MICHAELL.PARKERt AND FREDERICKA.EISERLING*
Departmentof Microbiology and Molecular BiologyInstitute, UniversityofCalifornia, LosAngeles,
California90024
Received 21 December1981/Accepted16December 1982
Bacteriophage SPO1, a structually complex phage with hydroxymethyl uracil
replacing thymine, hasbeenstudiedby structural andchemical methods with the aim of defining the virion organization. The contractile tail of SPO1 consists of a
complex baseplate, a tail tube, and a 140-nm-long sheathcomposed of stacked disks(4.1 nm repeat), each containing six subunits of molecular weight 60,300.
The subunits arearranged in six parallel helices, eachwith ahelicalscrew angle
(no)
of 22.50. The baseplate was shown to undergo a structural rearrangementduring tail contraction into ahexameric pinwheel. A mutation in gene 8 which produced unattachedheadsand tails alsoproducedtails of different lengths. The
tail length distribution suggests that the smallest integral length increment is a
single disk of subunits. The structural arrangement of subunits in long tails is
identicaltothatof normal tails, and the tails can contract.Many ofthelong tails showed partial stain penetration within the tail tube to apoint which coincides
withthe top of aunit-lengthtail.Theimplications of these findings with respect to
taillengthregulation arediscussed.
Bacteriophage SPOl ofBacillus subtilis, first
described by S. Okubo, has been extensively studied, with emphasis onits genetics and
con-trol ofgene
expression
(26, 27). The reviewby
Hemphill and Whiteley (14) also summarizes much of this work. Ourinterest inthestructureandassembly ofSPO1 is basedonthe
compari-son of this phage to other well-studied
phages
such as T4, X, and P22, with the view thatsimilarities will
permit generalizations
and thatdifferences mayrevealnew
principles.
Totheseends,wedescribeherethestructureof the main
componentsof the
phage
anddiscuss thediscov-eryofataillength
regulation
defectinmutantsincistron8 and the
implications
of thisfinding
for models of tailassembly.MATERIALS AND METHODS
Bacterial strains. B. subtilis 168M (indole-negative) wasthe nonsuppressor (su) host forgrowth ofSPOl wild-typephage and for defective mutant lysates. The
suppressor-containing strain HA101-B (his met) su+
leu was used for growth of suppressible mutants of SPO1 (25). When needed, spores were prepared as described below.
Bacteriophage. SPO1 wild-type phage and mutants 046-0 and 056 (26) were kindly provided by E. P. Geiduschek. All stocks werestored in Tris
stabiliza-tPresentaddress: DepartmentofGenetics, Universityof Washington, Seattle,WA98195.
tion buffer(see below). PhagesT4D and thefiberless mutant X4E were from the laboratory collection.
Culturemedia andbuffers.Medium CHT and Spizi-zen's saltsaredescribed in Gage and Geiduschek (12). One-half CHT contains half the amount of casein hydrolysate per liter. Medium MML contains (per liter) 5 g of glucose, 40 mg of Difco nutrient broth, 50 mg of L-leucine,and 5 x 10-4M CaC12 inSpizizen's salts. CHT or MML agar contains 15 g of Difco agar perliter; top agar is 0.9 g of Difco agar in 100 ml of
Spizizen's salts, and 1Ox NY contains 8 g ofDifco
nutrient broth and5g of Difco yeastextract in 100 ml ofwater. Sporulation medium was that of Hansen et al. (13).
Tris stabilization buffer (E. P. Geiduschek, personal communication) contains0.1 MTris-chloride (pH 7.5), 0.5 MNaCl,and 5mMMgSO4.Tris-magnesium buffer isstabilization bufferminus theNaCl.
Indicator bacteria. Spores are a useful source of stable plating bacteria. Cells of 168M were removed from an agar surface and inoculated into 500 ml of sporulation medium ina2,800-mlFernbach flask and incubated for 3 to 4 days at 37°C on a reciprocal shaker. The spores were purified by the method of Spudich and Kornberg(31) andwerekeptindistilled water at40Cat aconcentration ofabout 3x 109to5x
109 spores per ml. Spores (3 x 108)wereaddedtothe soft agar before plating. When exponential indicator was used, a saturated overnight culture ofHA101-B (14 to 18 h at 37°C) in MML was diluted 1:100into freshMMLandshaken at37°Cuntilanabsorbanceat 500 nm of between 0.7 and 1.0 was reached. Four drops of cellsuspensionwereused persoft agar tube. Preparation of phage stocks. Large quantities of SPO1wereprepared bysuspendingasingle plaque in 239
on November 10, 2019 by guest
http://jvi.asm.org/
20 mlof CHT or MML, to which5mlof exponential-phase bacteria at 8 x 107/ml was added. The culture was shaken at 37°C until lysis was complete. This lysate was added to1liter of cells in thesamemedium at8 x107/ml andwasincubatedon ashakerat37°Cfor 5min; then, 50mlof 1Ox NY mediumwasadded, and the culture wasfurther incubated with shaking until lysis was complete. The phage were harvested by differential centrifugation andweresuspended in Tris stabilization buffer. Phage suspensions were freed from cell debris by using CsCl step gradients (see below).
