0022-538X/08/$08.00⫹0 doi:10.1128/JVI.00630-08
Nonstructural Proteins 1 and 2 of Respiratory Syncytial Virus Suppress
Maturation of Human Dendritic Cells
䌤
Shirin Munir, Cyril Le Nouen, Cindy Luongo, Ursula J. Buchholz,
Peter L. Collins, and Alexander Bukreyev*
Laboratory of Infectious Disease, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
Received 20 March 2008/Accepted 12 June 2008
Human respiratory syncytial virus (RSV) is the most important agent of serious pediatric respiratory tract disease worldwide. One of the main characteristics of RSV is that it readily reinfects and causes disease throughout life without the need for significant antigenic change. The virus encodes nonstructural protein 1 (NS1) and NS2, which are known to suppress type I interferon (IFN) production and signaling. In the present study, we monitored the maturation of human monocyte-derived myeloid dendritic cells (DC) following inoculation with recombinant RSVs bearing deletions of the NS1 and/or NS2 proteins and expressing enhanced green fluorescent protein. Deletion of the NS1 protein resulted in increased expression of cell surface markers of DC maturation and an increase in the expression of multiple cytokines and chemokines. This effect was enhanced somewhat by further deletion of the NS2 protein, although deletion of NS2 alone did not have a significant effect. The upregulation was largely inhibited by pretreatment with a blocking antibody against the type I IFN receptor, suggesting that suppression of DC maturation by NS1/2 is, at least in part, a result of IFN antagonism mediated by these proteins. Therefore, this study identified another effect of the NS1 and NS2 proteins. The observed suppression of DC maturation may result in decreased antigen presentation and T-lymphocyte activation, leading to incomplete and/or weak immune responses that might contribute to RSV reinfection.
Human respiratory syncytial virus (RSV) is the most impor-tant viral agent of serious respiratory tract illness in infants and children worldwide (14, 15). It is estimated that, in the United States alone, the yearly number of hospitalizations of infants under 1 year of age due to RSV bronchiolitis or pneumonia is 51,000 to 82,000 and 22,000 to 44,000, respectively (72). One of the hallmarks of RSV biology is that it readily reinfects throughout life, sometimes even during the same epidemic season and frequently involving strains that are antigenically similar (26, 29; reviewed in references 14 and 15). Most infants are infected at least twice during the first 3 years of life (29). Reinfection also is common in adolescents and adults (20) and is an important cause of morbidity and mortality in the elderly and in hematopoietic stem cell transplant recipients (22).
The high incidence of reinfection by antigenically similar strains is generally taken as evidence that the immune response to RSV infection is deficient, but this important and complex issue is poorly understood (15). In the most extensively char-acterized example of RSV-mediated immune suppression, the viral nonstructural proteins 1 and 2 (NS1 and NS2) have been shown to inhibit the induction of alpha, beta, and lambda interferons (IFN-␣, -, and -) as well as signaling through the IFN-␣/ receptor (IFNAR) (10, 21, 44, 75, 76). Deletion of viral IFN antagonists can greatly reduce RSV growth in IFN-competent cells and in vivo, indicating their importance to viral replication (80). In addition, type I IFN generally might
en-hance innate and adaptive immune responses. Thus, a reduc-tion in IFN producreduc-tion and signaling may decrease the mag-nitude and quality of the adaptive response and decrease protection against reinfection. For example, with bovine RSV, deletion of the NS2 protein resulted in a virus that efficiently induced virus-neutralizing serum antibodies and virus-specific CD4⫹T lymphocytes despite a high level of attenuation, an effect thought to reflect increased immunogenicity (83). In mice, suppression of the type I IFN response by the human RSV NS2 protein was shown to inhibit the CD8⫹ T-lympho-cyte response (37). We note that the NS2 protein is the major IFN antagonist of RSV in the bovine and murine systems, whereas the NS1 protein plays the major role in humans, as shown in the present report.
In the present study, we investigated whether the RSV NS1 and NS2 proteins affect the maturation of immature myeloid DC during RSV infection. Immature DC reside in peripheral tissues and serve as sentinels that actively capture and process antigens. Following uptake of an antigen, DC undergo pro-found changes, called maturation, that greatly enhance their ability to present antigen in the context of major histocompat-ibility complex (MHC) type I and type II molecules to naive antigen-specific T cells. DC maturation is characterized by increased expression of MHC molecules and various accessory (costimulatory) molecules, which interact with their receptors on T cells. DC maturation is also accompanied by expression of various cytokines and chemokines that play important roles in attracting and activating T cells (reviewed in reference 5). Although plasmacytoid DC are known to be the major pro-ducers of type I IFNs in many tissues (12, 73), a recent study suggested that, during pulmonary viral infections, myeloid DC, * Corresponding author. Mailing address: Building 50, Room 6505,
NIAID, NIH, 50 South Dr., MSC 8007, Bethesda, MD 20892-8007. Phone: (301) 594-1854. Fax: (301) 496-8312. E-mail: AB176v@nih.gov.
䌤Published ahead of print on 18 June 2008.
8780
on November 8, 2019 by guest
http://jvi.asm.org/
along with macrophages, rather than plasmacytoid DC, are the major source of type I IFN (40). These data further support the interest in investigating the effects of NS1 and NS2 on myeloid DC. In the present study, we used human monocyte-derived DC, which are differentiated in vitro from peripheral blood monocytes. This is an appropriate source, since there is a growing appreciation that peripheral monocytes are an impor-tant progenitor of myeloid DC present in mucosal tissues such as the lung (84). Here, we report that the NS1 and NS2 pro-teins mediate suppression of DC maturation and the secretion of numerous cytokines and chemokines and that this effect depends, at least in part, on the suppression of type I IFN signaling. These data contribute to our understanding of the basic mechanisms of RSV immunopathogenesis, may help guide evaluation of vaccine candidates based on deletion of NS1 and NS2, and may help explain the high rate of RSV reinfections.
MATERIALS AND METHODS
Construction of recombinant viruses.The open reading frame (ORF) for enhanced green fluorescent protein (GFP; Clontech, Mountain View, CA) was PCR amplified to add a ClaI site and an RSV gene start signal (from the N gene) upstream of the ORF and an RSV gene end signal (from the G gene) and SalI site downstream of the ORF (Fig. 1). The ClaI-SalI fragment was cloned into a PstI-XhoI subclone (spanning from the P to SH gene) of the RSV antigenome cDNA in which the P-M intergenic region was previously mutated to contain ClaI and SalI sites. The resulting fragment, now including the GFP gene, was introduced into an AatII-PacI subclone (spanning from the upstream plasmid sequence to the SH gene) of previously described antigenomic cDNAs encoding wild-type (wt) RSV (16),⌬NS1 (80),⌬NS2 (79), and⌬NS1/2 (74). Finally, the AatII-XhoI fragment (spanning from the upstream plasmid sequence to the end of the SH gene) of each construct was used to replace the corresponding frag-ment in the full-length RSV cDNA plasmid D46/6120, in which silent mutations were introduced into the last three codons of the SH protein and the stop codon and 112 nucleotides of noncoding region of the SH gene downstream the ORF were removed in order to stabilize the plasmid for propagation in bacteria (11). Recombinant RSVs were recovered as previously described (16). All viruses are based on RSV strain A2.
Cells, virus growth, and purification.Vero cells were maintained in Opti-MEM I medium (Invitrogen, Gaithersburg, MD) supplemented with 5% heat-inactivated fetal bovine serum and 2 mML-glutamine (Invitrogen); for virus
propagation, the medium was further supplemented with 100 IU/ml penicillin and 100g/ml streptomycin sulfate (Invitrogen). Cells were infected at an input multiplicity of infection (MOI) of 0.5 (here and below, the MOI is indicated in PFU) and harvested by scraping when the cytopathic effect was extensive. The cells were collected by centrifugation at 200⫻gat 4°C for 10 min, subjected to freeze-thaw to release virus, and centrifuged again. The supernatants were lay-ered onto discontinuous 30 to 60% (wt/vol) sucrose gradients and subjected to centrifugation at 120,000⫻gat 4°C for 90 min. The virus-containing bands were collected and, in order to eliminate sucrose, were diluted with Advanced RPMI 1640 medium containing 2 mML-glutamine, pelleted by centrifugation at 8,000⫻g at 4°C for 2 h, and resuspended in the same medium. Virus aliquots were stored at⫺80°C, and the concentrations were determined by plaque assay as described previously (53). UV inactivation of wt RSV (UV-wt RSV) expressing GFP was performed by exposure to UV light in a Stratalinker UV cross-linker (Stratagene, La Jolla CA) using a total dose of 1,296 mJ, which was determined to be the minimum required for complete inactivation of the virus. The lack of residual infectivity was confirmed by plaque assay.
