Rochester Institute of Technology
RIT Scholar Works
Theses
Thesis/Dissertation Collections
9-1-1996
Modeling ventricular-vascular coupling in the
developing embryo
Michael Bauman
Follow this and additional works at:
http://scholarworks.rit.edu/theses
This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion
in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact
ritscholarworks@rit.edu
.
Recommended Citation
MODELING VENTRICULAR·VASCULAR
COUPLING IN THE DEVELOPING EMBRYO
by
Michael G. Bauman
A Thesis Submitted
III
Partial Fulfillment
of the
MASTER OF SCIENCE
III
Mechanical Engineering
Approved by:
Mark H. Kempski, Ph.D.
Department of Mechanical Engineering
Kevin B. Kochersberger, Ph.D.
Department of Mechanical Engineering
Michael P. Hennessey, Ph.D.
Department of Mechanical Engineering
Charles W. Haines, Ph.D.
Department of Mechanical Engineering
(Thesis Advisor)
(Department Head)
Department of Mechanical Engineering
College of Engineering
Rochester Institute of Technology
Rochester, NY 14623
Release
I, Michael G. Bauman, do hereby give permission to Wallace
Memorial Library to reproduce this Thesis, entitled Modeling
Ventricular-Vascular Coupling in the Developing Embryo, in whole or in part. Any
such reproductions may not be for commercial use or profit.
Signed,
Michael G. Bauman
9-
23-90
Table
of
Contents
List
of
Tables
List
of
Figures
Abstract
iv
Vll
1.
Introduction
1
1.1
Congenital Cardiovascular Malformations
1
1.2
Research Objectives
2
1.3 Thesis
Objectives
and
Goals
3
2.
Methodology
6
2.1
Modeling
6
2.1.1
Modeling
Hemodynamic Systems
6
2.1.2 Model Rationale
7
2.1.3 Model Simulation
11
2.1.4
Simulink
16
2.1.5 State Space
and
Block
Diagram Formulation
1
8
2.1.6 Initial Conditions
24
2.2
Optimization
Techniques
26
2.2.1 Design Variables
26
2.2.2 The Method
of
Least Squares
27
2.2.3
Numerical
Optimization
Techniques
29
2.3
Preparation
of
Data
35
2.3.1 Data Acquisition
35
2.3.2 Data
Filtering
and
Conversion
35
2.3.3 Assumptions
36
3. Results
41
3
.1 Verification
of
Simulation
and
Optimization
Routines
4 1
3.1.1
Benchtop
Circuit
Setup
41
3.1.2 Model 1 Test Results
42
3,1.3 Model 2 Test
Results
43
3.1.4 Model 3 Test
Results
44
3.1.5
Comments
45
3.2
Results
of
Blood Data
Simulation
and
Optimization
46
3.2.1
Convergence
Criteria
46
3.2.2
Model
1
Results
47
3.2.3 Model 2
Results
51
3.2.4 Model 3
Results
56
3.2.5
Multiple
Cycle
Simulation
Results
60
4.
Discussion
and
Conclusions
6.2
4.