Preparation of lysates.Asaturatedovernight culture of 168M (14to18 hat37°C) in CHTwasdiluted1:100 into 1/2 CHT and was grownto adensity of 2x108cells perml. Cellswerecentrifugedat4,000xgfor 6 minat room temperature and were suspended in one-sixth theoriginal volume of fresh /2 CHT. The concentrated cellswereincubatedon ashakerat37°C for 8 min and thenwereinfectedat aninput ratio of5 to 7phage per bacterium. The bacteria were either allowed to lyse spontaneously or were lysed 65 to 140 min after infection by the addition of KCN to a 5 mM final concentration (or sodium azide to 10 mM), DNase (Sigma DNase I, DN-100) to a final concentration of 10 ,ug/ml, followed5minlater byfreshly prepared Wor-thington lysozyme (code: LY71A)to afinal concentra-tion of 100 ,ug/ml. The lysate was centrifuged at low speed to removecell-size aggregates and was stored untreatedorfixed for electron microscopy (EM) for1 h witha0.05% (vol/vol) final concentration of glutar-aldehyde (Fisher Scientific biological grade, 50% [wt/wt], G-151).
Gradientcentrifugation ofphage components. CsCl stepgradients were used topurify intact phage. The gradient consisted of six 0.5-ml steps of 70 to 20% dilutions(vol/vol)of room temperature-saturated CsCl inTris-magnesium buffer (pH 7.5). Centrifugationwas for17minat30,000 rpm,20°C, inanSW50.1 rotor.
Purification of phage parts was done in two steps. The first purification separated cell membrane, pro-tein, and protein-nucleic acid complexes from one another.Samples (0.1to 0.5ml)werelayered on top of preformed linear CsCl gradients of density range 1.20
to1.65 g/cm3inTris-magnesium buffer (pH 7.5). The
gradients wereformed in SW41 centrifuge tubes, and sampleswerelayeredontop andcentrifugedat30,000 rpmfor4h at20°C. Bands visible bylightscattering werecollected onafractionating device developed by Coombs (7). Fractions were dialyzed against Tris-magnesiumbufferplus3%sucrose(wt/vol)and 10 mM azideatroomtemperature.
Fractionsof interestwerelayeredonlinear5to45% sucrose gradients prepared in Tris-magnesium buffer plus2.5 MNaCl. Samples upto 0.5mlwerelayeredon the sucrose gradients in SW41 centrifuge tubes and were centrifuged at 36,000 rpm for 20 min at 20°C. Bands visible by light scattering were collected and
prepared directly for EM or dialyzed against Tris stabilization buffer plus 10mMazide.
PreparationofSPOlcontracted tails.SPOl contract-ed tails were preparcontract-ed by the addition of5MNaClO4 and 0.005 M EDTA (final concentrations). Thesample wasimmediately dialyzed againststabilization buffer. Contracted tails were also prepared by adjusting a purified phage suspension (at 1011 PFU/ml) to pH 3.5 with glacial acetic acid. The pH was then rapidly returned to 6.0 with 6 N NaOH addeddropwise with vigorous stirring. DNase was added before treatment to digest ejected DNA, and the sample was then prepared formicroscopy.
Optical diffraction. Optical diffraction was per-formed on a folded diffractometer constructed by Baker (Ph.D. thesis, University of California, Los Angeles, 1976) whichwassimilartotheonedescribed by Aebi et al. (2). Optical diffractograms of micro-graphs of extended tailswereprepared and indexed as described by Smith et al. (30). The T4 diffraction pattern,aswellasthree-dimensionalreconstructions, has beenpreviously described (3, 30, 34).
Electronmicroscopy. The grids used for specimen preparation were covered with carbon-coated Parlo-dion films, made hydrophilic by glow discharge ion bombardmentorbytreatmentwithonedrop of 0.1% cytochromecimmediately after specimen adsorption. Onedrop of thespecimenwasallowedtoadsorbtothe grid, whichwasthenrinsed with10drops ofwaterand negatively contrasted with two drops of 1% uranyl acetate, pH 3.1 to 4.5. Metal shadowed specimens wereprepared by firstadsorbingavirussuspensionto agrid and thenwashingit withseveraldrops ofwater andallowing it to airdry. Thegrids were shadowed with platinum at an angle of about 300 ina vacuum evaporator. Micrographs oflysatesandgradientbands were recorded with a JEOL 100-B electron micro-scope equipped with an anticontamination device, operated at 80 kV, using aminimum electron beam exposure technique similar to that ofWilliams and Fisher (36).
RESULTS
Structure of extended tails. The tail ofSPO1
has a stacked-disk structure which measures
140.3 (±2.1)nmlong and 18.6 (±0.4) nm wide,
with about 33 to 36 annuli. The exact number
wasdifficulttocountbecausethe basaltail-plate
structure [24.7 (±4.3) nmlong, 59.3 (±3.7) nm
wide] obscured the distal end of the sheath.
Optical diffraction analysis of electron
micro-graphswas done on bothnegatively contrasted T4D wild-type and SPO1 in the same field.
