0022-538X/79/08-0537/09$02.00/0
Uptake of Minute Virus of Mice into Cultured Rodent Cells
P. LINSER, HELEN BRUNING, ANDR. W. ARMENTROUT*
Departmentof Biological Chemistry, University of Cincinnati College of Medicine, Cincinnati,Ohio45267
Receivedforpublication28December1978
The uptake of minute virus of mice into cells in tissue culture was examined biochemically andbyelectronmicroscopy. Cell-viruscomplexeswere formedat
40C,
anduptake of virus wasfollowed
after thecells
wereshifted to 37°C. The infectious particles appeared to enter cells at 370C by a two-step process. The first and rapid phasewasmeasured by the resistance of cell-bound virustoelution by EDTA. The bulk of the bound virus particles became refractory to elution with EDTAwithin 30 min of incubation at370C. The infectious particles became resistant to EDTA elution at the same rate. The second, slower phase of the uptake process was measured by the resistance of infectious particles to neutral-izationby antiserum. This process was complete within 2 h of incubation at370C. During this 2-h period, labeled viral DNA became progressively associated with thenuclear fraction of disrupted cells. The uptake of infectious virus could occur during the G1 phase of the cell cycle and was not an S phase-specific event. Theuptake process was notthe cause of the S phasedependence of minute virus of
micereplication. Inelectron micrographs, virus absorbed to any area of the cell surfaceappearedtobe taken into the cell by pinocytosis.
In many instances, the primary interaction between virus and cell occurs at a specific cell surface component present in limitedquantities (1). These virus receptors may have alimited distribution among the tissues of a susceptible organism, and, insomecases, the receptors ap-pear at specific times during development (3). The tissue specificityof virus infection by polio-virus and the adenopolio-viruses is controlled to some extentbythe presenceorabsence of these virus receptors (1). The nondefective parvoviruses also exhibita degree of cytotropism (6), anda specific virus receptor appearstobeinvolved in infection by theparvovirusminute virus of mice (MVM) (5). The cell receptors for MVM
satu-rate atabout 5 x 105 virus particlesbound per
cell,andthe presence of these saturablebinding
sites has been correlated withsusceptibilityto infection(5). Examination of the cell-virus com-plexes with an electron microscope suggested that the virusreceptor is randomlylocatedon thecell surface (5).
Inthisstudy we examined the early steps in virus infection beyond the initial binding
reac-tion. Afterbinding[3H]thymidine-labeled virus
tocellsat
40C,
we used biochemicaltechniques to measure the synchronousinternalization
of virus at370C. We also examined the uptake of virusby electron microscopic techniques in an attempt to further characterize the early steps oftheinfection.The replication of the nondefective
parvovi-ruses, includingMVM, requires host cell func-tions whichoccur during S phase (7). It is not clearexactlywhich step in MVMreplication is
subject tothiscellcycle control. Weexamined
the uptake process in synchronized cells and
showed that it is notrestrictedtoS phase but
mayoccurearlyin
Gi.
MATERIALS AND METHODS
Thecelllines used werethe A-9 mouse line and the RT-7ratbraintumorcellline (8). A-9cellsweregrown
inF-liminimalessentialmedium (Grand Island
Bio-logical Co.) supplementedwith 10% heat-inactivated
fetalcalf serum, 100 U ofpenicillinperml,and 100,g
ofstreptomycin perml in suspensionat densities of
from5x 104to 5x 105 cellsperml.RT-7cellswere
growninmonolayersin the above media.Suspensions of mitotic RT-7 cells were prepared as previously describedbymanually shakingculture flasks
contain-ingmonolayersat nearconfluency (8).Cellsprepared
in thisfashionarevirtuallyall in mitosisatthe time of detachment (8).
Virus. Plaque-purified MVM was originally ob-tained from Peter Tattersall. MVM and MVM labeled inits DNA with[methyl-3H]thymidineweregrownin RT-7 cells andpurified as previously described (5). The concentrations ofparticles in the purified viral
preparationswere calculated fromabsorption at280
nm(10).
Binding assay. Binding ofradiolabeled virus to
cellswasperformedinsuspensionasdescribed
previ-ously (5).Briefly,asmall volumeofviruswasadded
tocellsandincubatedat4°C,andthenthesuspension
was filtered through a membrane filter (Nuclepore 537
on November 10, 2019 by guest
http://jvi.asm.org/
Corp.). The amount of cell-bound virus was deter-minedby measuringtheradioactivityretainedonthe
washed filters by scintillation counting.