1
Validity
ofSimulations
62
4.2
Recommendations
for
Future
Studies
65
Appendices
68
Appendix
A
Algorithm Flow
Chart
68
Appendix B
MATLAB
m-files
69
B.l
INTERP.m
70
B.2
OBJILSC.m
71
B.3
OBJlLSL.ni
73
B.4
OBJILSC.m
75
B.5
OBJ2LSL.m
11
B.6
OBJ3LSC.m
79
B.7
OBJ3LSL.m
81
List
of
Tables
Table
Description
Page
Section 3
3-1
Model 1 Test Results
42
3-2
Model 2 Test Results
43
3-3
Model 3 Test Results
44
3-4
Model
1
Constant
Parameter Optimization
Results
49
3-5
Model 1
Linearly Varying
Parameter Optimization Results
50
3-6
Model 2 Constant Parameter Optimization Results
54
3-7
Model 2
Linearly Varying
Parameter Optimization Results
55
3-8
Model 3 Constant Parameter Optimization Results
58
3-9
Model 3
Linearly Varying
Parameter Optimization Results
59
List
of
Figures
Figure
Description
pase
Section
1
1-1
Formation
of
the
Heart
2
1-2
Schematic
of
Heart
and
Adjoining
Vasculature
4
Section
2
2-
1
Lumped Parameter Fluid I-C-R
Model
6
2-2
Windkessel Afterload
Model
8
2-3
Model 1:
Resistive-Capacitive
Model
of
Aortic Arches
9
and
Conotruncus
2-4
Model 2: Series Inertance Model
of
Aortic Arches
and
9
Conotruncus
2-5
Model 3: Parallel Inertance Model
of
Aortic Arches
10
and
Conotruncus
2-6
Basic R-C-R Circuit
1 1
2-7
Plot
of
Output Voltage for R-C-R Circuit Test Case
1
3
2-8
Plot
of
MATLAB Simulation Output for R-C-R Circuit
1 5
2-9
Block Diagram Representation
of aSystem Transfer
16
Function
2-10
Model 1: Resistive-Capacitive Model
of
Aortic Arches
18
and
Conotruncus
2-11
Model 1 Block Diagram
19
2-12
Model 2: Series Inertance Model
ofAortic Arches
and
20
Conotruncus
2-
1
3
Model 2 Block Diagram
2 1
Figure
Description
Page
2-15
Model 3 B lock
Diagram
23
2-16
Model 1: Electrical
Benchtop
Test Case Circuit
30
2-17
Plot
of
Benchtop
Test Results
Using
LEASTSQ.m
33
2-18
Unfiltered Pressure
and
Volume Data
35
2-19
Filtered Pressure
and
Flow Data
36
2-20
Multiple Pressure
vs.
Volume Traces (P-V
Loops)
38
2-2 1
Ventricular-Vascular
Coupling
Window
ofOpportunity
39
Section 3
3-1
Model 1
Benchtop
Optimization Results
42
3-2
Model 2
Benchtop
Optimization
Results
43
3-3
Model 3
Benchtop
Optimization
Results
44
3-4
Model 1 Constant Parameter Best Fit
48
3-5
Model
1 Linear
Parameter
Best Fit
48
3-6
Model
2 Constant
Parameter
Best
Fit
52
3-7
Model 2 Linear Parameter Best Fit
52
3-8
Model
2 Constant
Parameter
Worst
Fit
53
3-9
Model
2 Linear
Parameter
Worst Fit
53
3-10
Model
3 Constant
Parameter
Best Fit
57
3-
1 1
Model
3
Linear Parameter
Best Fit
57
Abstract
Congenital
cardiovascular
malformations
are
one
of
the
major
contributors
to
infant mortality
in
the
United
States
today.
Defects
in
the
cardiovascular
system
are
evident
at
very early
stages
in
the
developing
embryo,
yet
there
exists
no
substantial
diagnostic
tool(s)
for
recognizing
defects
at
these
early
stages.
Cardiac
morphogensis occurs
throughout the
pre-innervated period
of
embryonic
development.
It
is
speculated
that the
flow
of
blood
during
the
early
embryonic stages plays an
important
role
in
the
development
of
the
cardiovascular
system.
Blood
flow
is
a
possible
mechanism
for
communication
between
the
heart
and
vasculature,
contributing
to
cardiac
development.
The
objective
of
this
study is
to
investigate
ventricular-vascular
coupling
across
the
conotruncus
and
aortic
arches
using
mathematical
models
to
simulate
experimental
hemodynamic data from
a
Stage
21
White Leghorn
chick
embryo.
Lumped-parameter
hydraulic
models of
the
embryonic
vasculature
are
tested
for
their
ability
to
predict
experimental
data
using Least Squares
minimization
techniques.