Isolation and culture of human DC.Elutriated monocytes obtained from healthy adult donors according to an approved clinical protocol were provided by the Blood Bank of the National Institutes of Health Clinical Center. CD14⫹ monocytes were purified by positive selection using anti-CD14 monoclonal an-tibody-coated magnetic microbeads as per the manufacturer’s instructions (Automacs; Miltenyi Biotech, Auburn, CA). The purity of the CD14⫹cells was determined to be greater than 98% by flow cytometry using anti-CD14–phyco-erythrin (PE) monoclonal antibodies (BD Biosciences, San Jose CA). Monocytes were cultured in 12-well plates at 6⫻105cells per well in Advanced RPMI medium 1640 (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 2 mML-glutamine (Invitrogen), 0.05 mM
-mer-captoethanol, 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Leukine; Immunex, Seattle, WA), 16 ng/ml interleukin-4 (IL-4; R&D Systems, Minneapolis, MN), 200 IU/ml penicillin, and 200g/ml streptomycin sulfate (Invitrogen). Cells were incubated for 7 days at 37°C, 5% CO2. Following incubation, as determined by flow cytometry, the cells had phenotypic char-acteristics of immature DC, namely, CD1a⫹CD14low
CD38low
CD11chigh (43, 62, 69).
Infection of DC.On day 7 of incubation with IL-4 and GM-CSF, the immature DC were harvested and their viability was assessed by trypan blue staining. Cells were seeded at 6⫻105
cells per well in 12-well plates and inoculated at an input MOI of 2 with the recombinant viruses (wt RSV, UV-wt RSV,⌬NS1,⌬NS2, and
⌬NS1/2) orEscherichia coliO55:B5 lipopolysaccharide (LPS) at 1g/ml (Sigma, St. Louis, MO). The cells were incubated at 37°C in a 5% CO2incubator. In experiments requiring blockade of IFNAR, DC were preincubated with 30g/ml of blocking antibody specific to IFNAR subunit 2 (IFNAR2; CD118; PBL Lab-oratories, Piscataway, NJ) or mouse immunoglobulin G2a (IgG2a; R&D Sys-tems), as an isotype control (the respective endotoxin levels in the two antibody preparations were below 1 and 0.1 endotoxin units per 1g of antibody), for 1 h before virus inoculation. In the experiments involving exogenous IFNs, recom-binant human IFN-␣2a or IFN-1a (PBL Biomedical Laboratories, Piscataway, NJ), referred to in the text as IFN-␣2 or IFN-, respectively, was added to the DC suspension at 24 h after infection at a final concentration of 1,000 IU/ml or 800 IU/ml, respectively, and after another 24 h, cells were analyzed by flow cytometry.
Flow cytometry analysis of DC.DC were analyzed for cell surface expression of various maturation markers by flow cytometry at 40 h postinfection. Briefly, most of the cells were collected by pipetting, and the remaining cells, which were attached to the bottom of the plates, were collected by applying staining buffer (phosphate-buffered saline containing 2% fetal bovine serum and 2 mM EDTA). Cells were pelleted by centrifugation at 200⫻gat 4°C for 5 min, the buffer was removed, and the pellet was resuspended in staining buffer. The cells were incubated for 20 min on ice in the dark with the monoclonal antibodies anti-CD80–PE (clone L307.4), anti-CD83–allophycocyanin (APC) (clone HB15e), anti-CD86–APC (clone FUN-1), anti-CD38–APC (clone HIT2), anti-CD54–PE (clone HA58), anti-HLA-ABC–APC (clone G46-2.6), anti-HLA-DR–APC (clone G46-6); IgG1-APC, IgG1-PE, and IgG2a-APC were the respective isotype control antibodies (all from BD Pharmingen, San Diego, CA). Following incu-bation, cells were washed three times with the staining buffer and resuspended in 200l of the same buffer. Propidium iodide was added to the cells just prior to data acquisition to enable discrimination between live and dead cells. Data were acquired on a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA) and analyzed with FlowJo software version 8.4.6 (Tree Star, Inc., Ashland, OR).
[image:2.585.43.279.72.155.2]Cytokine assay.Culture supernatant aliquots were collected at 6, 20, and 40 h postinoculation. The concentrations of IFN-␣and IFN-were determined by enzyme-linked immunosorbent assay (ELISA; PBL Laboratories and Invitrogen, FIG. 1. Insertion of a transcription cassette expressing enhanced
GFP between the P and M genes of recombinant wt RSV and NS1/NS2 gene deletion RSVs. The diagram shows (from left to right) the end of the P gene, followed by an intergenic region, followed by the inserted GFP transcription cassette, in which the GFP ORF was engineered to be flanked with a copy of the gene start (GS) transcription signal from the RSV N gene and a copy of the gene end (GE) signal from the RSV G gene, followed by an intergenic region, followed by the start of the M gene. The sequence is positive sense, translation initiation and termination codons are in bold, and the ClaI and SalI restriction endonuclease sites used in the construction are italicized. In the orig-inal biological RSV strain A2 isolate on which the recombinant system is based, the intergenic region between the P and M genes has the sequence GGAAAGGGT. All of the viruses used in subsequent ex-periments were these GFP-expressing recombinants.
on November 8, 2019 by guest
http://jvi.asm.org/
respectively) according to the manufacturer’s instructions. A Luminex multiplex bead assay (Linco-Millipore, St. Charles, MO) was used to measure the concen-trations of the following panel of 30 cytokines, chemokines, and soluble proteins: IL-1␣, IL-1, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, transforming growth factor␣(TGF-␣), IL-10, IL-13, IL-12/23 p40 (which is a common subunit in IL-12 and IL-23), IL-12p70, IL-15, IL-17, G-CSF, GM-CSF, tumor necrosis factor alpha (TNF-␣), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), IFN-␥, soluble CD40 ligand (sCD40L), IL-8/CXCL8, monocyte chemoattractant protein 1 (MCP-1/CCL2), macrophage inflammatory protein 1␣ and - (MIP-1␣/CCL3 and MIP-1/ CCL4), IFN-␥-inducible protein 10 (IP-10/CXCL10), regulated upon activation normal T-cell expressed and secreted (RANTES/CCL5), fractalkine/CX3CL1, and eotaxin/CCL11. In some experiments, the concentrations of TNF-␣, RANTES/ CCL5, MIP-1/CCL4, and IP-10/CXCL10 were also determined by ELISA (R&D Systems).
Apoptosis assays.Immature DC were infected with wt RSV, UV-wt RSV,
⌬NS1,⌬NS2,⌬NS1/2, or mock infected, and at 24 and 48 h postinfection, apoptosis was evaluated by (i) flow cytometric detection of cell membrane phos-pholipid phosphatidylserine, measured by annexin-V–APC (Invitrogen) binding, (ii) flow cytometric detection of the activated form of caspase-3 using active PE-labeled caspase-3 antibody (BD Pharmingen), and (iii) quantitative analysis of genome fragmentation using a cell death detection ELISA (Roche Applied Science, Indianapolis IN). As a positive apoptosis control, cells were treated with 5M staurosporine and incubated for 4 h at 37°C. For flow cytometric analysis with annexin-V and caspase-3 antibodies, dead cells, which could have died due to necrosis or apoptosis (and which did not differ markedly in abundance among the samples) were identified by staining with propidium iodide and fixable red dead cell stain (Invitrogen), respectively, and were excluded from the analysis.
Statistical analysis.The data were analyzed using a one-way analysis of vari-ance with Tukey’s multiple comparisons test as a post test using Prism-5 software (GraphPad Software, Inc., San Diego, CA). The data were considered statisti-cally significant at aPlevel ofⱕ0.05.
RESULTS
Construction of recombinant wt RSV and NS1 and/or NS2 gene deletion viruses expressing enhanced GFP. We previ-ously generated recombinant RSV in which the NS1 and NS2 genes were deleted individually and in combination (74, 79, 80). For the present study, these gene deletion mutants and their wild-type parent were modified to express enhanced GFP from a gene cassette placed between the P and M genes (Fig. 1). The G gene end signal was chosen for use in this cassette
because it is among the most efficient of these signals and would have the minimum impact on the viral transcriptional program (41); the gene start signals are more highly conserved, and that of N is typical. The position between the P and M genes was chosen because it placed the foreign insert well downstream of the NS1 and NS2 genes, avoiding direct effects on the expression of NS1 and NS2. The viruses were propa-gated in Vero cells, expression of GFP by infected cells was confirmed by fluorescence microscopy, and the sequence of each virus was confirmed in its entirety. All of the experiments in this study used these GFP-expressing viruses, and for sim-plicity the GFP annotation will be omitted from the virus names.