Figure 1 shows electron micrographs ofa tail
fiberless (X4E) T4 phage and an SPOl phage
with theirrespective opticaldiffractionpatterns.
FIG. 1. Optical diffraction ofT4andSPO1 phage tails. Ontheleftareelectronmicrographsof a T4phage (top) andanSPO1 phage (bottom), with their respective optical diffraction patterns.Boxesmarkportions of tails optically diffracted. TheSPO1 optical diffractogram hasbeen indexed forthehelicalfamily generated bythe helicalscrewanglefl=22.51°.The(6,0)spot isonlayer linethree,atlatitude K. The annularrepeat distancein reciprocal space (1/p) equals (1/4.1nm). The spotsareindexed(n, m),where nisthe orderof the Bessel function generating the particular spotand mis the "branch"onwhich it lies(see reference30).
on November 10, 2019 by guest
http://jvi.asm.org/
,-
_..
_fAlm _
_S
_a
a
_ _W
a
e1w a=
20.
I
'.1
S4~
_m
Om-...
_p,
_ii_|_
lo__
_i. -4*1
_-_ x, _
4
qbe_mb
-0EW
100nm
Z M=1
R
_ -
-em
r
0400
on November 10, 2019 by guest
http://jvi.asm.org/
242 PARKER AND EISERLING
5
--100nm
(4.1
.7-FIG. 2. The "coarse" helices of SPO1 wild-type phage tails. Upper: SPO1 negatively contrasted with 1% uranyl acetate, and its tail diffraction pattern. Lower: SPOl shadowed with platinumatanangleof
approximately 30°. Coarse helices are seen in both
micrographs by viewing at a glancing angle in the direction of the arrows. The helicesareright-handed
andgive risetothestrong(-6,0), (6,0) pair ofspots.
Theannularrepeatdistance(p) of T4 and SPOl
phage tails was compared by measuring the
altitudeof the firstmeridionalreflection, (0,1)or
(0, -1), from the origin. TheT4 annularrepeat
(12 measurements)wasnormalizedto4.1nm(6,
23). The SPOl annular repeat (24
measure-ments) was determined in the same way and
found to be 4.068 (±0.028 standard deviation)
nm. The Studentttestwasusedtocomparethe
two annular repeats; no significant difference
wasfound. The helicalscrewangle(fQ) for SPOI
was -97.49°ascompared with the publishedT4
value of between -102.26 and -102.72° (30).
The longpitchhelicalgroovesin the sheathare
right-handed,as seen in metal shadowed
prepa-rations (Fig. 2), again thesame asforT4(30).
Viewing anSPOl tailat aglancingangle (see
arrow in Fig. 2), a set of parallel lines appears
which representtheso-calledcoarsehelices(4). Innegatively contrasted specimens, the micro-graph isaprojection of informatin from bothtop
and bottom sidesof the particle (withrespect to theelectronbeam). Hence, thesecoarse helices
canbeseen onboth sides oftheparticle (Fig.2).
Asdescribed by Smithetal.(30),"helicalfamily (II)" corresponds to these coarse helices. The
diffraction maxima (6,0) and (-6,0)represent a
sixfoldcontributiontothis helical family.There are twosuchpairs, eachrepresentingthe contri-bution from the front or the back side. When
contrastisapplied by metalevaporation ("metal
shadow") toSPOlphage,theresulting particles show only one-sidedimages. Figure 2also
com-pares the optical diffraction pattern ofa
nega-tively contrasted and a metal-shadowed phage.
The coarse helices of the tail are visible in the
electron micrograph, and diffraction ofthat
mi-crograph shows only two maxima, namely, the
(-6,0)
and the (6,0). The helices are right-handed with ascrewangle (fl0) of about220.
Mutant tail structure.Thesuppressiblemutant
of SPOl in gene 8(046-0[26]),whengrown on a
restrictive host, accumulates tails of different lengths as well as unattached full heads. Tails isolated by centrifugation on sucrose gradients and prepared forEM are shown in Fig. 3. The
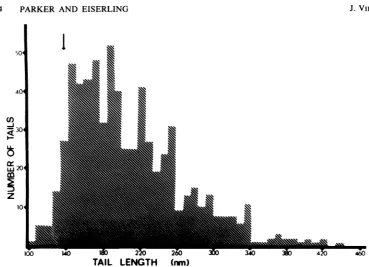
tail lengths vary from normal to three to four times normal. Figure 4 shows the tail length distribution formutant046-0. Thedistribution is skewed toward unit length, and there are no
apparent integral unit-length increases larger
than asingle ring of subunits.Wealsoobserved
long-tailed intact phageatverylowfrequency in preparations of
glutaraldehyde-fixed
lysates (Fig. 3). These particles were unstable withoutglutaraldehyde fixation; hence, their infectivity
was unmeasurable.
Measurementof
diffractograms
of 13 long tailsofSPOlgave an annularspacingof 4.1nmanda
fl of -97.46°. The helical screw angle wasthe
same asthatofthe wildtype(Fig. 5). Whenlong
tails wereisolatedon sucrosegradients
contain-ing low salt or were subjected to lowpH, they
contracted, and the tail tubesometimes
protrud-ed from below the basal structure or above the
topofthesheath. Thisindicatedthat in someof
these mutant tails, the tube was not firmly
attached to the neck region. Optical diffraction
of 046-0 contracted tails yielded an axialrepeat
of 1.7 nm and a f of
32.20.