This
Chapter 1
-Introduction
1.1
Congenital
Cardiovascular
Malformations
Congenital
cardiovascular
malformations
are
oneof
the
major contributorsto
infant
mortality
in
the
United
States
today
(Clark
and
Takao 1990).
Cardiovascular
malformationsmay
also present
themselves
as a major
health
risk
later in life.
Detecting
birth
defects,
andin
particular
those
related
to the
cardiovascular
system,
early in fetal development has been
a
goal of
many
researchers.
Thus far
there
have been very few
tools to
act as
indicators
ofcardiac malformations
during
embryonic morphogenesis.
The heart is
the
first
functioning
organ
in
the embryo,
beginning
to
beat
wellbefore
the
existence
of a nervous system.
In
the
human,
the
heart begins
to
beat 22 days
after
conception.
At only
a
day
after
the
heart
starts
beating,
it
begins
to
fold
and
develop
into four
distinct
chambers
known
as
the
right and
left
atrium and
right andleft
ventricle.
From
a
primitive muscle-wrapped
tube,
the
heart
undergoesa
very
complex and continuous period of
development (see Figure
1-1),
and
if
all
goes
right,
ending
witha
four-chambered blood
pump,
complete with
valvesand neural control system.
During
the
morphogenesis of
the
heart,
there
exist
numerous(and
quite
possibly
infinite)
occasions
for
malformations
to take
place,
thus
making it
remarkablethat
a
majority
of
hearts
turn
out
"correct."
This
raises
the
important
question,
"Why
do
mosthearts (and
infants)
turn
out
fine,
while others
do
not?"
What
are
the
factors
that
contributeto the
development
of
the
heart,
and
what
is
the
controlling
mechanismin
cardiovascularmorphogenesis when
there
is
an absence of
acentral
Figure 1-1.
Formation
of
the
heartfrom
a musclewrapped
tube.
While
cardiovascular malformations are not
limited
to
any
one
specificarea,
it
has
been
shown
that
ventricular
septal
defects
are
perhaps
the
most
commonof
allheart
malformations
occurring in live-born infants (Human
Embryology
1993).
Along
withthis
are malformations such as
double
outlet
left
ventricle and
transposition
ofthe
great
vessels.Some
malformations
may
not
be detected
throughout
embryonicdevelopment,
and
only
become painfully
apparent when a person
has become
anadult.
Anomalies
withthe
mitral
valves are quite common
in
adults,
yet are
rarely life
threatening
(Human
Embryology
1993).
Early
detection
of such malformations often allows
treatment
and
in
some casesthe
saving
ofa
life,
thus
a good
understanding
ofthe
processes
andfunctions involved
with morphogenesis
become
a great
concern.1.2
Research
Objectives
The
long
term
and
overall scope ofthe
researchpresented
in
this
thesis, along
withparalleled
research,
is
to
foster
the
prevention oflife
threatening
birth defects
through the
sciences of
engineering
and medicine.The
short
term
objectives associated withthis
researchare aimed at
understanding
the
hemodynamics
occurring in
the
pre-innervatedembryo,
and
to
find
methods of
recognizing
The
focus
is
to
utilize
engineering
tools
as
a
meansof
describing
hemodynamic function
within
the
normally developed
embryonic
cardiovascularsystem.
1.3
Thesis
Objectives
and
Goals
The
primary focus
of
this thesis
is
to
investigate
the
existence of
ventricular-vascularcoupling
in
stage
21
White
Leghorn
chicken embryos
through
various
hydraulic modeling
scenarios.
Stage 21
represents
about
3.5
days
into
the
gestation
period
for
the
chicken
embryo,
which
translates
to
about
45
days
into
the
human
gestation
period,
according
to the
Hamburger-Hamilton
(Hamburger
and
Hamilton
1951)
scale
of embryonicdevelopment.
At
this
stage,
as mentioned
before,
there
exists no autonomic
nervous systemto
control cardiacfunction.