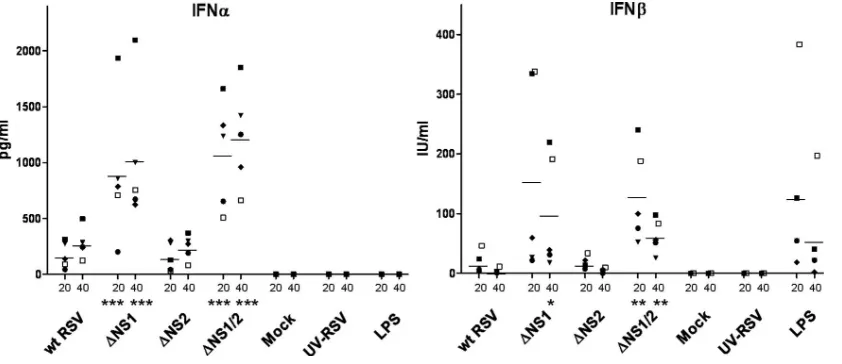
NS1 is a suppressor of the type I IFN response in human DC.To investigate whether NS1 and/or NS2 suppresses the production of type I IFN in DC, as they do in human epithelial cells and macrophages (75), we inoculated immature DC with wt RSV, the NS1/NS2 gene deletion mutants, or wt RSV that had been inactivated by UV irradiation. As controls, additional cells were mock treated or were treated with LPS. Cells from five donors were analyzed in separate experiments. The DC culture supernatants were sampled at 6, 20, and 40 h postin-oculation, and the concentrations of IFN-␣ and IFN- were determined by ELISA (Fig. 2; note that the 6-h time points are not shown because little or no IFN-␣and IFN-was detected in any of these samples). UV-RSV did not induce IFN-␣or - secretion. In DC infected with wt RSV, only a small induction of IFN-␣ and IFN-was observed, an increase that was not statistically significant. In contrast, infection of DC with the
[image:3.585.82.503.68.250.2]⌬NS1 or⌬NS1/2 virus resulted in increased induction of IFN-␣ and IFN-in each of the donors: for ⌬NS1 and⌬NS1/2, the average increase in IFN-␣ at 20 h postinfection was 5.5-fold and 8-fold, respectively, and for IFN- it was 36.3-fold and 39-fold, respectively, compared to wt RSV. Similar increases were observed at 40 h postinfection. In contrast, the increase observed with⌬NS2 was minimal and comparable to that in-duced by wt RSV. There was no strong correlation between the FIG. 2. Concentration of IFN-␣(in pg/ml) and IFN-(in IU/ml) in the medium of DC 20 and 40 h after inoculation with the indicated viruses at an input MOI of 2, or mock treatment, inoculation with an equivalent amount of wt RSV that had been inactivated with UV irradiation, or treatment with 1g/ml LPS. Immature DC from five donors were analyzed in separate experiments; the mean values are indicated by horizontal bars. Statistically significant differences compared to wt RSV are indicated:*,P⬍0.05;**,P⬍0.01;***,P⬍0.001. The various symbols represent individual donors, with the same symbol used in the two panels.
on November 8, 2019 by guest
http://jvi.asm.org/
level of production of IFN-␣and IFN-in DC from the same donor, although some donors had consistently high levels of production of both IFNs. These data indicate that, in human DC, the NS1, but not NS2 protein, is the major antagonist to the production of IFN-␣ and IFN-, which is similar to the effects of these proteins in human respiratory epithelial cells and macrophages (75, 76).
Infectivity of NS1/2 gene deletion viruses in DC.To inves-tigate possible effects of the NS1 and NS2 proteins and the differences in induction of type I IFNs on the ability of RSV to infect human DC, we inoculated immature DC derived from multiple donors in separate experiments with the panel of viruses at an input MOI of 2 and analyzed the expression of GFP 40 h later by fluorescence and conventional microscopy (Fig. 3A) and flow cytometry (Fig. 3B to D). Infection with any of the live viruses or stimulation with LPS resulted in DC aggregation starting at ⬃4 to 10 h postinfection (Fig. 3A, second row; stimulation with LPS is not shown). No changes in cell morphology or size due to infection or stimulation were evident by light microscopy (Fig. 3A, third row). However, we did observe an increase in forward scatter (indicative of an increase in size) for approximately half of the population of virus-infected or LPS-stimulated cells, but not mock-infected cells, by flow cytometry, presumably reflecting aggregation due to maturation. There was no difference in the expression of maturation markers between these populations (data not shown). Analysis of GFP expression by fluorescence micros-copy (Fig. 3A, top row) showed that a small fraction of cells was positive in cultures infected with each of the viruses. This was quantified by flow cytometry using the GFP marker, as shown by representative data from a single donor in Fig. 3B. The combined data (Fig. 3C) showed that approximately 2 to 4% of the cells inoculated with wt RSV were positive for GFP. Compared to wt RSV, the mean percentage of positive cells was reduced by 20% and 12% for⌬NS1 and⌬NS1/2, respec-tively, and was increased by 26% for ⌬NS2 (Fig. 3C). This modest reduction in percentages of GFP-positive cells for the viruses lacking the NS1 protein is consistent with the increased levels of the type I IFN in these cultures (Fig. 2). Infection with the UV-inactivated RSV did not result in any GFP-positive cells, as expected (not shown). In cultures infected with any virus, the GFP-positive cells exhibited a range in the magni-tude of GFP expression (Fig. 3B). This heterogeneity in ex-pression level, along with the low percentage of GFP-positive cells, presumably reflects variable susceptibility of individual cells to RSV due to a mechanism yet to be identified. In addition, flow cytometry analysis (Fig. 3B and D) demon-strated a difference in the mean GFP intensity of the GFP-positive cells for the various viruses, with the following grada-tion of intensity: wt RSV⬎ ⌬NS2⬎ ⌬NS1⬎ ⌬NS1/2. Previous studies indicated that deletion of the NS1 and NS2 genes did not have a significant impact on the kinetics and magnitude of viral gene expression, as measured in cells that do not produce type I IFN (32, 79). This suggests that the reduced expression associated with these deletions here was not due to altered viral machinery but rather might be due, at least in part, to the IFN-mediated antiviral state, including possible effects on pro-tein synthesis and mRNA stability (reviewed in reference 27). The NS1 and NS2 proteins suppress maturation of DC.We next investigated whether deletion of NS1 and/or NS2 affected
the ability of RSV to induce maturation of immature DC. Cells were inoculated with the panel of viruses at an input MOI of 2 or with the following controls: an equivalent amount of UV-inactivated wt RSV, mock treatment, or treatment with LPS. The cells were analyzed 40 h later by flow cytometry for surface expression of seven functionally diverse maturation markers: CD80, CD83, CD86, CD38, CD54, HLA-ABC, and HLA-DR. CD80 and CD86 (also known as B7.1 and B7.2 molecules) serve as ligands for T-cell costimulatory receptor CD28 in activated T cells, thereby contributing to their activation (61), or as ligands for cytotoxic T-lymphocyte antigen 4 (CTLA4) in nonactivated T cells, resulting in their silencing (48). CD83 (or HB15) (91) is one of the most important markers for fully mature DC (90; reviewed in reference 57) and functions as an enhancer of T-cell activation (39). Engagement with CD83 ligand induces prolonged expansion of CD8⫹T cells and pref-erential enrichment for antigen specificity (30). CD38 is an ectoenzyme that synthesizes ADP-ribosyl messengers involved in calcium signaling and also functions as a receptor that binds its counter-receptor, CD31. CD38 regulates DC activation, is functionally involved in the expression of CD83 and secretion of IL-12, plays an important role in DC chemotaxis and transendothelial migration, and contributes to the survival of mature DC (24). CD54, or intercellular adhesion molecule 1, is important in leukocyte adhesion, such as during endothelial migration and during contact between DC and T cells engaged in T-cell activation (70). HLA-ABC and HLA-DR are two respective representatives of the MHC class I and class II molecules that present the bound antigen to and activate CD8⫹and CD4⫹T cells, respectively, and are widely used for characterization of human DC maturation.