We note that many long tails, both free and
attached to heads, show stain penetration into
thetail tube. Thispenetrationends 140nmfrom
" 1:
..::,zv'i,.,
V4.'.
.1. .,3f
.A/
i
'a q,.
I .e
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.490.47.241.80.453.2]a
b
At
C
.
d
E
f
... ts t~~~~~~~~*
., - .~~~~~~~~:
r
FIG. 3. Electronmicrographs of SPOlwild-typeandmutant046-0structures.(a)and(m)Wild-type SPOt. (b) Wild-type SPOt showing contracted tail. (c) Wild-type tailas isolatedfrom awild-typelysate. Note the absence ofa connector.(d) through (g)Wild-type"necked"tails isolatedbyCsCl shockprocedure.(h)through
(1)
046-0 tails isolated by sucrose gradient sedimentation from mutant infected restrictive host. (n) through (r)
Glutaraldehyde-fixed046-0mutantphage isolatedon aCsClequilibriumgradient.Barin(a)represents 100nm.
..,
i.-N . -. ..w---
N?
N.VW
1
..r
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.490.56.443.70.610.2]EISERLING
50
40'
Cl)
-j
-304
fr-wLj204
10
l
[image:6.490.62.431.53.320.2]TAIL LENGTH (nm)
FIG. 4. Tail lengthdistribution of 046-0mutanttails.
thebaseplate,atthepositioncorrespondingtoa
unit-length tail (Fig. 3i, j,1,q,r). Five tailswhich
showed stain penetration in the core of the
upperpartofthe tailwerediffractedtwice:once
near thebaseplateand once near thetopof the
tail (see reference 33). The Student t test
indi-catednodifference between thescrewanglesof the upper and lower portions of the tails. The
average helical screw angle (fl0) ofsus 046-0
longtails is (95% confidence)22.54 ± 0.15°.
Long tails ofphage T4 have also been
pro-ducedinvitroby Tschoppand Smith(33).These
long tails were formed spontaneously in a
con-centrated suspension of normal-length tails by thebreakdown ofsome structures andthe sub-sequent reassembly of the subunits of both sheath and tail tubeontounit-length structures. Structure of contracted sheath. Aftertreatment
with acetic acid, contracted sheaths are
abun-dant. Thewild-type contracted sheath was 63.4
(±3.7) nm long and 26.7 (±1.3) nm wide. The
coreprotrudesfromthe distal end of the sheath
andwas142.1(±3.8)nmlongand8.8(±0.6)nm
in diameter.Contracted tails of SPOl wild-type
phagewerenotlong enoughtoyieldanalyzable
diffractionpatterns. Mutant sus 046-0 long tails
did contract and were sufficiently longto yield
good optical diffractograms. Figure 6 shows a
negatively contrasted sus 046-0 contracted long
tailanditsdiffractionpattern.Thehelicalscrew
anglewascalculatedby using only the altitude of
themaximum(6,1) (layerline6)with respect to
fr 3O
*o
43o
460Arrowrepresents unitlength(140nm).
the R axis. Eight particles were analyzed. The
family of helices with a screw angle of32.3 +
0.70
(95%
confidencelimits)correspondedtothesmallest screw angle (fl0) of 2.30. When
con-tracted sheaths werepreparedbyNaClO4 treat-ment (see above), they occasionally attached end-ontothespecimensupportsurface(datanot
shown). The sheath clearly had 12 "arms"
which radiate out from a central core. Moody
(21, 22) hasexamined similar contractedsheaths
from phage T4. The "arms" are apparently the
family of helices generated by the fl screw
angle.Thesesheaths, whichappearascylinders from the side, seem to form cones, their large diameterbeing in contact with the support film
and the upper taper being caused by stain
shrinkage aboutthe sheath. The contracted tail
has 12 "coarse" helices generated by a helical
screw angle of2.30.
Wefound that thebasaltail-plate (which
cor-responds to thebaseplate on otherphages)
un-derwent a major structural transition when the
sheath contracted. Views perpendicular to the
tail axis showed that the somewhat amorphous
basal structure on the extended sheath was
transformed intoadouble-plate structureonthe
contracted sheath (see Fig. 3a and b). Isolated
basal structures from contracted sheaths could
be seen lyingflat onthegridor at an angle still
attached to a contracted sheath (Fig. 7). The
"contracted"basalstructure wascomposedofa
central ring, perhaps part of the tail tube,
sur--~~~~~~N -o
on November 10, 2019 by guest
http://jvi.asm.org/
edby a strong secondringofsixspots, and that
was in turn surrounded by six elongated spots
pointing in a counterclockwise direction. The
baseplates show a preferred orientation on the
supportfilm, sincemost if not allofthem show
this "counterclockwise" arrangement. Inthose
favorable cases in which the contracted sheath
or phage ghost was still associated with the
baseplate (Fig. 7), the same orientation was
maintained.Ourspecimen preparationand pho-tographic processing conventions represent the
sampleonthefar sideofthe supportfilmas seen
from the electronsource.This orientation ofthe
baseplate correspondedto
viewing
anadsorbed phageonthe cell envelopefromwithin the cell.Thus, the baseplates attach to the carbon sup-portfilm by using the same surface with which theyadsorbtothe cell. Sincethelong-pitch helix
ofthetail sheath wasright-handed,thebaseplate
projections and the long-pitch helix happen to
point in the same direction. This arrangement
wasthesame as that foundforthe T4baseplate
and sheathby Crowther etal. (9). The complex baseplate structure seems to combine the
fea-tures of both the baseplate and tail fibers of
phages likeT4. No evidence for the long, thin, rigid tail fibers found on T4have been reported
foranyBacillus bacteriophage.