The
material presented
in
this thesis
is
an extension of previouswork as
wellas
anexploratory
effort
in hemodynamic
modeling.
It is
speculated
that the
flow
ofblood
during
the
early
embryonic
stages
plays
animportant
role
in
the
development
of
the
heart
and vasculature
(Hu
and
Clark 1989). In
some
cases,
deliberate shunting
of
blood
flow
or
slight
changes
in
the tissue
composition
(i.e.
treatment
with retin
A)
has induced drastic
changes
and malformations
in
cardiovascular
development (Broekhuizen 1995). This
researchhas
pointed
in
the
direction
of
blood flow
as
being
a medium
for
physiologic
communicationduring
morphogenesis.
In
the
pre-innervated
embryo,
there
existsno
autonomic nervoussystem
to
controlblood
flow,
yetthere
exists
strong
evidence
to
supportthe
theory
ofhemodynamic
regulation on abeat
to
beat basis
(Kempski
et
al1993).
Hemodynamic
pressurehas been
hypothesized
to
respond
to
aresistance
to
flow
associatedwith
vascularimpedance,
indicating
possible
hemodynamic
During
early
stages
of
development,
the
vasculature
just
proximalthe
left
ventricleundergoes several
changes.
The
outflow
tract
from
the
left
ventriclebegins
as
a
bulb-like
formation
known
as
the conotruncus
(bulbus
cordis),
and
eventually
septatesand transforms
into
the
mature semilunar valve.
During
early
cardiovascular
development
several vascularbranches known
as
the
aortic arches sprout
from
the
early
outflow
tract, known
as
the
aorticarches which
transform
during
subsequent
development
stages
into
what will
later become
the
major
arteries
in
the
adult.
Further
downstream
is
a
multitude
of
vesselsthat
willeventually
extend
throughout
the
adult
body.
Conotruncus
Aortic Arches
flightand lett-anterior cardinal veins
Ventricle
Rightandleftumbilical arteries
Figure 1-2. Schematic
representationof human heart
and vasculature atapproximately 25
days
afterconception.
Studies have
shown
that
ventricular-vascularcoupling may
exist
early
on
in
cardiac
development,
and
may
act as
a
regulatory
mechanism
for
cardiac
development.
Even in its
earliest
form,
the
conotruncus acts
to
regulate
blood flow
out of
the
heart,
and
keep
blood
from regurgitating back into
the
ventricle.
The
conotruncusmay play
a
vital
role
in
1989).
The function
and
health
of
the
conotruncus,
therefore,
may
act as a
mechanism and/orindicator for
the
development
of
malformations
associated
withthe
left
ventricle andthe
adjoining
vasculature.
Developing
amathematical model
to
representthe
physiology
withina
section of
the
vasculature which
includes
the
ventricle,
conotruncus,
aorticarches,
and
distal
vasculature
then
becomes
the task
athand (Matalevich
1995,
Mates
etal
1982,
Kempski
et al
1993,
1995).
Previous
work
has
demonstrated
the
use of electrical circuit-type models
in
simulating
the
dynamics
of
blood flow
through
various parts
of
human
and
non-human vasculature.Lumped
parameter models
involving
resistors,
capacitors,
and
inductors have been
used
to
simulate
the
resistive, elastic,
and
inertial
characteristics,
respectively,
associated withthe
blood
and/or
blood
vessels.Varied degrees
ofsuccess
have
been
achievedin using linear
forms
of
these
models,
such
as
the
classic
Windkessel
model,
to
representvascular
impedance
and
coupling
associated with
blood flow
and pressure
(Kempski
1994,
Matalevich
1996).
This study
will
investigate
the
application of
these
linear lumped
parameter circuit
models
to
simulate
physiologic
occurrences
across
the
conotruncus
andaortic
arches.
Various
models
willbe
postulatedand
tested
for
goodness
of
fit,
parameter
sensitivity,
linearity,
and
physiological relevance.This study
willalso
evaluatethe
use of commercial
PC
-based softwareas a
tool
for
cardiovascular research.
