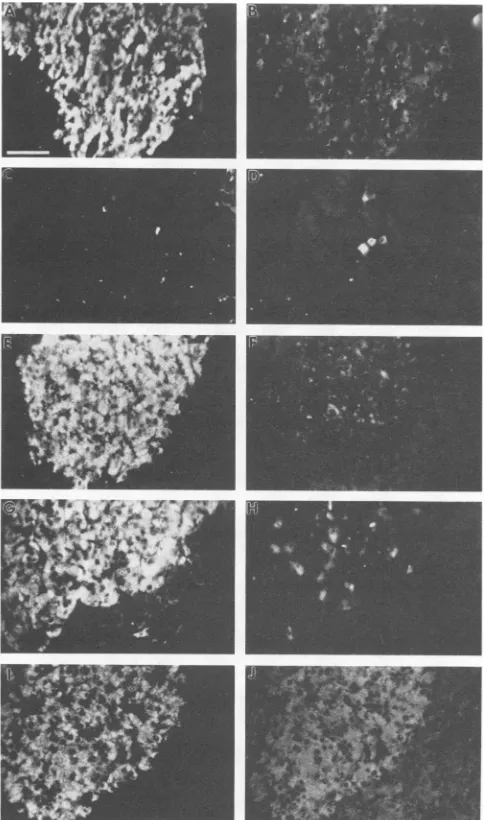
FIG. 4. Serum antibody reactivity for pancreas cells. The two
panelsineachrow show doubleimmunofluorescence assays ofthe
samefield ofpancreascells.(A, C,E,G,and I)Serum from thefinal
blood sample (14 weeks postinfection) ofa DHBV-infected duck (infection at 1 day after hatching). (B, D, F, and H) Rabbit
anti-surface antigen serum and pancreas cells from a4-month-old
congenitally DHBV-infected duck(anaislet isshown) (B),exocrine
pancreasfroma3-week-old congenitallyDHBV-infected duck (D), pancreas cells from a 4-month-old uninfected duck (an a islet is
shown) (F), and pancreas cells from the duck that was used to
provide the test serum (an a islet is shown) (H). (J) Rabbit
antiglucagon serumandpancreascellsfrom the duckthatwasused
to provide the test serum. The antiglucagon serum has previously
been described (1) and, as used here, was diluted 1:5 with
phos-phate-buffered saline. Barequals 20 p.m.
demonstrable reactivity, antibodywasdetectable at2weeks
postinfection, and for the remaining duck, antibody was
detectable at 4 weeks postinfection. As with the ducks
infected at 8 days afterhatching, reactivity was blocked by
competition with purified preparations of immature viral
cores but not by competition with preparations of surface
antigen particles. In addition to the lower percentage of antibody-positive ducks among the group infected at 1 day
after
hatching,
two sets ofobservations indicated that the anti-coreantigen
humoral response wasconsiderably
weakerintheseducks than in theducksinfectedonday 8:
(i)
a more
rapid
waning of antibody production (8 of the 12 ducksinfectedonday1thatexhibited antibody reactivityat 2 weeks postinfection had lost detectable reactivity by 4 weeks postinfection) and (ii) a 2- to 3-fold-lower titer ofserum
antibody
at2weekspostinfection
(ontheorder of1:3 fortheantibody-positive
ducksinfected onday 1versus 1:6 or 1:9fortheantibody-positive
ducks infected onday8).
Assays fora-islet cellreactivitywerethencarriedoutwith sera obtained from the
ducks
infected on day 1 at timessubsequent
to thewaning
ofdetectable reactivity forviral-antigen-positive
acinar cells. As tested withviral-antigen-positive
islettissue,
mostofthese sera yielded apatternof a-islet-associated fluorescence indistinguishable from that obtained with sera from uninfected ducks. However, seraobtained from one duck bothat 12 weeks postinfection and when theduckwaskilledat14weeks postinfectionexhibited reactivity to virtually all a-islet cells, including those not
detectably
viralantigen
positive(Fig.4Aand B show results ofthe assay ofthefinal blood sample from this duck). This serumdid not exhibit detectable reactivity forviral-antigen-positive
or-negative
acinarcells(Fig.
4C and D) orhepato-cytes (datanot
shown).
The recognition by this duck serum of viral-antigen-negative a-islet cells suggested that reactivity was directed
to
antigen
expressed in uninfected a-islet cells. Thispossi-bility was confirmed in assays of the serum with pancreas tissuefrom age-matcheduninfectedducks, with virtuallyall a-islet cells showing fluorescence above background level (Fig. 4E and F; positive fluorescence was detectable at serum dilutions of up to 1:81). The recognition of viral-antigen-negative a-islet cells in pancreas sections from the autologous duck
(Fig.