Separationof nuclei andcytoplasm.Isolation of nuclei was performed by a modified version of the
method of Wray (11). Cellssuspendedinmediawere
centrifugedat4°Cand2,000rpmfor 5minand
resus-pendedat106cellsperml in buffercontaining50jtM PIPES[piperazine-N,N'-bis(2-ethanesulfonic acid)],1 mM CaCl2, and0.5Mhexylene glycol (2-methyl-2,4-pentanediol; Eastman Organic Chemical Div.,
East-manKodakCo.)atpH6.5. Thesuspensionwas incu-batedat37°Cfor 5 min and thenpassedtwicethrough
a25-gauge needle to burst the swollen cells. Nuclei werepelletedasdescribedabove,and the supernatant
wascollected.The pelletwasresuspendedin 1mlof
isolation buffer and centrifuged at 1,000 rpm. The supernatantsof this and the previouscentrifugation
were combinedasthecytoplasmicfraction. The
nu-clear pellet of the finalcentrifugationwasresuspended
in 1 ml of isolation buffer. These nucleiare90to95%
free of cytoplasmictags(8).Acid-insoluble radioactiv-ityin each fractionwasmeasuredbyprecipitatingthe material with 10% trichloroacetic acid at4°Cfor 1h, using 50,ul ofa 1:10 dilution of fetal calfserum as
carrier. Precipitates were collected by filtration on
WhatmanGFC filters and solubilized with Soluene-100(Packard).Radioactivitywasmeasuredby
scintil-lation spectrophotometry in toluene-based scintilla-tioncocktail.
Electron microscopy. Cells were embedded for
electron microscopy in LuftEpon as previously de-scribed(5).Ultrathin sectionswerecutperpendicular totheplaneofgrowth,mountedon400-meshcopper
grids,and stained withuranylacetateandlead citrate
asdescribed previously (5). Micrographswere taken
with eithera Zeiss EM-9A electronmicroscope ata
40-kVaccelerationvoltageoraJeolJEM 100 B
elec-tronmicroscopeata60-kV accelerationvoltage.
RESULTS
Elution of virus particles from the cell
surfaceby EDTA. We have previously shown
that MVM bound to A-9 cells at 4°C can be
eluted from the cell surface by washing with
Ca2+-Mg2+-free phosphate-buffered saline
(PBS) containing EDTA (5). The earlystagesof
theuptakeprocesswereexamined byfollowing
the elutionof labeled virus fromthecellsurface
using this wash procedure. To observe the
up-take processin thelargest number ofcells,the
cell-virus complexes were formed at 4°C and
subsequently incubatedat37°C (1). Most of the
MVMboundat4°C isatthe cell surface (5).
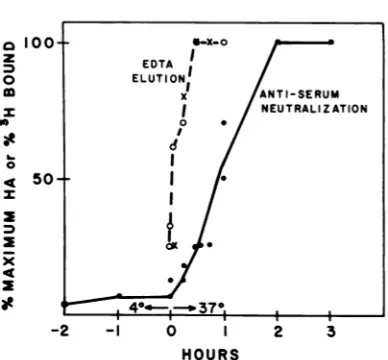
As Fig. 1 shows, virtually all of the virus
particles bound toA-9 cells at4°C became
re-sistant toEDTAelutionwithin 30min of
incu-bationat37°C.