The
workpresented
in
this
paper
will
be
used as a
benchmark for future
studies
in
the
area
ofventricular-vascular
coupling
and
Chapter 2
-Methodology
2.1
Modeling
2.1.1
Modeling
Hemodynamic
Systems
Blood flow
through the
human
vasculature
is
a
hydraulic
orfluid
systemin its
simplest sense.
Blood flow is dependent
upon pressure variations
throughout the vasculature,
which
in
turn
is
a
function
of vascular resistance
to
flow,
distensibility
of
the
vesselwalls,
and
inertial
effects associated with
the
blood itself.
Though
numerous
factors
contributeto
the
blood flow
characteristics
in any
part
ofthe vasculature,
simple
"lumped"
parameter
models of a
fluid
system can
be
used
to
represent a
sumtotal
ofthese
factors for
the
ease ofsimulating
the
dynamic
performance
within a particulararea of
the
vasculature.Figure 2-1
shows a
typical
lumped
parameter modelof a
fluid
system.
f
~
c
='
K
s/
////7//S7
Pa
X
\
'Piston and
Spring
(Ap
=pistonarea)
Rf
sFluid
"Slug"(Acs
=crosssectional area ofslug)
'/
=pL
cs /
figure 2-1. Lumped
parameterfluid I-C-R
model.
Volumetric flow from
the
heart,
Qh
is
the
Model
Parameters
for
a
Hydraulic
(Fluid)
System:
q
:
volume
[ml]
Q
:
volumetric
flow
rate
[ml /
sec]
P
:
pressure
[mmHg]
R
:
resistance
[mmHg
sec
/ml]
C
:
capacitance
[ml
/mmHg]
I
:
inertance
[kg
/mm4]
As
with
any
dynamic
system,
there
exists a set of
governing
equations whichdescribe
how
the
system
behaves.
For
a
hydraulic
system, the
elemental equations
relating fluid flow
and pressure
(differential)
to
the
system parameters are as
follows:
Pbc=*/Q3
(2-1)
Pab=//iir
(2,2)
dP
Q2
=C,
-f-(2.3)
dt
And
by
the
laws
of
continuity,
we can
writean expression
relating
the
flow
through
various parts
ofthe
system
as:Qi
=Q2
+
Q3
(24)
When
the
elemental equationsare
usedin
the
continuity
equation,
adifferential
equation results
which
describes
the
dynamics
of
the
system.
Pb+^Pb+^Pb=^Pa+^Pc
(2-5)
CjRj
Cj Ij
Cj Ij
Cj Rj
2.1.2
Model Rationale
Lumped
parametermodels,
typically
in
the
form
of electrical circuit analogs
to the
hydraulic
circuits,
have been
usedwith
successin modeling
the
hemodynamics
through
both
been
used
to
simulate
the
blood
flow
through
various
vasculaturebeds (Mates
1982,
Burkoff
[image:18.562.63.506.108.287.2]1988,
Kempski
1994),
and
has
proved
to
be
a valid model
in
multiple studies(Figure 2-2).
Figure 2-2.
Three-element Windkessel
model shown as an electrical circuitanalog
withcurrent source
representing
aflow driver.
At
particular stages of embryonic
development
andsections
ofthe
vasculaturebed,
the
Windkessel
modelfits
quitewell,
while
it
seemsto
lack
performancein
other areasof
study.
At
stage
21
of
the
Leghorn
chick
embryonicdevelopment,
the
vasculaturebed
includes early forms
of
the
conotruncus and aortic arches.
At
this
stage
the
conotruncusis
not much
morethan
a
bulbus
muscleformation
ratherthan
afull
fledged
valve.The
aortic
arches are
arterialbranches
that
eventually
combine
during
morphogenesisto
form
a single
aorta.