Tostudythe head-tail connector structure, we
prepared "neck tails" by an osmotic shock method similartothatof Coombs and Eiserling (8), but with l/2 saturated CsCl (approximately 33% [wt/wt]). Figure 3 shows tails from phage
which were decapitated in this manner. The
head-tailconnector was 23 nmlong (measured
from the top of the sheath) and contained the
collarand twodisk-like structures. The dimen-sions were similar to that of the T4 connector
(8),although SPOl lacks the whisker-like
struc-turesfoundin T4. The two topdisksmayanchor
the tail into the head. This type of simple
an-choringstructurecouldcircumvent theassumed
symmetry mismatch between the sixfold
sym-metric tail and the fivefold symmetric vertex of
theicosahedral head.
DISCUSSION
SPOl tail structure. Abacteriophage tail is a
complex structurewhich recognizes specific
re-ceptors on the cell surface and penetrates the
host cell envelope, delivering the viral genetic
material contained in the protective head shell.
Tail structures vary from the rudimentary
six-subunit structureof Salmonella phage P22 (the
lOOnm
..
_.~~~~~~~A4rs_ fi
[image:7.490.253.449.72.461.2]41A
FIG. 5. Optical diffraction of sus 046-0 long tails.
Theupperhalf of this figure shows an SPOl wild-type
phage and its optical diffractogram. The lower half
showsamutant sus 046-0 long tail with its diffraction
pattern.Thetwopatternsareessentially identical. See
the text for a detailed comparison of the two
struc-tures. Arrows indicate region of tails which were
diffracted.
simplest) to complex contractile tails such as
thatfound inphage T4.
We now have detailed structural information
forfour viruses with contractile tails.
Bacterio-phageT4has been studied in great detail
(3,
18,
21, 22, 24, 30). Bacteriophage Mu (1) and B.
subtilis phage G (11) have also been studied.
Althoughthese viruses havedistinctly different
hosts,developmental patterns, and tail
lengths,
theorganizationof sheath subunitsappearstobe
very similar. The annular repeats are: 4.1 nm
(T4), 4.1 nm (SPO1), 3.8 nm (G), and 3.0 nm
- NW I., 1. ...
r.,
on November 10, 2019 by guest
http://jvi.asm.org/
OOQnm (41A)
FIG. 6. Opticaldiffraction ofsus046-0longtails. This shows acontracted tail contrastedwith 1% uranyl acetateandits indexed optical diffractionpattern. Note stain penetrationin theexposed tail core only to the extentofthe length ofa normaltail(lengthindicated bywhite bar)by viewingup core axis at aglancingangle.
(Mu). The helical screw angle (fQ) is also very
similar: -102.2° (T4). -97.5° (SPO1), -99.1° (G), and -96.8° (Mu). These two parameters
describe the packing arrangements of extended sheath subunits. Allofthesheaths have sixfold
symmetry,andthe coarsehelicalgrooveis
right-handed. An interesting difference is in the
mo-lecular weight of the sheath subunit: 65,000 for
T4 (32), 60,000 to 61,000for
SPOl,
and52,000forMu(1). The sheathsubunitmolecular
weight
forphageG is unknown. A decrease in
molecu-lar weight corresponds to a decrease in the
diameter of the extended sheath: 19.8nm(T4),
18.6 nm(SPO1), and18.0 nm (Mu). Tokeepthe
same packing geometry while decreasing the
molecular weight of the subunit, the diameter
should also decrease. The same molecular
weight of the Mu sheath subunit may also result intheobserveddecrease in annular repeat
com-paredwith the otherphages.Thediameter ofthe tail tube (core) seems to be constant (about 9
nm)in all of thephages described above.
Asimilaranalysisof contracted sheath shows
the same conservation of packing geometry.
Phages T4,
SPOl,
G, and Mu have almostidentical helical screw angles (32, 32, 27 and330,
respectively), and the annular repeat is the
iden-tical 1.7nm in T4, SPOl, and G, although it is
uncertain in Mu(1, 3, 11). Analyses ofpartially
contractedtailsshow that contraction proceeds
upward from the baseplate (11, 24). We have
alsoobservedpartially contracted tails ofSPOl,
andthe contraction starts at thebaseplate.