Elution ofinfectious virus from the cell
surfaceby EDTA. In these virus preparations,
theratio ofinfectious particlestototal particles
ranged from 1:600to1:3,000.Theinteractions of
the labeled particles with the cell surfaces did
a z 0 x 0 49 4c oo0-
50--2 -I I 2 3
HOURS
FIG. 1. Comparison oftherate atwhich cell-bound virus becomes resistanttoelutionbyEDTA with the rate atwhichthe bound particles become resistantto inactivationbyantiserum.Symbols:0,rate atwhich
labeled virus boundtorandomly growingA-9 cells became resistanttoelution byEDTA. A-9cells(106 cells in1mlof serum-freeF-l1medium)werereacted
with [3H]thymidine-labeled virus(2.4 x 1010
parti-cles) for1 hat4°C. Afterthe incubationat4°C,the cellsuspensionswereincubatedat37°C for the indi-cated times. Suspensions were thenfiltered as de-scribed in thetextand washedfor5minat0°Cwith Ca2+-Mg2+-free PBS containing1 mM EDTA. The
cell-associated radioactivity, after cold incubation andwashingwith normalPBS,wasdefinedas100%
(22,500 cpm) for comparison with EDTA-PBS-washed samples. The input quantity ofvirus
con-tainedapproximately23,000cpmofacid-precipitable radioactivity.x,Rateatwhich infectiousvirus bound
tosynchronizedRT- 7 cells became resistanttoelution byEDTA. RT- 7 cellsweresynchronizedand allowed
toattachtoTflasks for2 hat37°C.Themonolayers
wereplacedat4°Candexposedto virusfor2h at
4°C. The cell-viruscomplexeswereshifted to37°C, andatintervals cellsampleswerereleasedfromthe
surface oftheflask with PBS-EDTA solution. The cellswererinsed twice in PBS-EDTAby centrifuga-tion andwerereplated at37C in medium.After2 daysthe cellsweredisrupted,and theamountofviral protein produced was determined by HA titration. The dataarepresentedaspercentagesofthe maxi-mum HA titer obtained (4,096). 0, Rate at which
infectious virus bound to synchronized RT-7 cells became resistanttoneutralizationbyantiserum. Syn-chronized RT-7 cells were allowed to attach to T
flasks and exposed to MVM at 4°C as described above.At intervalsduringthe4°Cabsorption period andduringthesubsequent 37°Cincubation,all
sam-pleswereexposedto19hemagglutination inhibition units ofrabbit anti-MVM antiserum. After 4 h of
exposure to antiserum, themonolayers wererinsed withfreshmedium without antiserumand incubated inantiserum-freemediumfor2days.Thecellcultures
werethenharvested, and theextentof infectionwas
determinedbyHA titration. The dataarepresented
aspercentages ofthe maximum HA titer obtained
(8,192). EDTA I ELUTIONI X ANTI-SERUM I NEUTRALIZATION 0 0 I I
I
I, I 4°1_ 37°1 1 1
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.504.266.460.68.248.2]UPTAKE OF MVM INTO RODENT CELLS 539
not necessarily reflect the interactions of the
infectious particles. To study the fate of the infectious particles after the cell-virus complexes
were shifted to370C, we examined therate at
which the infectionprocessbecame resistantto
EDTA elution. Inthese experiments,
synchro-nizedRT-7cells wereusedtodetermine whether
uptake of infectious virus couldoccurduring the
G1 phase. RT-7 cells in mitosis had
approxi-mately thesamenumber of viral receptors per
cell(5x105)asrandomly growing A-9cells(Fig.
2).
Synchronized RT-7 cellswereexposedtovirus
at40Candshiftedto370C,andatintervalscell
samples were washed with 1 mM EDTA. This
procedure removed loosely bound virus aswell
as detaching the cells from the surface of the
flask.The cellswerewashed and replated into T
flasksat370C.After 48h, the cell sampleswere
harvested, and the accumulated viral protein
wastitrated by hemagglutination (HA). As Fig.
1shows, therate atwhich thetotal viral particle
population (radioactivity) became resistant to
EDTAafter the shiftto370Cwasapproximately
the same as the rate at which the infectious particles (HA production) became elution resist-ant.
Movement of infectious virus into the
cell. Resistance of bound virus to elutionfrom
the cell surface by EDTA does notnecessarily
result from internalization of the virusparticles.
To measure internalization, we examined the rate atwhich bound virus became resistantto
inactivationby antiserum after incubation of the
cell-viruscomplexesat370C.
When the cell-virus complexeswere exposed
toantiserumbefore incubationat370C,the
cul-tures were infected, but only at a low level, indicating that the bulk of the infectious
parti-cles remainedvulnerabletoneutralizationatthe
cell surface at 40C (Fig. 1). However, after
in-cubationat370C, theinfection processbecame
progressively more resistant to the antiserum
until, after 2 h, the antiserumnolongerinhibited
infection.
The bulk of the virus particles, as well asthe
minor fraction of infectious particles, became
resistanttoEDTAelution fromthe cell surface
atthe same relatively rapidrate. However,
re-sistance ofinfectivitytoantiserum proceededat
aslowerrateandwasmaximal only aftera2-h
incubationofthe cell-viruscomplexesat370C.
The formation ofavirus-receptor complex stable
toEDTAelutionappearedto representarapid
initialstepwhichoccurredat370C before
inter-nalization of theinfectious virus.