4Gand H) established thatreactivity
was mediated by an autoantibody. The reactivity ofrabbit antiglucagon serum for these target cells (Fig. 41 and
J)
served in turn to directly identify them as a cells. Under conditions ofcompetition with acommercial preparation of bovine glucagon (1), the reactivity for a cells ofthe rabbit antiglucagon serum but not ofthe autoreactive duck serum was blocked (data not shown).
Pancreas sectionsfromthe age-matched uninfected ducks were used to screen a large number of duck sera for a comparable reactivity. In addition to the duck described above,a second duckfrom the group of 30 infected at 1day after hatching exhibited serum antibody reactivity for DHBV-negative a cells; this reactivity was detected in the blood sample obtained at 14 weeks postinfection (serum antibody titer of 1:9) but not in earlier or subsequent samples. Antibody was also detected in the final sample obtained at 16 weeks after hatching from one of the 7 congenitally DHBV-infected ducks examined(antibody titer of 1:27) but in none of the samples obtained from the 20 ducks infectedon day 8. All blood samples fromuninfected ducks wereantibody negative, with the test sample compris-ing the following three groups: (i) 21 uninoculated controls described above, which weremonitored at 2-week intervals for up to 16 weeks after hatching; (ii) 30 ducks from the SPAFAS breeding flock, which were bled at 12, 16, and20 weeks after hatching; and (iii) 60 ducks from the SPAFAS flock, ranging in age from 7 to 12 months, which were bled once.
Thepresence of autoantibodyin a small percentage of the DHBV-infected ducks examined here and its absence inthe uninfected ducks tested raises the possibility that DHBV
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.58.300.71.481.2]920 NOTES
infection isapredisposing factor for the induction of a-cell autoreactivity. If this suggestionproves correct, the DHBV system may represent a useful model for the study of the induction of autoimmunity in the contextofahepadnavirus infection.
WethankElsa Aglowfor preparations of the tissue sections and
Frank Southwelland Pat Frazerfor technical assistance.
Thsinvestigation wassupported byUnited States Public Health
Service grants AI-22065, CA-10815, AI-18641, CA-06551, and
RR-05539 from the National Institutes of Health andgrant185113
from theJuvenile Diabetes Foundation.
LITERATURE CITED
1. Halpern, M. S., J. Egan, W. S. Mason,andJ. M.England. 1984. Viral antigen in endocrine cells of the pancreatic islets and
adrenal cortex of Pekin ducks infected with duck hepatitis B
virus. VirusRes. 1:213-223.
2. Halpern,M.S., J. M. England, D. T. Deery, D. J. Petcu, W. S. Mason, and K. L. Molnar-Kimber. 1983. Viral nucleicacid
syn-thesis and antigenaccumulation inpancreasandkidneyofPekin
ducks infectedwith duckhepatitisBvirus.Proc.Natl.Acad. Sci.
USA80:4865-4869.
3. Halpern, M. S., J. M. England, L. Flores, J. Egan, J. Newbold, and W. S. Mason. 1984. Individual cells in tissues of
DHBV-infected ducks express antigens cross-reactive with those on
virussurface antigen particlesandimmatureviralcores.Virology
137:408-413.
4. Halpern, M. S., S. B. McMahon, W. S. Mason, and A. P. O'Connell. 1986. Viral antigen expression in the pancreas of
DHBV-infected embryos and young ducks. Virology
150:272-286.
5. Mason, W. S., C. Aldrich,J.Summers, and J. M. Taylor. 1982.
Asymmetric replication of duck hepatitis B virusDNAin liver
cells: free minus strand DNA. Proc. Natl. Acad. Sci. USA 79: 3997-4001.
6. Mason, W. S., M.S.Halpern, J. M. England,G. Seal, J. Egan, L. Coates, C. Aldrich, andJ. Summers. 1983. Experimental
trans-mission of duck hepatitisBvirus. Virology131:375-384.
7. O'Connell, A. P., M. K. Urban, and W. T. London. 1983.
Naturally occurring infection of Pekin duck embryos by duck
hepatitisB virus. Proc. Natl. Acad. Sci. USA80:1703-1706.
8. Urban, M. K., A. P. O'Connell, and W. T. London. 1985.
Sequenceofeventsin natural infection of Pekin duckembryos withduck hepatitis Bvirus. J. Virol. 55:16-22.
9. Vyas, G. N.,J. L.Dienstag,andJ.H.Hoofnagle (ed.).1984. Viral
hepatitisand liver disease. Grune& Stratton, Orlando,Fla. J. VIROL.