The levels of cell surface expression of the maturation mark-ers CD80, CD83, CD86, CD38, and CD54 as measured by flow cytometry are shown in Fig. 4. Figure 4A shows representative primary data for wt RSV,⌬NS1/2, and mock treatment; Fig. 4B summarizes data for the complete panel of viruses and treatments using cells derived from seven different donors (note that theyaxis in Fig. 4B indicates the level of expression relative to wt RSV as 100%). Compared to the mock-treated DC, inoculation with wt RSV resulted in a prominent upregu-lation of all of the maturation markers except CD83. Infection with UV-inactivated wt RSV did not increase the expression of any of the markers, indicating that virus replication is required to activate DC maturation. Some experiments were also per-formed with UV-inactivated⌬NS1 RSV, which also did not increase the expression of any marker (data not shown). Inter-estingly,⌬NS1/2 induced a statistically significant increase in expression of all of the maturation markers compared to wt RSV, and⌬NS1 induced a statistically significant increase in expression of CD80 and CD38, but not the other markers. This was the case even though, as already shown in Fig. 3D, the level of intracellular viral gene expression by⌬NS1/2 and⌬NS1 in the DC was significantly reduced compared to wt RSV, as assayed by GFP fluorescence. In contrast, infection with⌬NS2 RSV did not increase the expression of any of the five markers compared to wt RSV. This indicates that the higher expression of the maturation markers induced by⌬NS1/2 RSV was mostly due to the lack of expression of NS1 rather than NS2 and that NS2 enhanced the antimaturation effect of NS1 but had no significant effect on its own. Expression of MHC-I and MHC-II
on November 8, 2019 by guest
http://jvi.asm.org/
FIG. 3. Infectivity of wt RSV and the NS1/NS2 gene deletion mutants for immature DC, MOI of 2. A. Photomicrographs of DC from a single donor 40 h after infection with the indicated viruses at an input MOI of 2, or mock treated. First row, fluorescence microscopy captured at 200⫻ magnification; second and third rows, bright-field microscopy, captured at 200⫻ and 500⫻, respectively. Small green dots seen in the wt RSV-infected DC preparation likely represent autofluorescence of negative cells due to the high brightness of fluorescence of the GFP-positive cells (larger round spheres). DC infected with the UV-inactivated wt RSV looked essentially like the mock-infected cells and are not shown. B. Representative primary flow cytometry analysis of DC from a single donor 40 h after inoculation with the indicated viruses. C and D. Summary of flow cytometry analysis of DC from six donors (panel C) or five donors (panel D) 40 h after inoculation with the indicated viruses, with mean values indicated by horizontal bars. (C) Percentage of GFP-positive cells; (D) GMI of GFP fluorescence in the GFP-positive populations from panel C. Statistically significant differences compared to wt RSV are indicated:*,P⬍0.05;***,P⬍0.001. Individual donors are indicated with different symbols, and same donors/symbols were used in panels C and D.
on November 8, 2019 by guest
http://jvi.asm.org/
was evaluated in a separate set of experiments with DC derived from five different donors. At the given MOI (2), no significant upregulation of either of these two molecules due to infection with any virus was detected (data not shown). We also com-pared expression of the maturation markers in the GFP-posi-tive and -negaGFP-posi-tive populations, with GFP expression taken as evidence of robust viral infection. Interestingly, after infection with wt RSV, expression of maturation markers by GFP-neg-ative cells was only slightly lower than that by the GFP-positive cells. Moreover, the percent increase in the average geometric mean intensity (GMI) of CD80, CD83, CD86, CD38, and CD54 for ⌬NS1/2 RSV relative to wt RSV was similar in GFP-positive and -negative cells and was statistically
signifi-cant for all markers except CD86 in GFP-positive cells (Table 1). We also observed elevated expression of these markers in both GFP-positive and GFP-negative fractions of DC infected with⌬NS1 RSV, compared to wt RSV, but the percent in-crease was statistically significant only for CD80 expression in GFP-negative cells (Table 1). Taken together, these data sug-gest that the NS1 and NS2 proteins have a suppressive effect on DC maturation and that there was not a substantial difference between the GFP⫹and GFP⫺populations.
RSV NS1 protein suppresses the expression of a broad array of cytokines and chemokines by DC.DC maturation is char-acterized by upregulation of the expression of an array of cytokines and chemokines in addition to type I IFNs. To eval-FIG. 4. Cell surface expression of five maturation markers 40 h after inoculation of immature DC with the indicated viruses at an input MOI of 2, or an equivalent amount of UV-inactivated wt RSV, mock treatment, or treatment with 1g/ml LPS. A. Example of primary flow cytometry data for wt RSV and⌬NS1/2 in DC from one donor. B. GMI of expression of the five maturation markers in DC inoculated with the indicated viruses and controls, relative to wt RSV as 100%. Seven donors are represented by different symbols, with the same donors/symbols in each panel. Expression levels of CD80, CD83, and CD54 for LPS-treated DC were analyzed in the same experiments as the viruses; however, they are shown on a different scale, as GMI values were substantially higher for LPS than other treatments. Statistically significant differences compared to wt RSV are indicated:*,P⬍0.05;**,P⬍0.01;***,P⬍0.001.
on November 8, 2019 by guest
http://jvi.asm.org/
uate possible effects due to the NS1 and/or NS2 proteins, we inoculated immature DC with the panel of viruses or with the following controls: UV-inactivated wt RSV, mock treatment, or LPS treatment. Cell culture supernatants of cells from three representative donors were collected at 20 and 40 h postinocu-lation and assayed for a panel of 30 cytokines, chemokines, and soluble proteins using a bead-based Luminex assay, with con-firmation in several instances by ELISA (see Materials and Methods for the complete list).
Compared to the mock-treated control, inoculation with wt RSV induced an increased elevation in the levels of the cyto-kines TNF-␣and IL-6 (Fig. 5A) and IL-1␣(data not shown) and the chemokines IL-8/CXCL8, RANTES/CCL5, IP-10/ CXCL10, MIP-1␣/CCL3, and MIP-1/CCL4 (Fig. 5B) and MCP-1/CCL2 (data not shown). The cytokines IL-12/23 p40, IL-10, and TGF-␣ (Fig. 5A) and VEGF, G-CSF, GM-CSF, IL-4, and IL-7 (data not shown) were not induced or were marginally induced by wt RSV compared to the mock-infected cells. Other cytokines and chemokines represented in the 30-member panel (see Materials and Methods) were below the level of detection in all wt RSV samples (data not shown). UV-inactivated RSV induced little or no secretion of any of the cytokines and chemokines and was essentially the same as the mock treatment.
In contrast, infection with the ⌬NS1 and ⌬NS1/2 viruses resulted in an increase in the expression of all of the cytokines and chemokines mentioned above except for G-CSF, IL-7, and VEGF. For many cytokines and chemokines, the increase in expression due to the lack of the NS1 protein was dramatic: for example, at 40 h after infection with⌬NS1/2 RSV, the mean levels of TNF-␣and RANTES/CCL5 exceeded that of wt RSV by 5.8- and 13.8-fold, respectively (Fig. 5). However, infection with⌬NS2 RSV resulted either in a lack of increase or only a marginal increase of cytokine/chemokine expression compared to wt RSV. These data suggest that the antagonistic effect of the NS1 protein on the immune response of the host is not limited to the suppression of type I IFN production but extends to a broad range of cytokines and chemokines.
Blocking type I IFN signaling results in increased infection of DC.It was of interest to investigate possible effects of type I IFN signaling on the ability of the viruses to infect DC. We used a neutralizing monoclonal antibody against IFNAR2 to block type I IFN signaling, since the antiviral activities of type I IFNs correlates well with their binding activities to IFNAR2 rather than IFNAR1 (31). The IFNAR2-blocking antibody, or
an isotype control, was added to immature DC at a concen-tration of 30g/ml and incubated for 1 h at 37°C, followed by inoculation with the panel of viruses at an input MOI of 2 or mock infection. At 40 h postinfection, the percentages of GFP-positive cells and the mean fluorescent intensity were deter-mined by flow cytometry (Fig. 6). This showed that, for each of the viruses (wt RSV,⌬NS1,⌬NS2, and⌬NS1/2), the percent-age of GFP-positive cells increased 1.5- to 2-fold in DC that had been treated with the IFNAR2-blocking antibody com-pared to the isotype control. This increase was statistically significant for each virus except for⌬NS2, for which there was significant donor variability and for which the overall increase in the percentage was smaller. Interestingly, the mean fluores-cence intensity of the infected cells was increased⬃3-fold for wt RSV with the antibody blockade, while smaller increases that were not statistically significant were observed for the deletion mutants (Fig. 6). The increase in the number of GFP-positive cells and in the level of GFP expression within this population in response to the antibody blockade presumably reflects a reduction in the IFN-mediated antiviral state, as might be expected with reduced signaling through IFNAR. Following the IFNAR blockade, the intensity of GFP expres-sion remained greater for wt RSV than for the NS deletion mutants. Since only IFNAR, and not the IFNreceptor, was blocked in these experiments, it may be that there was a re-sidual antiviral effect due to IFNsignaling, which would be more efficiently inhibited by wt RSV than by the mutants lacking one or more of the NS proteins, resulting in greater GFP expression with wt RSV (21, 59).