Be-causethepacking geometries of sheath subunits
in both T4 and SPOl are nearly identical, the
contraction mechanisms areprobably thesame.
In the extended sheath, the annular repeat
dis-tanceis 4.1 nm, and each annulus is rotatedby
about 17 to 220 to the right relative to the one
below it. Aftercontraction, the annular repeat
decreases to 1.7 nm, and the twist angle between
anannulus and the one below it is about 320 to
the right. Moody (24) determined that T4 tail
contraction starts at thebaseplate andmovesin
a wave up the sheath through helical
arrange-ments which are intermediate between the
ex-tended and contracted structures. He
deter-mined that each annulusrotatesabout 150 tothe
right relative to the one below it and that
sub-units of each extendedannulusinterdigitatewith
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.490.74.426.78.380.2]'-I,,
qt.
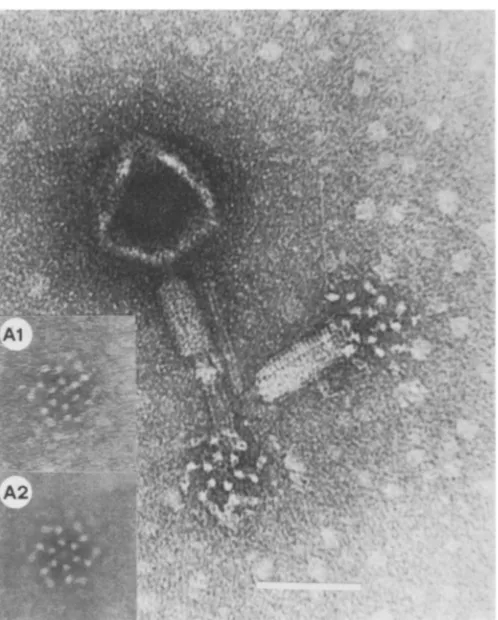
FIG. 7. Wild-type SPOl particles, showing basalstructures associated with contracted sheath. The basal structureshowssixfold rotational symmetry which has been enhancedphotographically in the inset. Al is the original micrograph;A2 hasbeenrotationallyaveraged byrotatingtheimage six times. Rotations of fiveor seven showednoenhanced structure. Barrepresents 100nm.
subunits of annuli above and below. It seems
probable that the complex mechanical
require-ments for phage tail contraction require that
suchproteinstructuresbebuiltin the same way.
Sheath proteins from different genetic origins
have highly conserved packing geometry, but
the bonds could be different as long as their
strengths andangular interactions among
them-selves,and with the core andbaseplatesubunits,
weresimilar.
The samestainpenetrationwehaveobserved
inSPOl tailtubes wasalsoseenby Tschoppand
Smith (33) in
long
T4 tailproduced
in vitro. Opticaldiffractionanalysisshowed no structural differences between the stained and unstainedparts ofthe tail. What is thesignificance of this
limited stain penetrationin terms ofmodelsfor
tail length regulation? An internal structural component could occupy the 3-nm diameter
central hole, or the ends ofthe normal-length
tube could be plugged. The firstpossibility
im-plies a length-determining component (17),
whichextends from the baseplate and fixes tail tubelength by anassembly interactionwith the
tube protein or by providing a unique binding
siteforaterminatorprotein(15, 17). Ifthe ends
are plugged or capped, mechanisms of length determination involving cumulative strain (16) orkinetic regulation (35) wouldbe morelikely.
The nature of the lesion in cistron-8
mu-tants is unknown, but it could affect one of a
number ofpossible sites, including a baseplate
protein, a possible length-determining protein,
thetail tube subunit itself, or aterminator
pro-tein. The mutant phenotypeincludes normal as
well as altered-length tails, but we do not yet
know whether this is due to "leakage" or to
more complex reasons.
SPOl baseplate structure. The baseplate of a
contractile-tailed bacteriophage is the most
structurally complex component of the virion
and seems tofunction both in therecognitionof
receptors on the host cell surface (19) and as a
triggering device for sheath contraction leading
to tail tube penetration into the periplasmic
space of the cell membrane, with subsequent
release ofthe DNA (5, 28, 29). The tail is a
metastablecomplex. Attachment to the cell
sur-A2
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.490.125.374.72.382.2]face causes the T4 baseplate to expand from a
compact hexagon to a thin, six-pointed star (9,
29).This expansion causes the irreversible
con-traction of the sheath. Phage T4 baseplates are
highly ordered structures containing six long
fibers(140 nm)which are the first phage
compo-nent to bind to the cell; thisinteraction may lead
to activation of the baseplate and subsequent
triggering ofthe hexagon-to-star transformation
when the short tail fibers (35 nm) made of gp12
contact the cell surface (9).
B. subtilis bacteriophage do notusually show
thehighly ordered baseplatesexemplified by the
T-even phages. This may be due to specimen
preservation problems. The common appear-anceof theextendedtailbasestructure is rather
amorphous (10), whereas the contracted tail
baseplate is highly ordered. The contracted tail baseplate of SPOl appears to be identical to
contractedtailbaseplatesseen onmany otherB.
subtilis phage (F. A. Eiserling, Ph.D. thesis,
University of California, Los Angeles, 1963).