Association of viral DNA with the
nu-clear fractionofdisruptedcells.Do the bulk
ofthe virusparticlesenterthe cellatthe same
8-2
2 3 4 5
I.M. x 10-6
FIG. 2. Binding of MVM to mitotic RT-7 cells. A totalof 2 x105RT-7 cellsharvested by mechanical detachment of mitotic cells were reacted with the indicated multiplicities of [3H]thymidine-labeled MVMparticles per cell (input multiplicity[I.M.1)for 2h at4°C in 1 ml of PBS. The bound multiplicity (B.M.) wasdeterminedafter filtration, asdescribed inthe text.
ratethat the infectiousparticles are taken up?
Toanswerthisquestion,wedetermined therate
and extent to which input viral label became
associated with the nuclear fraction of infected
cellsafter in vitrodisruption. Association of viral
label with the nuclear fraction is taken simply
as an indication that the viral particles have
entered the cell. Nuclear association does not indicate whether viral particles have actually penetratedtothe nucleus of the intactcellsnor does it give any information on the physical
state or infectivity of the virus particle which
containsthelabel.Intheseexperiments,the cell-viruscomplexeswereagainformed at4°C; the cellswerethen incubatedat370Ctoallow viral
uptake,andatintervals thecellsweredisrupted
andseparatedinto nuclear and
cytoplasmic
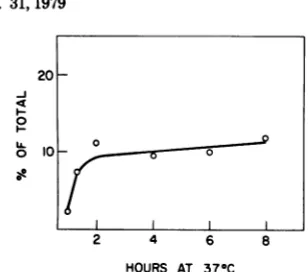
frac-tions. Theproportions of the bound viral label in the nuclear and cytoplasmic fractions were determined from the acid-precipitable radioac-tivity. In randomly growing A-9 cells, roughly 27% of thecell-bound viral label became associ-ated withnuclei after2 hat370C, andfurther increasesoccurredatarelativelyslowrate(Fig. 3).
Wetake the rateofassociation ofvirallabel with the nuclear fraction as
representing
therateofentry of theviralparticlesinto the cell.
Theinitial rate of entry of the labeledparticles
was not markedly different from the rate of
internalizationof the infectiousparticles(Fig. 1
and3).
Nucleus-associated viral label isin viral
DNA. In the experiments describedabove,the
bulk of the
[3H]thymidine-labeled
viralmaterialwhich wasfoundassociatedwiththecellnuclear
fraction resided in intact single-stranded viral
VOL. 31,1979
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.504.274.434.61.208.2]o 60_
0
0
* 40
2 4 6 8
HOURS AT 370C
FIG. 3. MVM uptake and compartmentqlization in A-9cells. A total of1.4 x 106 A-9 cells in1 mlof serum-free F-li mediumwerereactedwith 4.8x1010
[3HJthymidine-lobeledvirus particles for1h at 4°C.
After the cold incubation, cell suspensions were in-cubated at 37°C with agitation for the indicated times. After thewarm incubation, suspensions were transferred to new siliconized glass tubes at 0°C, and thecells werepelleted. The nuclear and cytoplasmic fractions were isolated as described in the text. Tri-chloroacetic acid-precipitable radioactivity was measuredin the supernatant (unbound) fraction as well as the nuclear and cytoplasmic fractions (see text). Trichloroacetic acid precipitation of the input quantity ofvirus yielded a total of 41,000 to 46,000 cpm. The total radioactivity present in samples for eachtime point(100%) was between38,000and47,000
cpm.Symbols:0, nucleus; 0, cytoplasm.
DNA.A-9cellswereinfected with labeledvirus at40C.Atintervals after incubationat
370C,
the total DNA was extracted from the cells. Over 95%of thecell-bound labelwasrecoveredas16S DNAaftersucrosegradientcentrifugation
(Fig.
4). DNA from each sample was
digested
withsingle-strand-specific Si nuclease under
condi-tions which didnotdigestdouble-strandedDNA (9). None of the DNAwasresistantto
SI
diges-tion; the DNAfragments
resulting
fromSi
diges-tionwere5Sorsmallerin size
(Fig.
5).
Thus, at times during the infection when25
to35% of the virallabelwasassociated withthe
nuclearfraction, virtuallyall of the
cell-associ-atedviral label wasin single-stranded DNA of
MVMgenomelength.
Association of viral DNA with the nu-clear fraction of
synchronized
cells. Theassociationof viral labelwith thenuclear
frac-tion ofthecellswasusedtoexamine the
move-mentof the bulk of the bound virus into
syn-chronizedcells.AswiththeA-9cells,therateof
associationofvirallabel with the nuclear
frac-tionwasmaximalduringthefirst2hof
incuba-tionat37°C (Fig. 6).