[image:7.585.40.543.82.156.2]Suppression of DC maturation is associated with suppres-sion of type I IFN production.Since expression of the NS1 and, to a lesser degree, NS2 protein was associated with reduced expression of type I IFNs in DC and reduced activation of DC maturation, we next investigated whether these events are linked. Immature DC were treated with the IFNAR2-blocking antibody and inoculated with the panel of viruses or mock infected, as already described, and the surface expression of CD86, CD83, and CD38 was evaluated by flow cytometry 40 h later (Fig. 7A and B). We found that, in DC treated with the IFNAR2-specific antibody, expression of the markers of mat-uration was reduced compared to the cells treated with the isotype control antibody. We also analyzed GFP-positive and -negative populations for maturation marker expression. Inter-estingly, these data demonstrated a reduction in expression of maturation markers in response to the IFNAR blockade in TABLE 1. Expression of maturation markers in GFP-positive and -negative DC populations
Virus
% Change in avg GMI relative to wt RSVa
CD80 CD83 CD86 CD38 CD54
GFP⫹ GFP⫺ GFP⫹ GFP⫺ GFP⫹ GFP⫺ GFP⫹ GFP⫺ GFP⫹ GFP⫺
⌬NS1 11 15c 21 35 ⫺12 9 1 26 4 9
⌬NS2 ⫺10 2 ⫺1 10 ⫺9 ⫺2 53c 22 4 12
⌬NS1/2 29c 20c 67c 77b 21 36b 86c 63c 39c 39c
a
Values represent results from seven individual experiments using DC from different donors. For wt RSV, the average GMI values for the GFP⫹/GFP⫺populations were as follows: CD80, 59/41; CD83, 6/4; CD86, 61/34; CD38, 35/29; CD54, 440/320. Statistical analysis was performed using a one-way analysis of variance with Tukey’s multiple comparisons test.
b
Significantly different from wt RSV (P⬍0.01). c
Significantly different from wt RSV (P⬍0.001).
on November 8, 2019 by guest
http://jvi.asm.org/
FIG. 5. Concentrations of selected cytokines (A) and chemokines (B) in the medium 20 and 40 h after inoculation of immature DC with the indicated viruses at an input MOI of 2 or an equivalent amount of UV-inactivated wt RSV, mock treatment, or treatment with 1g/ml LPS. Three donors are represented by different symbols, with the same donors/symbols used in each panel. The DC supernatants were analyzed in a Luminex bead assay except in the case of IP-10/CXCL10, which was determined by ELISA. Note that the IL-12/23 p40 subunit is shared with IL-23, a cytokine that enhances IFN-␥production but mainly in memory rather than naı¨ve T cells.
on November 8, 2019 by guest
http://jvi.asm.org/
both the GFP-positive and -negative cells (Fig. 7C). Taken together, these results suggest that the increased maturation of DC due to the lack of NS1 protein is related, at least in part, to the increased production of type I IFNs. While robust in-fection of a fraction of the DC population was necessary for significant maturation of the total population, whether or not a given cell was robustly infected did not significantly affect its maturation.
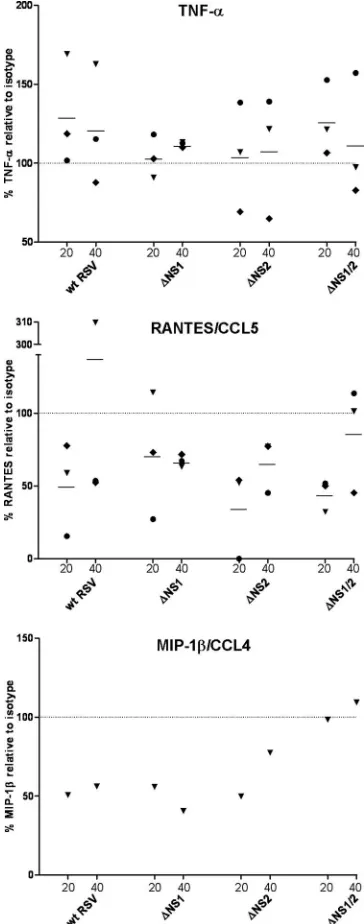
Suppression of chemokine expression is associated with suppression of type I IFN. The observed suppression of the expression of multiple cytokines and chemokines by the RSV NS1 protein might be caused by two distinct mechanisms. First, the suppressive effect of NS1 could be a consequence of a direct suppressive effect on the activation of transcription fac-tors that regulate gene expression of these cytokines and che-mokines. Second, the suppressive effect may be a result of suppression of type I IFN production and signaling, which directly or indirectly control transcription of multiple genes involved in inflammation, including cytokines and chemokines, and which may be required for maturation of DC (reviewed in reference 82). To distinguish between these possibilities, im-mature DC were treated with the IFNAR2-blocking antibody or isotype control antibody and were inoculated with the panel of viruses, as already described. At 40 h postinfection, ali-quots of culture supernatants were collected, and the concen-trations of the cytokine TNF-␣and chemokines RANTES/CCL5
and MIP-1/CCL4 were analyzed by ELISA (Fig. 8). DC from three individual donors were analyzed in three separate exper-iments. The blockade of IFN signaling did not appear to affect the levels of TNF-␣. In contrast, there was a marked reduction of RANTES/CCL5 expression for most of the donors, al-though the average values were not statistically significant due to a high variability between the samples from individual do-nors. The levels of MIP-1/CCL4 in DC from two of three donors were only slightly upregulated by wt RSV or any dele-tion mutant (data not shown), which was similar to the low-level secretion of MIP-1by DC from one donor, as shown in Fig. 5B, presumably reflecting a low responsiveness of these donors. For the third donor, with a substantial upregulation of MIP-1in response to the viruses, its level was markedly re-duced in response to IFNAR2 blockade (Fig. 8). These data suggest that at least in part, suppression of cytokine/chemokine expression by the NS1 protein is a consequence of the type I IFN suppression.
[image:9.585.137.448.67.343.2]Effect of exogenous IFN on the maturation of mock- and virus-infected DC.Next, we investigated the effects of exog-enously added IFN on mock-infected and virus-infected DC. Immature DC were mock inoculated, or inoculated with the panel of viruses, or treated with LPS. Twenty-four hours later, exogenous IFN-␣2 or IFN-was added to a final concentration of 1,000 IU/ml or 800 IU/ml, respectively. The cells were in-cubated for an additional 24 h, and the level of maturation was FIG. 6. Infectivity of wt RSV and the NS1/2 gene deletion mutants (input MOI of 2) in immature DC following treatment with the IFNAR2-blocking monoclonal antibody or an isotype control antibody, assayed by flow cytometry 40 h after virus inoculation. (A) Percent change in the number of GFP-expressing cells in the total population (left panel) and the GMI of GFP expression in the GFP-positive cells for IFNAR2-blocking antibody-treated DC from four donors, expressed relative to the isotype control as 100% (right panel). Individual donors are indicated with different symbols, and the same donors/symbols are used in the two panels. B. Example of primary flow cytometry data showing GFP expression in the GFP-positive subpopulation of DC, representing a single donor; cells were treated with a IFNAR2-blocking antibody or the isotype control antibody and infected with wt RSV or⌬NS1/2 RSV.
on November 8, 2019 by guest
http://jvi.asm.org/
FIG. 7. Effect of IFNAR2 blockade on the cell surface expression of maturation markers on DC in response to infection with wt RSV or the various gene deletion viruses. A. Expression of maturation markers CD86, CD83, and CD38 by DC, from three donors, in which IFNAR2 was blocked prior to infection. The values are expressed relative to the control cells that had been treated with the isotype control antibody prior to infection, which was assigned the value of 100%. The mean values are shown by horizontal bars, and statistical significance of each reduction compared to the corresponding isotype antibody control is shown by asterisks:*,P⬍0.05;**,P⬍0.01;***,P⬍0.001. Individual donors are indicated with different symbols, and the same donors/symbols are used in the three panels. B. Representative primary flow cytometry data showing expression of the maturation markers by DC. C. Expression CD38 by GFP-positive and GFP-negative fractions of DC, with primary flow cytometry data for wt RSV and⌬NS1/2 in GFP-positive and GFP-negative fractions of DC from one representative donor.
8789
on November 8, 2019 by guest
evaluated by analysis of CD83, CD86, and CD38 expression by flow cytometry (representative results for CD38 are shown in Fig. 9). We found that the addition of IFN-␣2 upregulated expression of CD38 in mock-infected DC by 161⫾82% and in DC treated with UV-inactivated RSV by 131 ⫾42% (mean percent increase based on DC from three donors⫾standard
error). Similarly, addition of IFN- increased expression of CD38 by 348% and 333% (mean percent increase based on DC from two donors), respectively. These increases were com-parable to those induced by wt RSV and ⌬NS2 but were approximately half those induced by⌬NS1 and⌬NS1/2. The addition of exogenous IFN to any of the virus-infected cultures or to the LPS-treated cultures did not augment the level of expression of CD38 (Fig. 9). Although exogenous IFN upregu-lated the expression of CD38, it had no effect on the expression of CD83 or CD86 in any of the cultures, including mock-inoculated and virus-infected cells, at least for these two spe-cies of IFN (not shown). These results are consistent with published studies showing that type I IFN enhances matura-tion of DC in response to exogenous stimuli but is not a potent inducer of DC maturation on its own (46) and that the pro-duction of IFN by DC in response to stimulation with LPS or poly(I-C) results in saturation of IFN-stimulated signaling and downregulation of IFNAR expression, making the cells refrac-tory to further stimulation with IFN (71).
