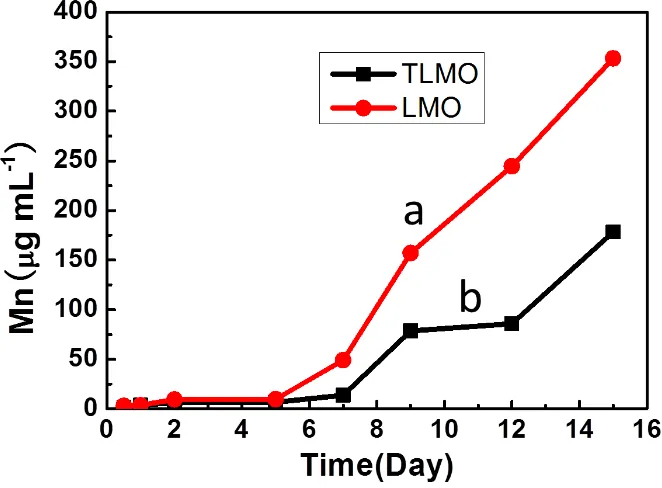
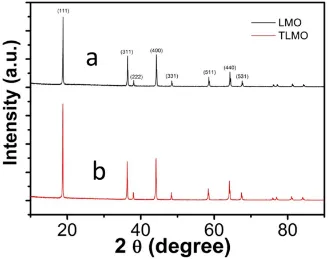
TiO2-Modified Spinel Lithium Manganate for Suppressing Mn Ion Dissolution in Lithium Ion Batteries
12
0
0
Full text
Figure



+4
Related documents
To overcome the low lithium ion diffusion and slow electron transfer, a hollow micro sphere LiFePO 4 /C cathode material with a porous interior structure was synthesized via
Its excellent electrochemical performance as anode material for LIBs shows that this design is an efficient way not only to buffer the volume expansion of ZnO during cycling