0O HOURS A 3 HOURS B 30
-20
-N 1
6HOURS
0 10 - ~~~~~16S
(~~) tO ~~23S
20 5 S
I10
-10 20 10 20
[image:4.504.77.235.58.225.2]FRACTION NUMBER
FIG. 4. Sizeof labeled viral DNA recovered from
infectedcells. A-9 cells were exposed to
[3H]thymi-dine-labeled MVM at 4°C for 2 h. The cell-virus complexeswereshiftedto37°C, and at zero time and 3and6htotal cellular nucleic acid was purified by proteaseK digestion and phenol-chloroform extrac-tion. Aportion of each sample was then centrifuged on aneutral15to30%sucrosegradient (SW40, 16 h at25,000rpm). The labeled material was located by scintillation counting a samplefrom each fraction. The sizeof the labeled viral material was determined relative to 3H-labeled Escherichia coli ribosomal RNAruninaparallel gradient (D).
A B
0 HOURS 3 HOURS
44
-cm 2
-0
a 6HOURS
04- ~~~23SA
4
~~~~~55
2
l0
20 10 20FRACTION NUMBER
FIG. 5. SI nuclease sensitivity of labeled viral DNA recoveredfrom infected cells. A portion of the samples from the experiment described in the legend toFig. 4wasdigested withS nuclease (1 h,
37QC)
and then centrifuged on neutral sucrosegradients (see legendtoFig. 4). Samples of each gradient frac-tion weredirectly assayed for radioactivity by scin-tillation counting. LabeledE. coli ribosomal RNA was runinaparallel gradient (D).on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.504.285.432.64.270.2] [image:4.504.286.435.408.579.2]UPTAKE OF MVM INTO RODENT CELLS 541
20
-J
I.-0
U. 0 lo0
2 4 6 8
HOURS AT 37°C
FIG. 6. Accumulation of virallabel in the nucleus of RT-7 cells. A total of 4.4 x 105 mechanically detachedmitotic RT-7cells in 1mlof serum-free
F-11 medium werereactedwith 6.2x 1010 [3Hlthymi-dine-labeled virus particles for1hat4°C.Afterthe
cold incubation, the cellsuspensionsweretransferred
to 25-cm2 Falcon T flasks and incubated for the
indicated times at 37°C. The nuclei were isolated
fromthese culturesasdescribedin thetext,and the
trichloroacetic acid-precipitable nucleus-associated
radioactivitvwasmeasured.
Changes in infectivity of viral particles
duringtheuptakeprocess.Weobserved that
apercentage of the virus boundat40C
sponta-neously eluted from thecellsurfaceat370C.For
example, in theexperiment shown in Fig. 3, 9%
of the input label didnotbindtocellsandwas
found freeinthe supernatant afterabsorptionat
40C. When the cells were incubated at 370C,
17% of the input label was recovered in the
supernatantafter 30min.Between8 and 10% of
thebound virus eluted from the cells when the
temperaturewasraised. The eluted virus
appar-ently remained free from the cell during the
subsequentincubation at370C, asthe
percent-age of unboundvirus remained relatively
con-stant once the temperature was raised. Virus
which elutedat370Cretained itsabilitytobind
tocellsandwas asinfectiousastheoriginalvirus
(data not shown). These results may indicate
that the binding of virus to the cell surface at
40Cdoesnotinactivatetheparticles.
It has been shown that the interaction of
poliovirus andadenovirus withthe cellsurface
at 370C results in loss of infectivity, and this
maybe theinitial stepin theuncoatingprocess
forthese viruses (1, 2, 4). In thecase ofMVM,
we showed that the particles absorbed to the
cellat40Cbecametightly boundwithin 30min
after the cells were shifted to 370C. Does the
formation of this stable cell-virus complex
dis-ruptthe bound viralparticles? As Table1shows,
incubationofthecomplexat370C hadverylittle
effect onthe infectivity of the boundvirus. In
theseexperiments, viruswasrecovered from the
infected cells at intervals up to 4 h after the
complexes were shifted to 370C,when the
up-takeprocess wasessentially complete. The
spe-cific infectivity of therecovered virus decreased
byonlyabout50%, indicating that the formation
ofatight complex with the cells at 370Cdoes not resultin a loss of infectivityfor the bound
particles. Although those experiments did not
necessarily examine the infectious process, the kinetics of inactivation of the bound particles roughly follow the uptake of labeled particles. It
appears that the bound viruses lose infectivity
during or after their internalization. Whether thisloss of infectivity is relatedtoviral uncoating requires further investigation.