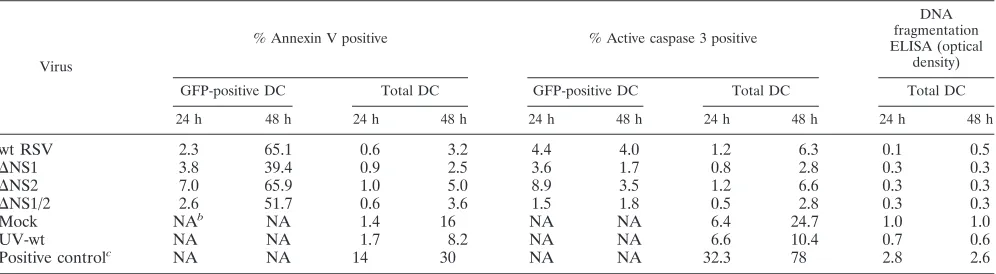
[image:11.585.71.253.70.532.2]NS1 and NS2 are not antiapoptotic in human DC. It was recently demonstrated that, in A549 human lung epithelial cells and primary human epithelial cells, the RSV NS1 and NS2 proteins suppress apoptosis at early (24 h) but not late (48 h) times postinfection (8). We confirmed this finding with the present set of viruses (data not shown). To evaluate possible effects of RSV NS1 and NS2 proteins on apoptosis in DC, we inoculated immature DC with the panel of viruses at an input MOI of 2 or with a mock treatment. As a positive control of apoptosis, we treated cells with staurosporine, an inhibitor of protein kinases (78) that is a powerful inducer of apoptosis (34). The level of apoptosis was evaluated at 24 and 48 h postinfection by three methods (Table 2): (i) flow cytometric analysis of cell membrane phospholipid phosphatidylserine, which is translocated from the inner to the outer leaflet of the plasma membrane during early stages of apoptosis (50, 60) and was detected by staining with annexin-V, which binds the phos-pholipid; (ii) flow cytometric analysis of the activated form of caspase-3, which is one of the major executioner caspases and is expressed as an inactive proenzyme that must be activated by cleavage by self-proteolysis or by upstream caspases (reviewed in reference 56); and (iii) quantitative analysis of cellular ge-nome fragmentation, which typically occurs during the later stages of apoptosis (1, 89), using an ELISA specific for cyto-plasmic histone-associated DNA fragments (mono- and oligo-nucleosomes). In the two assays involving flow cytometry (an-nexin V and activated caspase-3), both the total population and the GFP-positive subpopulation were analyzed; in the DNA fragmentation assay, only the total DC population could be analyzed (Table 2). By all three assays, the total populations of DC inoculated with wt RSV and the various mutants exhib-ited less apoptosis than the mock-treated or UV-inactivated virus-treated controls. The antiapoptotic effect of infection might be explained by the observation that RSV infectivity was necessary to induce maturation and that maturation is known to protect against apoptosis (47). On the level of the total cell population, there were no consistent differences in the amount of apoptosis among the various viruses. Analysis of the GFP-positive populations showed that⌬NS2 tended to be associated with a somewhat increased number of apoptotic cells com-pared to wt RSV at 24 h, but not 48 h. In any event, the level FIG. 8. Concentrations of the cytokine TNF-␣and the chemokines
RANTES/CCL5 and MIP-1/CCL4 in the medium from DC that were treated with the IFNAR2-blocking antibody, or an isotype control, prior to infection with the indicated viruses at an input MOI of 2. Medium samples were taken at 20 h and 40 h after virus inoculation and were analyzed by ELISA. The values for DC from three individual donors (TNF-␣and RANTES/CCL5) and one donor (MIP-1/CCL4) are shown and are expressed relative to the isotype control taken as 100%, with mean values shown as horizontal bars. Individual donors are indicated with different symbols, and the same donors/symbols are used in the three panels.
on November 8, 2019 by guest
http://jvi.asm.org/
of apoptosis associated with⌬NS2 was comparable to but not greater than that of the total mock-treated or UV-inactivated RSV-inoculated culture. Here, too, the level of apoptosis seemed to depend inversely on the level of DC maturation: apoptosis was greatest among the deletion mutants in the case of⌬NS2, where maturation was the least, and this increase was reversed when the NS2 deletion was combined with the mat-uration-promoting NS1 deletion.
DISCUSSION
Several viruses, including measles virus (35), Ebola virus (9, 49), herpes simplex virus (39, 58), cytomegalovirus (52), simian virus 5 (SV5) (2), and influenza virus (23), have been shown to induce DC maturation, at least to some extent, but also were shown to block full maturation. RSV has been demonstrated in previous work (18, 25) and in the present study to induce maturation of DC, at least to some extent, but RSV-matured FIG. 9. Effect of exogenously added IFN on DC maturation. Results show expression of CD38 by DC infected with the indicated viruses at an input MOI of 2, or treated with 1g/ml LPS, and then treated 24 h later with 1,000 IU/ml of IFN-␣2 (A) or 800 IU/ml of IFN-(B). Representative data are shown from one of three (A) or two (B) experiments performed with DC from different donors. GMI values of CD38 expression for IFN-nontreated and -treated DC are shown in each plot.
on November 8, 2019 by guest
http://jvi.asm.org/
DC have been reported to have a strongly impaired capability to activate CD4⫹T lymphocytes (18), implying that there is an RSV-specific suppression of maturation. We hypothesized that the RSV interferon antagonists NS1 and NS2 might be factors that impair DC maturation. Indeed, in the present study we found that deletion of the NS1 protein resulted in an increase in DC maturation, implying that the protein normally plays a suppressive role. The increase in maturation associated with the deletion of NS1 was augmented by the further deletion of NS2, although deletion of NS2 alone did not have a significant effect.
DC maturation was monitored by the cell surface expression of several biologically diverse markers of maturation and by assay for the production of an array of cytokines and chemo-kines. Among the maturation markers tested, CD83 and CD38 were the most dramatically upregulated by the lack of NS1 and NS2 (Fig. 4). Suppression of CD83 by the NS1/2 proteins may result in a weaker stimulation of T cells due to less effective costimulatory function, as well as reduced expansion of the antigen-specific CD8⫹cells (30). Suppression of CD38 may result in a reduced survival of mature DC and a reduced polarization of T cells toward Th1 (24). TNF-␣and RANTES/ CCL5 were among the cytokines and chemokines whose ex-pression was upregulated the most by the lack of NS1 and NS2. TNF-␣is a pleiotropic proinflammatory cytokine that has mul-tiple biological effects. In response to RSV infection, TNF-␣ also is produced by human alveolar macrophages (7) and pe-ripheral blood mononuclear cells (4). TNF-␣is an important mediator of RSV-induced lung pathology and plays an impor-tant role in RSV clearance (64). RANTES/CCL5 is a proin-flammatory CC chemokine which is strongly upregulated by RSV infection in various cell lines, including upper (65) and lower (54) airway epithelial cells and macrophages (17). In the mouse model, RANTES/CCL5 has also been shown to con-tribute to exacerbation of allergic airway inflammation by RSV (33) and plays a central role in lung inflammation during RSV infection (17). Expression of many of these cytokines and che-mokines, including TNF-␣, IL-12, IL-10, RANTES/CCL5, MIP-1␣/CCL3, and MIP-1/CCL4, is known to be induced by
type I IFNs (reference 81 and references therein). It also is noteworthy that deletion of NS1 resulted in a substantial in-crease in the expression of IL-12/23 p40 and IL-10. Expression of IL-12 or IL-10 by DC is associated with an enhanced acti-vation of Th1 or Th2 cells, respectively, and it is not clear what the effect would be of upregulating both of these antagonistic Th-polarizing cytokines.
We found that the suppression of the expression of DC surface maturation markers and cytokines associated with the NS1 and NS2 proteins depended, in large part, on antagonism of the type I IFN response. This was demonstrated by the ability of an IFNAR2-blocking monoclonal antibody to sub-stantially block the increase in maturation marker and cytokine expression associated with deletion of NS1 and NS2. IFNAR2 blockade also reduced the level of maturation induced by wt RSV, indicating that IFN contributed to maturation even in the presence of NS1 and NS2. The conclusion that type I IFN is important for DC maturation during wt RSV infection was supported by a recent study in vivo, which was published while the present manuscript was in preparation, showing that IFNAR signaling is required for wt RSV-induced expression of DC maturation markers and optimal cytokine/chemokine pro-duction in the mouse model (63). IFN-␣/have been described as strongly enhancing DC maturation in combination with var-ious other activation stimuli, such as ligation of toll-like recep-tors or costimulatory molecules or viral antigens (46, 66, 85; reviewed in reference 82). In contrast, on its own, IFN induces only partial maturation of DC, characterized by some upregu-lation of CD40, CD80, CD86, and MHC class I and II, but not CD83 (46). In our hands in the present study, IFN-␣2 and IFN-failed to induce upregulation of CD86 as well as CD83 in uninfected DC.