Thetwobaseplate conformations seen onSPOl particles imply that the baseplate also triggers
SPO1 tail contraction. As mentioned in the
previous section, partially contracted tails of
SPOl have been observed, and the contraction
starts atthebaseplate.
There are nolong tail fibers, asexemplifiedby
T4,associated with any B. subtilis phage studied
to date. Short tail fibers cannot be ruled out
becausetheymaybepartoftheamorphous base
structure. Indeed, contracted baseplates do
show small fibers. The extended tailbase struc-ture maybemoreregular in design, but flatten-ing durflatten-ing specimen preparation for EM may
destroy thisorder.
Conclusions. The head-tail connector
struc-ture is apparently similar in all bacteriophages,
atleastatthe2-nmlevel ofresolution.
Contrac-tilesheath structureisremarkably similar inthe
twophagesSPOl andT4,and eachphage shows
amajorrearrangementofthebaseplate structure
which precedes sheath contraction. The base-plates both show striking sixfold rotational
sym-metry. Tail tube length regulation is probably
similar in SPOl and T4, although no length
variants of T4 tails other than thosedescribed in
vitro are known. Onemajordifferencebetween
the twophagesis the lack oflong,thin tailfibers
in
SPOt.
Perhaps there iscoupling, in T4assem-bly, between the length of the tail and the
assembly ofthe tail fibers (37) such that fibers
cannot be attached efficiently to longer or
shorter tails. These fibers give T4 a significant
competitive advantage,andsuchlengthvariants
wouldbe eliminated from the phage population.
Since
SPOt
lacks these fibers, such selectivepressurewould benil, andgreater tolerance for
taillength variationcould exist. Presumably,the
nature of the cell surface receptors in B. subtilis determines the lack of long tail fibers in these phages.
Phage taillength is precisely determined, and
viruses would seem to be ideal model systems
for studying such control. We have
character-izedan SPO1 mutant which makes abnormally
long tails. We show that the sheath subunit packinggeometryof the mutanttail is thesame asthat of thewild-type tail, even in theregion which extends beyond normal length, and that
longtailsappear toexcludestainintheregion of
the core whichcorresponds tounit-length tails.
These two observations are identical to those described for in vitro-produced T4 long tails
(33).
Complementation data (unpublished) indicate that thelong tails produced by mutations ingene 8could be duetofailuretoterminate tailgrowth effectively, sincesome mutant046-0tailscanbe complemented to produce infectious phage. Alternatively, if the gene 8 product interacts with the baseplate, then different mechanisms
may apply. A length determiner model which invokes a template moledule(s) in or along the
corewould fitthestain exclusion seenin T4and
SPOl long tails (17, 35).Similarly, acumulative strainmodelinwhichtail tubepolymerization is halted when bonddeformations accumulatetoa
critical levelcannotbe ruled out(16), since the baseplate may setthelimit of the
required
bond strain.The constraints employed to conserve
pack-ing geometry and function, in terms of tail
contraction and DNA delivery, appear to be independent of tail length, since the taillengths of different viruses are not the same. Whyare
tails of different viruses different lengths, and
how isthetaillength ofeachvirussostringently controlled?Theanswerstothese
questions
mayallowus to understand
principles
whichgovernthe genesis ofsupramolecular structures of de-fined size andshape.
ACKNOWLEDGMENTS
Our sincere thanks to E. PeterGeiduschek for continued encouragementandsupport.Caroline Beard did much ofthe preliminarywork incharacterizing SPO mutants.
This workwassupported byaPublic Health Servicegrant from the National Institute ofAllergy and InfectiousDiseases, and by grants from the Genetic Biology Program of the National Science Foundation. The EMBOcourse in Image Processing and the staff of the Microbiology Department, Biozentrum, Basel, Switzerland, providedM.L.P.with valu-ablehelp in analysis ofSPO structure, aswellasfinancial support.
LITERATURE CITED
1. Admiraal, G.,andJ.E.MeUema.1976. Thestructureof the contractilesheathofbacteriophageMu.J.Ultrastruct. Res. 56:48-64.
2. Aebi, U., R. R. Smith, J. Dubochet, C. Henry, and E.
on November 10, 2019 by guest
http://jvi.asm.org/
40:703-718.
5. Benz,W., and E. B. Goldberg. 1973. Interaction between phage T4 adsorption intermediates and thebacterial enve-lope.Virology 53:225-235.
6. BUlenga,R.,U.Aebi,and E.Kellenberger.1976. Proper-ties and structure ofagene24-controlledT4giant phage. J.Mol.Biol. 103:469-498.
7. Coombs, D. H. 1975. Density gradient fractionation by piston displacement.Anal. Biochem. 68:95-101. 8. Coombs, D. H., and F. A. Eiserling. 1977.Studies on the
structureprotein composition and assembly of the neck of bacteriophage T4. J. Mol. Biol. 116:375-405.
9. Crowther,R. A.,E. V.Lenk, Y.Kikuchi,and J. King. 1977. Molecular reorganization in the hexagon to star transition of the baseplate ofbacteriophage T4.J. Mol. Biol.116:489-523.