Electronmicroscopyof virusuptake.
Be-cause of the low ratio of infectivityto particles
inourMVMpreparations, itwasnotpossibleto distinguish the infectious interactions from the fate of the bulk of the virusparticles by electron microscopy. However, the experiments de-scribed above indicate that there are no gross differences in therate atwhich themassof the virusparticlesenterthecell and therateofentry
TABLE 1. Infectivityof cell-bound virus during the uptakeprocessa
Radioac-
Spe-Infectivity t... cific in- % Spe-TimneTune
Ii(ectCIDty
(TCID50)toVlty
fectiv- ificin (total ity fectivity cpm)(Xl104)
5min 6.5x107 5,400 1.2 100 30min 5.6x107 5,900 0.9 75
1h 3.8 x107 5,100 0.8 67
2h 2.0 x 107 3,200 0.6 50
3h 2.8x107 4,000 0.7 58
4h 4.4 x107 8,800 0.5 42
aRT-7
cells
weresynchronizedbymitotic detach-mentandallowed to attachtothesurface of T flasks for2hat37°C.Labeled viruswasabsorbedtocells for 2hat4°C,theinoculumwasremoved,and the mono-layers were rinsedtwice in 4°C serum-free mediumandplacedat370C.Atintervals after the shiftto37°C,
the cells were removed from the surfaces of the T flasks in PBS-EDTA solution, which also removed loosely bound virus. Thecellswerewashed twice in serum-freemedium,resuspended in0.01 MTris,pH 9.0,and frozen. The viruswasextracted from the cells byDouncehomogenization, DNasedigestion (1hat
370C,50,gof DNase perml,1mMMgCl2),and Freon
extraction. Samples of the extracted virus were
as-sayedforradioactivityandinfectivity.The 50% tissue culture infective dose(TCID5o)isthereciprocalofthe dilution of the extracted virus whichgivesapositive HA titer in 50%oftriplicateinfected cultures(5).The
specificinfectivity is theTCID5odividedbythe
acid-precipitablecountsper minutepresentin thesample
ofviral extract used for the TCIDso assays. At the time ofextraction from the infected cells,the input viruspreparation hadaspecificinfectivityof1.1 x104
(108 TCID50per9,100cpm).
0
O
31,
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.504.71.225.60.196.2]ofthe infectious particles. On this basis, it re-mainspossible that thecellhandles the uptake ofboth kinds ofparticles in the same fashion, andelectron micrographs of the uptake of the bulk of the particles might also represent the uptakeprocessfor the infectiousparticles.
To visualize the processof virusuptake, A-9
cell monolayers were incubated with
approxi-mately 106 particlesper cell at4°C.The cultures were then incubated at 37°C for 10 min and processed for electronmicroscopy.InFig.7 and
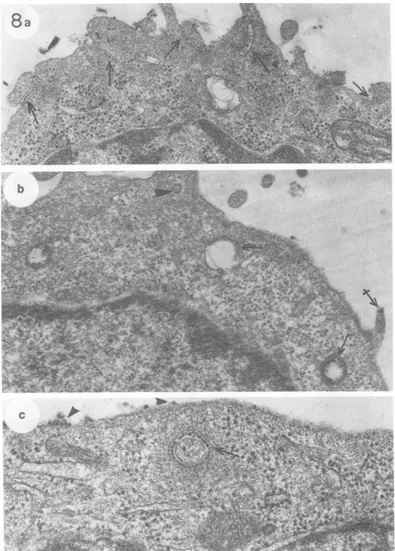
8theuptake of MVM into A-9cells appearsto
occurin severalways. Single
virions,
aswellasgroups of virus, are taken into coated vesicles
viaendocytoticclefts. We have shownpreviously
that MVM binds to these clefts at 40C in the absenceofuptake (5).The clefts bubble into the cell andpinchofftoformfree
cytoplasmic
vesi-cles(Fig. 7).The submembranous
thickening
oftheoriginalcleftnowforms the
rough
coating
ofthevesicle. Thesecoatingvesiclesaregenerally seenclosetothecell surface.
Virus particles are also taken up in smooth vesicles. In Fig. 7a and d and 8a, the virus particles appear to act as multivalent ligands, binding adjacent regions of the
plasma
mem-brane together. Filipodia often become ligated to the cell surface in such a manner. Often afilipodiumcompletelycoated withvirusappears
tobe internalized bythe cell (Fig. 7d and 8b).