While secreted IFN clearly played a substantial role in DC maturation in RSV-inoculated cultures, it probably did not fully account for maturation. Although a substantial reduction in maturation marker expression was observed upon blocking IFNAR2 signaling, it did not completely block DC maturation (Fig. 7). This might reflect leakiness in the blockade, but it also suggests that other factors may play a role. This also is sug-TABLE 2. Markers of apoptosis in DC inoculated with wt,⌬NS1,⌬NS2, or⌬NS1/2 RSVa
Virus
% Annexin V positive % Active caspase 3 positive
DNA fragmentation ELISA (optical
density)
GFP-positive DC Total DC GFP-positive DC Total DC Total DC
24 h 48 h 24 h 48 h 24 h 48 h 24 h 48 h 24 h 48 h
wt RSV 2.3 65.1 0.6 3.2 4.4 4.0 1.2 6.3 0.1 0.5
⌬NS1 3.8 39.4 0.9 2.5 3.6 1.7 0.8 2.8 0.3 0.3
⌬NS2 7.0 65.9 1.0 5.0 8.9 3.5 1.2 6.6 0.3 0.3
⌬NS1/2 2.6 51.7 0.6 3.6 1.5 1.8 0.5 2.8 0.3 0.3
Mock NAb NA 1.4 16 NA NA 6.4 24.7 1.0 1.0
UV-wt NA NA 1.7 8.2 NA NA 6.6 10.4 0.7 0.6
Positive controlc NA NA 14 30 NA NA 32.3 78 2.8 2.6
a
Immature DC were inoculated with the indicated viruses at an input MOI of 2 PFU/cell, and percentages of annexin V-positive or caspase-3-positive cells were determined for both the total DC and GFP-positive DC populations at 24 and 48 h postinfection. The extent of DNA fragmentation was determined for the total population at 24 and 48 h postinfection by ELISA. Three donors are represented: each assay involved a different donor.
b
NA, not applicable, since no green fluorescent cells were detected in the samples. c
The positive control in the annexin V and activated caspase-3 assays was immature DC treated with 5M staurosporine; the positive control for the DNA fragmentation ELISA was provided with the kit.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:13.585.44.542.81.218.2]gested by the observation that CD83 and CD86 were not up-regulated significantly in response to IFN-␣2 and IFN- but were significantly upregulated in response to ⌬NS1/2. The magnitude of upregulation of CD38 induced by IFN alone was approximately half that induced by⌬NS1/2, tempting one to conclude that type I IFN might account for approximately half of the maturation induced by that mutant. However, one can-not reliably make this comparison, since the virus-inoculated cultures presumably produce multiple species of IFN as well as other potentially synergistic factors, such as TNF-␣, that con-found direct comparison. We had anticipated that activation of intracellular signaling pathways by intracellular viral RNA syn-thesis might be important in the activation of maturation, es-pecially in cells that were robustly infected and GFP positive. However, we were surprised to find that there was little differ-ence in the extent of maturation between GFP-positive and GFP-negative cells. We also were surprised to find that the antibody-mediated blockade of IFN signaling reduced the mat-uration of GFP-positive as well as GFP-negative cells. Thus, it appears that both the GFP-positive and -negative populations depended, in large part, on exogenous type I IFN for matura-tion (Fig. 7C).
The implication of a role for type I IFN in DC maturation in response to RSV infection differs from recently published data involving infection of human immature DC with SV5 or influ-enza A virus (2, 45). In those studies, deletion of the respective viral antagonist of the type I IFN response, namely, the NS1 protein of influenza A virus (which is unrelated to the NS1 protein of RSV) and the V protein of SV5, augmented the expression of DC maturation markers and cytokines, but this effect was not blocked by the inclusion of antibodies to IFN-␣ and -in the cell culture medium (2, 45). Thus, these previous studies concluded that, while the type I IFN signaling pathway was important in DC maturation in response to those viruses and was inhibited by the respective viral antagonists, secreted IFN-␣ and - were not necessary for DC maturation. The formal possibility exists that the IFN-␣/ blockade in these previous studies was incomplete due to incomplete neutraliza-tion. It also is possible that another IFN was involved, such as IFN-, a type I IFN that is expressed in leukocytes and binds to IFNAR (19), or IFN-, a family of several cytokines that signal type I IFN-like antiviral responses through a distinct receptor and which are strongly suppressed by the RSV NS1 (76) and might similarly be suppressed by the IFN antagonists of SV5 and influenza viruses. However, the likeliest interpre-tation is that maturation of DC in response to RSV indeed differs from the situation with SV5 and influenza virus in being substantially dependent on secreted IFN. The basis for this difference remains unknown: likely, it is not due to the low infectivity of RSV, since GFP-negative and -positive cells did not greatly differ in the extent of maturation, as already noted. The role of the NS1 and NS2 proteins in RSV immunobi-ology has received added interest because the current live attenuated RSV vaccine candidates include ones involving de-letion of NS2 in combination with a menu of attenuating mu-tations (88) or involving deletion of NS1 on its own (unpub-lished data); deletion of both may be overattenuating and is not presently being considered. As already mentioned (see the introduction), there is evidence from experimental animals suggesting that deletion of NS1 and/or NS2 provides for
en-hanced adaptive immune responses (37, 83). The finding that deletion of NS1 and NS2 provides for increased DC matura-tion would be consistent with this idea. However, the role of type I IFN in the development of immune effector mechanisms remains controversial. While some studies have demonstrated positive effects of type I IFN on T-cell activation, clonal ex-pansion, and memory formation (36, 77), other studies have reported a negative effect on the development of T-cell re-sponses (13, 51, 68). In our case, deletion of the NS1/2 inter-feron antagonist proteins resulted in a⬃50% increase in the expression of maturation markers (Fig. 4) and a greater (up to severalfold) increase in cytokine and chemokine secretion (Fig. 5). These effects are comparable with those described for SV5, where mutations disabling the viral IFN antagonist gene re-sulted in a⬃50 to 100% increase in expression of human DC maturation markers. In that case, the enhanced DC maturation was associated with two- to eightfold more effective prolifera-tion of autologous T cells (2). In our case, the potential effect of the upregulated expression of maturation markers, cyto-kines, and chemokines on T cells remains to be evaluated. In addition, the increased expression of proinflammatory cyto-kines associated with the NS1 deletion in the present study raises the possibility that an NS1-based vaccine might have increased reactogenicity compared to one based on the com-plete complement of RSV genes or based on an NS2 deletion. It will be important and interesting to monitor the responses to these candidate vaccines during clinical trials, although in in-fants and young children it is difficult to obtain the necessary clinical samples in sufficient quantity and frequency.
A recent study showed that silencing the expression of the RSV NS1 and NS2 proteins during RSV infection in epithelial cells resulted in enhanced apoptosis (8), a finding that we confirmed with the present set of NS deletion viruses (data not shown). This previous study showed that, during wt RSV in-fection, the expression of NS1 and NS2 activates the antiapop-totic phosphoinositol 3-kinase pathway and that silencing the expression of these proteins ablates this protective effect. How-ever, in the present study, inoculation of DC with wt RSV and each of the NS deletion mutants conferred protection against apoptosis compared to mock-treated and UV-inactivated RSV-inoculated cells. This difference between epithelial cells and DC may be due to the strong antiapoptotic effects of DC maturation. Activation of DC maturation by antigen is known to result in the induction of antiapoptotic proteins of the Bcl-2 family and to confer protection from apoptosis (47). More recently, the engagement of toll-like receptors 4 and 6 in SV5-infected DC was shown to promote maturation through
NF-B-dependent signaling and to confer protection from apop-tosis (3). RSV is known to engage toll-like receptor 4 (42) and induce antiapoptotic proteins of the Bcl-2 family (38), and infection induces NF-B signaling that is augmented by the NS2 protein through an unknown mechanism (76). When the GFP-positive fraction of RSV-inoculated DC was examined, there was some increase in the level of apoptosis associated with deletion of NS2, which might have been due to a possible loss of activation of the phosphoinositol 3-kinase pathway and/or the reduction in NF-B signaling associated with deletion of the NS2 protein. However, the observed level of apoptosis did not exceed that observed in mock-treated or UV-inactivated RSV-inoculated cultures. Furthermore, the
on November 8, 2019 by guest
http://jvi.asm.org/
increase in apoptosis associated with the NS2 gene deletion was reversed by combination with the NS1 deletion, indicating that the promaturation, antiapoptotic effect of the latter was dominant. These findings with RSV are reminiscent of studies with SV5, for which introduction of mutations that ablate the type I IFN antagonism of the V protein resulted in an in-creased apoptosis in A549 human lung epithelial cells, but not in human DC (2, 86, 87), suggesting that the balance between the pathways that determine apoptosis in these two types of cells is different.