10. Davidson, P. F.1963. The structure of bacteriophage SP8. Virology 21:146-151.
11. Donelli, G., F. Guglielmi, and L. Pagoletti. 1972. Structure and physicochemical properties of bacteriophage G. I. Arrangement ofprotein subunits and contraction process of tail sheath. J. Mol.Biol. 71:113-125.
12. Gage, L. P., and E. P.Geiduschek. 1971. RNAsynthesis duringbacteriophage SPO1 development. Six classes of SPOlRNA.J. Mol.Biol. 57:279-300.
13. Hansen, R. S., J. Blicharska, and S. Szjulmaster. 1964. Relationship between the tricarboxylic acid cycle en-zymes andsporulation ofB.subtilis.Biochem. Biophys. Res.Commun. 17:1-7.
14. Hemphill, H. E., and H. R. Whiteley. 1975. Bacterio-phages ofBacillus subtilis. Bacteriol. Rev. 39:257-315. 15. Katsura,I.1976.Morphogenesis of bacteriophage lambda
tail. Polymorphism in the assembly of the major tail protein.J.Mol. Biol. 107:307-326.
16. Kellenberger,E.1972.Assembly in biological systems and mechanisms of length determination in protein assem-blies, p. 189-206 and 295-298. In Wolstenholme and O'Conner (ed.), Polymerization in biological systems. Associated ScientificPublishers,Amsterdam.
17. King, J.,E.Lenk, and D. Botstein.1971. Mechanism of head assembly and DNA encapsulation in Salmonella phageP22.Morphogeneticpathway. J. Mol. Biol. 80:697-731.
18. Krimm,S.,and T. F.Anderson.1967. Structure of normal and contracted tail sheathsof T4 bacteriophage. J. Mol. Biol. 27:197-202.
19. Lindberg, A. A. 1973. Bacteriophage receptors. Annu. Rev. Microbiol. 27:205-241.
20. Markham, R.,S.Frey,andG.J.Hills.1963. Methods for the enhancement ofimage detail and accentuation of structurein electronmicroscopy. Virology 20:758-760.
study of tail tubes from bacteriophage T2L. J. Mol. Biol. 150:217-244.
24. Moody, M. F. 1973. Sheath of bacteriophage T4. III. Contraction mechanism deduced from partially contract-ed sheaths.J.Mol. Biol.80:613-635.
25. Okubo, S.,and T. Yanagida. 1968. Isolation of a suppres-sor mutantin Bacillus subtilis. J. Bacteriol. 95:1187-1188. 26. Okubo, S., T. Yanagida, D. J. Fujita, and B. M. Ohlsson-Wilhelm. 1972. The genetics of bacteriophage SPOl. Biken J. 15:81-97.
27. Rabussay, D.,and E. P.Geiduschek. 1977.Regulation of geneaction in the developmentof lyticbacteriophages, p. 1-196. In H. Fraenkel-Conrat and R. R. Wagner (ed.), Comprehensive virology, vol. 8. Plenum Press, New York.
28. Simon, L. D., and T. F. Anderson.1967. Theinfection of Escherichia coli by T2 and T4 bacteriophages as seen in the electronmicroscope. I. Attachment and penetration. Virology32:279-297.
29. Simon, L. D., and T. F. Anderson. 1967.The infectionof E.coliby T2 and T4bacteriophages as seen in theelectron microscope. II. Structure and function of thebaseplate. Virology 32:298-305.
30. Smith,P.R., U. Aebi,R.Josephs,and M. Kessel. 1976. Studies of the structure of the T4 bacteriophage tail sheath. I. The recovery of three-dimensional structural information from the extended sheath. J. Mol. Biol. 106:243-275.
31. Spudich, J. A., and A. Kornberg. 1968. Biochemical studies of bacterialsporulation and germination. J. Biol. Chem. 243:4588-4599.
32. Tschopp, J., F. Arisaka, R. Van Driel, and J.Engel.1979. Purification, characterization andreassembly of the bac-teriophage T4D tail sheath protein P18. J. Mol. Biol. 128:247-258.
33. Tschopp, J., and P. R.Snith. 1978. Extra long T4 tails producedin in vitro conditions. J. Mol. Biol. 114:281-286. 34. Venyaminov, S. Yu.,L. P.Rodikova,A. L.Metlina,and
B. F. Poglazov. 1975. Secondary structure change of bacteriophage T4 sheath protein duringsheath contrac-tion. J. Mol. Biol. 98:657-664.
35. Wagenknecht, T., and V. A. Bloomfield. 1977.In vitro polymerization of bacteriophage T4D tailcoresubunits. J. Mol.Biol. 116:347-359.
36. Williams,R.C.,andH. W. Fisher.1970. Electron micros-copyof tobacco mosaic virus under conditions of minimal beamexposure. J. Mol. Biol. 52:121-123.
37. Wood, W. B., and J. King. 1979. Genetic control of complex bacteriophage assembly, p. 581-624. In H. Fraenkel-Conrat and R. R.Wagner (ed.),Comprehensive virology, vol. 13. Plenum Press. New York.