Virus-filled channels running into the
cyto-plasmarefrequently seen (Fig. 8a) and may be the product ofvirus-mediated interconnection ofmembranes. Such channelsmaypinchoff and fuse with other vesicles to form large vacuoles linedon their innersurfaces with viruses. Such large virus-lined vacuoles are frequently seen (Fig. 7 and 8), usually located deeper in the cytoplasm than the coated vesicles. Smaller smooth vesicles containing virusesarealso fre-quently seenatvarying depthsin thecytoplasm (Fig.7and 8).
In Fig. 7b, small vesicles containing single virus particlesare seen.However,intermediate
stages of
single
particle uptake(monocytosis)
werenotobserved,andthese vesicles with
single
virusparticlescould representcrosssectionsof thechannelsofvirusdescribed above. The fact
that activemonocytosiswasnotobserved may
be duetothe
difficulty
ofaccuratelyidentifying
single
particlesofthis sizeatthecell surface.Double-walled,
smoothvesicleswereoftenob-served. These vesicles appearedtobe channels
ofviruswhich had circularized.The central
re-gion
of suchvesicles couldeitherbeempty,asavacuole, orenclose amatrixverysimilartothe
surrounding cytoplasm (Fig.8b and
e).
When virusbindinganduptakewereallowed
toproceed simultaneously at 370C, the uptake
process was notnoticeably different from that observedwithpriorviralabsorptionat40C
(data
not
shown).
UninfectedA-9 cells wereembeddedin
mono-layersandsectionedperpendiculartotheplane
ofgrowth(datanotshown).Thecellsurfacewas
clear ofparticulates. Theplasmamembraneof
these cells frequently exhibited thickened
re-gions which appeared to give rise to coated
vesicles. In the absence ofvirus these regions andvesicleswerefreeofparticles.
DISCUSSION
In this study, we examined some aspects of
the uptake of MVM by cultured rodent cells.
Theuptakeoftheinfectious particlesappearsto occurbyatwo-step process. The majorityof the
particles,aswellastheinfectious particles,first
form an EDTA-resistant complex at the cell
surface at 370C. Formationofthis initial
com-plex is relatively rapid and is complete by 30 min.Theinfectious particles then leave thecell
surface and become resistant to antiserum
in-activationat a slower rate. Infectiousparticles leavethe cell surfaceatapproximatelythesame ratethat viral labelbecomesassociated withthe nuclearfractionofdisrupted infected cells. From
thesedata,theinfectious particlesappear to be
FIG. 7. MVM uptakebyA-9cells. ViruswasappliedtoA-9cells(106particlesper cell) in amonolayerat
4°Cfor1h.Approximately2 x 105particleswereboundpercell, asmeasuredby aparallel binding assay.
Thecultureswereincubatedat37°Cfor 10mintoallow viral uptakeandwere thenprocessedforelectron
microscopy. (a)Arrowsindicatestages of virus uptake into coatedvesiclesfrominvagination(right and left)
toautonomous vesicle (center).Arrowheads show patchesofviruscontinuouswithfilipodialandflat surface plasma membrane. x65,000. (b) Aportionof acoated vesiclecontaining virus (arrow)isseenadjacentto an apparentmonoendocytotic vesicle (arrowhead). Crossed arrows indicatealarge, smooth vesicle coatedon its inner surface with virusand asmaller, smoothvesicle onlypartially coatedon itsinner surface with virus.
x54,000. (c) Small arrow indicates a coated vesicle containing virus fairly deep in the cytoplasm. This
representsasdeepapenetrationof thecytoplasmaswaseverobservedforcoatedvesicles. Crossed arrows indicatealarge vesicle andasmaller, smoothvesiclethat mayactuallybecontinuous with each other.The largearrow atthebottom (alsoseeinset)indicatesasmooth vesicle stillin theprocessofinvagination. Virus completely fills the resultant vesicle. Virus in a patch is also evident at the cellsurface(arrowhead).x59,000.
(d) Afilipodium, apparently coated with virus. Thefilipodiumseems to be bound to the adjacent surface
membrane via itscoating ofvirus. x68,000.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
INTO RODENT CELLS 543
2a.
1..
zol*-
A-W`-s* - 4,
X.v;St Aas ..-dta ~
.d At
4 I
.-W
k
.404I;**.. I
w
*-;4,.
6A
on November 10, 2019 by guest
http://jvi.asm.org/
Atrt 7 '~aor~ V
O.