While
the
Windkessel
modelmay accurately
predictdynamics
downstream
of
the
arches,
also referred
to
as
"afterload",
it is
speculatedthat
additionalparameters need
to
be
introduced
to
represent
the
dynamic
characteristics associatedwith
the
additionalphysiologic
features.
Assuming
a complianceis
associatedwith
both
the
aortic
arches
and
conotruncus,
along
withan
impedance due
to the
additionof
the
aortic
arches,
wecan postulate a
^Taa
Rvi
AA/V
[image:19.562.59.505.440.619.2]c
Figure 2-3.
Model 1:
resistive-capacitive
modelof
aortic arches and conotruncus withWindkessel
model
representing
afterload presented
by
the
distal
vasculature.Rvl, Rv2,
andCv
representthe
parameters associated
withthe
downstream
vasculature(afterload).
Though
the
vessels are
very
small
during
this
stage
in
embryonic
development,
the
movement of
the
blood may impose
a
relatively
significant
momentumassociated
witha
"slug"
of
fluid
(blood)
within
the
flow.
A
slight modification
to
Model 1 in Figure
2-3
to
include
an
inertance
term,
lb,
would
allowfor any inertial
effects
having
to
do
with
the
mass
of
the
blood.
Figure 2-4
shows
the
schematic
definition
ofModel
2
withthis
inertial
systemparameter.
Figure 2-4.
Model 2:
series
inertance
modelof
aortic archesand
conotruncus
withIf
inertance
associated
with
the
mass of
the
blood
is
a
significantfactor,
then
it is
unclear as
to
whether
it
occurs
just
posterior
to the
conotruncus,
as
portrayedin Model
2,
or
if
there
exists an
inertial
effect right out of
the
left
ventricle
itself.
If
the
latter is
the case, the
model needs
to
be
modified
further
to
simulate
this
effect and
to
also
include
a
resistanceimposed
by
the
conotruncus.
Anticipating
the
effect of such a
system,
a
configurationof an
[image:20.562.62.502.270.478.2]inertor
(inductor)
in
parallel with a resistor would simulate
the
dynamics
quite
accurately.Figure 2-5
shows
this
configuration
as
Model
3,
once
again
using
the
three
elementWindkessel
model
to
represent
the
downstream
vasculature.
Figure 2-5.
Model 3:
parallelinertance
modelof
aortic archesand
conotruncus withWindkessel
model
representing
afterload presentedby
the
distal
vasculature.With
three
models
postulated,
it is inevitable
that
one
model
will
represent
the
dynamics
of
the
system
better
than the
others.To determine
the
validity
of each
model,
actual
data
needs
to
be
passed
through
a
simulationof each representative
system,
and
the
system
parameters
need
to
be
optimized
such
that there
is
a
good
match
between
the
from
the
left
ventricle, Qs.
The
output
is
seen
as
the
left
ventricularpressure,
Pa,
and
is
represented
by
the
pressure at node
(a)
in
all
three
models.
Before using
the models,
each system must
be
converted
to
a usableform,
that
is,
a
set of
differential
equations
representing
the
dynamics
of each system.
The methodology
of
doing
this
can
be
found
in
the
next section.
2.1.3
Model
Simulation
There
are
several methods
for solving for
the
output(s)
of a
dynamic
system.A
typical
system can
be
represented with one or more
differential
equations
in
terms
of state
variables and some sort of
forcing
function.
We'll
use an example of a
basic R-C-R
circuit(Figure 2-6).