Several mechanisms of immune evasion have been identified for RSV. They include, but are not limited to, antagonism of type I IFN induction and signaling (10, 75, 76), suppression of the innate response by the cysteine-rich region of the G protein (28), interference with normal macrophage and DC functions (6, 18, 55, 67), sequence diversity in the G protein, one of the two major protective antigens (15), and tropism for the super-ficial cells of the respiratory lumen, where the efficiency of the immune defense is reduced. The data presented here provide evidence that the suppression of type I IFN mediated by the RSV NS proteins suppresses maturation of DC and may be a factor in suppressing the adaptive immune response and pro-viding for reinfection throughout life.
ACKNOWLEDGMENTS
We thank Ronald Rabin and Brian Murphy for reviewing the manu-script and for useful suggestions. We also thank Christine Winter for sequencing of the viral constructs, Lijuan Yang for ELISAs, and David Stephany for help with flow cytometry.
This study was funded as a part of the Intramural Research Program of NIAID, NIH.
REFERENCES
1.Arends, M. J., R. G. Morris, and A. H. Wyllie.1990. Apoptosis. The role of the endonuclease. Am. J. Pathol.136:593–608.
2.Arimilli, S., M. A. Alexander-Miller, and G. D. Parks.2006. A simian virus 5 (SV5) P/V mutant is less cytopathic than wild-type SV5 in human dendritic cells and is a more effective activator of dendritic cell maturation and func-tion. J. Virol.80:3416–3427.
3.Arimilli, S., J. B. Johnson, M. A. Alexander-Miller, and G. D. Parks.2007. TLR-4 and -6 agonists reverse apoptosis and promote maturation of simian virus 5-infected human dendritic cells through NFkB-dependent pathways. Virology365:144–156.
4.Arnold, R., B. Konig, H. Galatti, H. Werchau, and W. Konig.1995. Cytokine (IL-8, IL-6, TNF-alpha) and soluble TNF receptor-I release from human peripheral blood mononuclear cells after respiratory syncytial virus infection. Immunology85:364–372.
5.Banchereau, J., and R. M. Steinman.1998. Dendritic cells and the control of immunity. Nature392:245–252.
6.Bartz, H., O. Turkel, S. Hoffjan, T. Rothoeft, A. Gonschorek, and U. Schauer.2003. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology
109:49–57.
7.Becker, S., J. Quay, and J. Soukup.1991. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J. Immunol.147:4307–4312.
8.Bitko, V., O. Shulyayeva, B. Mazumder, A. Musiyenko, M. Ramaswamy, D. C. Look, and S. Barik.2007. Nonstructural proteins of respiratory syn-cytial virus suppress premature apoptosis by an NF-B-dependent, interfer-on-independent mechanism and facilitate virus growth. J. Virol.81:1786– 1795.
9.Bosio, C. M., M. J. Aman, C. Grogan, R. Hogan, G. Ruthel, D. Negley, M. Mohamadzadeh, S. Bavari, and A. Schmaljohn.2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis.188:1630–1638. 10.Bossert, B., S. Marozin, and K. K. Conzelmann.2003. Nonstructural
pro-teins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol.77:8661–8668.
11.Bukreyev, A., I. M. Belyakov, J. A. Berzofsky, B. R. Murphy, and P. L. Collins.2001. Granulocyte-macrophage colony-stimulating factor expressed by recombinant respiratory syncytial virus attenuates viral replication and
increases the level of pulmonary antigen-presenting cells. J. Virol.75:12128– 12140.
12.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanza-vecchia, and M. Colonna.1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med.
5:919–923.
13.Chi, B., H. L. Dickensheets, K. M. Spann, M. A. Alston, C. Luongo, L. Dumoutier, J. Huang, J. C. Renauld, S. V. Kotenko, M. Roederer, J. A. Beeler, R. P. Donnelly, P. L. Collins, and R. L. Rabin.2006. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J. Virol.80:5032–5040.
14.Collins, P. L., and J. E. J. Crowe.2007. Respiratory syncytial virus and metapneumovirus, p. 1601–1646.InD. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. 15.Collins, P. L., and B. S. Graham.2008. Viral and host factors in human
respiratory syncytial virus pathogenesis. J. Virol.82:2040–2055.
16.Collins, P. L., M. G. Hill, E. Camargo, H. Grosfeld, R. M. Chanock, and B. R. Murphy.1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5⬘proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. USA92:11563–11567.
17.Culley, F. J., A. M. Pennycook, J. S. Tregoning, J. S. Dodd, G. Walzl, T. N. Wells, T. Hussell, and P. J. Openshaw.2006. Role of CCL5 (RANTES) in viral lung disease. J. Virol.80:8151–8157.
18.de Graaff, P. M., E. C. de Jong, T. M. van Capel, M. E. van Dijk, P. J. Roholl, J. Boes, W. Luytjes, J. L. Kimpen, and G. M. van Bleek.2005. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J. Immunol.175:5904–5911.
19.de Weerd, N. A., S. A. Samarajiwa, and P. J. Hertzog.2007. Type I interferon receptors: biochemistry and biological functions. J. Biol. Chem.282:20053– 20057.
20.Dowell, S. F., L. J. Anderson, H. E. Gary, Jr., D. D. Erdman, J. F. Plouffe, T. M. File, Jr., B. J. Marston, and R. F. Breiman.1996. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infec-tion among hospitalized adults. J. Infect. Dis.174:456–462.
21.Elliott, J., O. T. Lynch, Y. Suessmuth, P. Qian, C. R. Boyd, J. F. Burrows, R. Buick, N. J. Stevenson, O. Touzelet, M. Gadina, U. F. Power, and J. A. Johnston.2007. Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J. Virol.81:3428–3436.
22.Falsey, A. R., P. A. Hennessey, M. A. Formica, C. Cox, and E. E. Walsh.2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med.352:1749–1759.
23.Fernandez-Sesma, A., S. Marukian, B. J. Ebersole, D. Kaminski, M. S. Park, T. Yuen, S. C. Sealfon, A. Garcia-Sastre, and T. M. Moran.2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol.
80:6295–6304.
24.Frasca, L., G. Fedele, S. Deaglio, C. Capuano, R. Palazzo, T. Vaisitti, F. Malavasi, and C. M. Ausiello.2006. CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood107:2392– 2399.
25.Guerrero-Plata, A., A. Casola, G. Suarez, X. Yu, L. Spetch, M. E. Peeples, and R. P. Garofalo.2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol.34:320–329.
26.Hall, C. B., E. E. Walsh, C. E. Long, and K. C. Schnabel.1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis.
163:693–698.
27.Haller, O., G. Kochs, and F. Weber.2007. Interferon, Mx, and viral coun-termeasures. Cytokine Growth Factor Rev.18:425–433.
28.Harcourt, J., R. Alvarez, L. P. Jones, C. Henderson, L. J. Anderson, and R. A. Tripp.2006. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1⫹T cell responses. J. Immunol.176:1600– 1608.
29.Henderson, F. W., A. M. Collier, W. A. Clyde, Jr., and F. W. Denny.1979. Respiratory-syncytial-virus infections, reinfections and immunity. A prospec-tive, longitudinal study in young children. N. Engl. J. Med.300:530–534. 30.Hirano, N., M. O. Butler, Z. Xia, S. Ansen, M. S. von Bergwelt-Baildon, D.
Neuberg, G. J. Freeman, and L. M. Nadler.2006. Engagement of CD83 ligand induces prolonged expansion of CD8⫹T cells and preferential en-richment for antigen specificity. Blood107:1528–1536.
31.Jaks, E., M. Gavutis, G. Uze, J. Martal, and J. Piehler.2007. Differential receptor subunit affinities of type I interferons govern differential signal activation. J. Mol. Biol.366:525–539.
32.Jin, H., H. Zhou, X. Cheng, R. Tang, M. Munoz, and N. Nguyen.2000. Recombinant respiratory syncytial viruses with deletions in the NS1, NS2, SH, and M2-2 genes are attenuated in vitro and in vivo. Virology273:210– 218.
33.John, A. E., A. A. Berlin, and N. W. Lukacs.2003. Respiratory syncytial virus-induced CCL5/RANTES contributes to exacerbation of allergic airway inflammation. Eur. J. Immunol.33:1677–1685.