-.7
"
'4
FIG. 8. MVMuptake intoA-9icells. The experimental conditions are the same as those describedin the legend to Fig. 7.(a)Arrows indicate severalplaces where channels of virus have been formed by the apposition of membrane surfaces. Filipodia are involved in some of these. x30,000. (b) Arrows indicate several large vesicles with smooth surfacescontainingnumerous virus particles. One suchvesicle(center)appearsto consist of two membrane surfacesinterconnectedwithvirus,forming acircularchannel. The crossed arrowindicates a smallpatch of virus that has not been internalized. The arrowhead indicates a coated vesicle possibly containinga singlevirusparticle. x42,XX0.(c)Arrow indicates a circularchannelof membrane-bound virus. This may be a cross sectionthroughthe base of a filipodium that is sunken into thecytoplasm.Arrowheads indicateviruses that have not yet beeninternalized. x80,OOO.
544
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.504.61.456.40.591.2]taken into the cells asrapidly as the bulk of the
viral particles whichdo not initiate infection.
The uptake of infectious virus occurs in the earlyG1 phase of the cell cycle and is not limited to the S phase. Therefore, neither the binding nor the uptake of virus is the cause of the S phase restriction of MVM replication.
Spontaneous elution of virus from the cell surface has been observed with adenoviruses (1) and picornaviruses (2, 4). In these cases, virus bound to the cells at40Candeluted at370C is no longer capable of binding again to the cell due to somephysical change in the viral particles (1, 2). Approximately 8 to 10% of the MVM
particles boundtoA-9cellsat4°C eluteat37°C
and remain unbound even after an additional 8 h of incubation at 370C. However, binding of parvovirus to cells at 40C does not appear to alter theparticles in any significant way, as the eluted virus remains fully infectious. In addition, the formation of the initial stablecell-virus com-plexes at370Cdoes notdisrupt the bound virus. Virtually all of the bound virus has formed an EDTA-resistant complex within 30 min at370C, and theviruses recovered from thecellsatthis time have lost little of their infectivity. On the other hand, there is a progressive decline in the infectivity of virus recovered from cells during
theinternalizationprocess. It remains to be seen
whether this loss ofinfectivity in the particles taken into the cells canbe relatedto an infec-tiousuncoatingprocess.
ACKNOWLEDGMENTS
The assistanceofRandyRichards intheanalysisofviral
DNA isgratefully acknowledged.
Thisinvestigation was supported by Public Health Service grants 1K04 CA-00134 and5RO1 CA-16517from the Na-tionalCancer Institute and by grant 1-396 from the National Foundation March ofDimes.
lTERATURE CITED
1.Dales, S. 1973. Early events in cell-animal virus interac-tions.Bacteriol. Rev. 37:103-135.
2. Fenwick,M. L,and P. D.Cooper.1962.Early interac-tions betweenpolio virus and ERKcells: some obser-vations on the nature and significance ofthe rejected particles. Virology 18:212-223.
3. Holiand,J. J. 1961.Receptor affinities as major deter-minants ofenterovirus tissue tropism in the human. Virology15:312-326.
4. Joklik, W. K., and J.E.Darnell,Jr.1961.The absorp-tion and early fateofpurifiedpoliovirusinHeLacells. Virology 13:439-447.
5. Linser, P. L.,H. Bruning, and R. W.Armentrout. 1977. Specific binding sites for aparvovirus, minute virus of mice, on cultured mousecells. J. Virol. 24:211-221.
6. Lipton, H.L.,and R. T. Johnson.1972.The pathogen-esisof rat virus infections in the newborn hamster. Lab. Invest. 27:508-516.
7.Rhode,S. L., m. 1973.Replicationprocess ofthe parvo-virus H-1. I. Kinetics in aparasynchronous cell system. J.Virol. 11:856-861.
8. Richards, R.,P.Linser,and R. W. Armentrout.1977. Thekinetics of assembly of a parvovirus, minute virus ofmice, insynchronized rat brain cells. J. Virol. 22: 778-793.
9. Sutton,W. D. 1971. A crude nucleasepreparationsuitable for use inDNA reassociation experiments. Biochim. Biophys. Acta 240:522-531.
10. Tattersall, P.,P.J.Cawte,A. J.Shatkin,and D.C. Ward. 1976.Three structuralpolypeptidescoded for by minute virus ofmice,aparvovirus.J.Virol. 20:273-289.
11. Wray,W. 1975. Parallelisolationproceduresfor meta-phase chromosomes, mitotic apparatus, and nuclei. MethodsEnzymol.40:75-89.
31,
on November 10, 2019 by guest
http://jvi.asm.org/