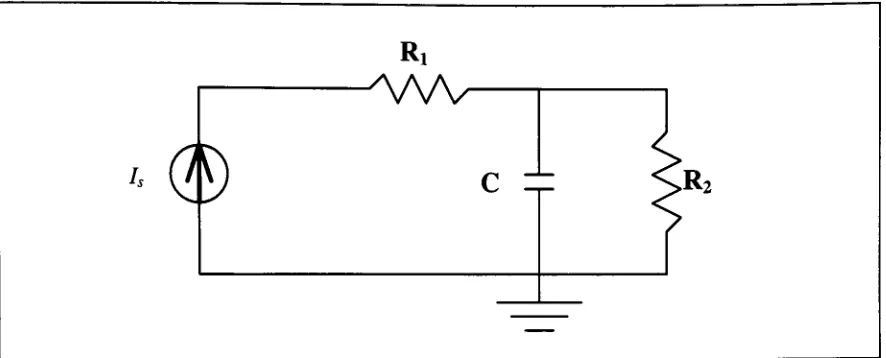
Figure 2-6. Basic R-C-R
electricalcircuit
Assuming
the
input
source
is
a
currentdriver
as shown
in
the
diagram,
the
governing
differential
equation
for
the
system
is:
vb
+
1
1
Where
vb
is
the
output voltage at node
(b). The
system
time
constant
is
given
by
the
inverse
of
the
coefficient
on
the
second
term:
T
=Ci/?2
(2.7)
It
should
be
mentioned
that
since
the
circuit
is
set
up
with
a
current
driver,
the
resistance
Ri
has
no
bearing
on
the time
constant
if
vb
is
the
desired
output.
For
a
harmonic
input,
(i.e.
Is(t)=
Is
sin(cot)),
a closed
form
solution can
be
expressed as:
Ji
+
iC^coy
From
equation
(2.8),
the
output voltage
vb
can
be
calculated
directly
from
the
input Is.
If
the
voltage
at
node
(a)
is
the
desired
output,
the
previous
expression
can
be slightly
modified
through the
use of
Ohm's law
to
give a closed
form
expression
for
va:va-vb
=iA^>vb
=vcl-i1R1
(2.9)
va
(t)
=7,/J,
sm(cot)
+
Is
. 2sin(a
-<p)
(2.10)
jl
+
iC^a))2(where
Is
is simply
the
sine amplitude
of
the
current.)
This
form
of
the
solution adapts quite well
to
least
squares optimization of circuit
parameters
using EXCEL
or other spreadsheet
solvers.
To demonstrate
this
method,
the
circuit shown
in Figure 2-6
was
built up
on
a
bread-board
and used
to
produce an output
voltage at node
(a)
whenexcited
by
a sinusoidal
input
current,
Is
(The
current source
wascreated
by
passing
the
output
voltagefrom
a
function
generatorthrough
a
resistance, thus
producing
a
proportionally
scaled
current.The
signalwas
a
zero-mean
sinusoid
of
approximately
1Hz
nominal
frequency.
Q, Ri,
and
R2
were
set
to
10u/,
17.58kQ
and
LabVIEW data
acquisition algorithm,
and
imported
directly
into
EXCEL
withno
filtering
necessary.
The
current amplitude
Is
was
determined
from
the
data
series,
and
the
signalfrequency,
co,
was verified as
being
6.15rad/sec
(/5=0.98Hz).
With arbitrary
values givenfor
Ci,
Ri,R2,
and
$,
a
sum-of-the-squares
minimization was performed
using
the
recordeddata
for
va
as a reference.
Figure
2-7
shows
the
outcome of
the
minimization with
resulting
valuesfor
Q, Ri,R2,
and
0
as expected.
Though relatively
simple and
straightforward,
this
methodproves
to
be
very unaccomodating
when more complex systems are
being
investigated.
The
closedform
solutions
to
multi-order systems with several parameters are
difficult
to
arriveat,
soa
state-space
formulation
of
the
governing differential
equations
is
developed,
and numericaltechniques
are used
to
solve
the
system.
Output Voltage
(Va)
for R-C-R Circuit
0.4
-0.4
Time,
seconds-Actual
Voltage
[image:23.562.66.491.361.570.2]Predicted Voltage
Figure 2-7. Analytic
simulationof R-C-R
electricalcircuit.MATLAB
employs
severaldifferent
numericaltechniques
to
solve multi-order state
space systems
of
differential
equations.Most
commonly,
MATLAB
makes use of
and solve
for
the
state
variables
at
discrete
time
points.
The
example
shown above could alsobe
put
into
state-space
form
and solved
using
the
numerical routines
withinMATLAB.
Essentially,
we
have
a
first
order state-space
equation,
where
the
voltagevb
is
the
statevariable,
and an output equation
for
va
using
the
same state space:
dx
dt
'
-1 "
v0
+
"1
"Lc>.
/,
=[A]*(iO
-I-[fi](0
differential
equations)
y(t)
=va=vb+
[R\ ]l,
=[C]x(t)
+
[D]u(t)
outputequation(s)
MATLAB
needs
only
the
A,B,C,
and
D
matrices
along
with a
vectorof
the
initial
conditions
(see Sect.
2.4,
Initial
Conditions)
on
the
states
to
produce an
outputgiven
aninput
u(t).
This
method makes
it very easy
to
go
from
the
state space
equationsto
a
numericaloutput without
having
to
find
a closed
form
of
the
solution
for
the
system.
For
the
R-C-R
circuit
example,
we
only have
one state
variable,
vb.An
expression canbe
writtenfor
the
initial
value of
vb
based
upon
the
input
Is
and
the
known
(experimental)
value of
va
at
time
t=0:
v<0) = v
-RJl0)
(2.11)
To
demonstrate
the
useof
the
state-space
solution, the
same
system shownin Figure
2-6
is
solved
using MATLAB. A function
called
by
LSIM,
shortfor linear
simulation,
is
usedto
integrate
the
state space
equationsat
discrete
timepoints
using
an
array
of
valuesfor
the
input,
u(t),
whichin
this
case
would representthe
currentin
the
R-C-R
circuit.
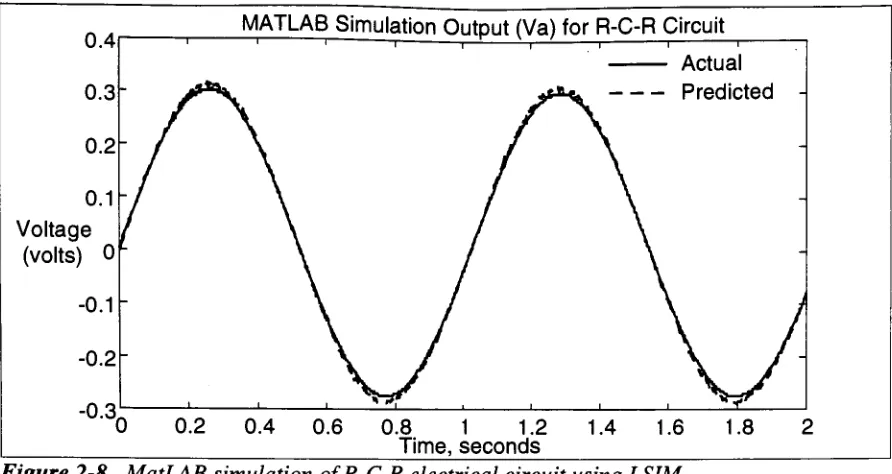
Figure 2-8
0.1
Voltage
(volts)
0LMATLAB
Simulation
Output
(Va)
for R-C-R Circuit
Actual
[image:25.562.58.504.48.285.2]Predicted
Figure 2-8. MatLAB
simulation
of R-C-R
electrical circuitusing
LSIM.
For
a
direct
simulation of
the system,
the
A, B, C,
and
D
matrices
aredefined
withthe
system parameters
Cl, Rl,
and
R2. For
a case where
manipulationof
the
system
parametersis
necessary,
such as
during
optimization,
the
matrices can
be defined in
terms
of
variables,
which are assigned values
somewhere
withinthe
MATLAB
workspace.Optimization
can
then
take
place
by
varying
the
system parameter variables
to
minimizea particular objective
function. The
model parameters used
in predicting blood flow
need
to
be
optimized such
that
there
is
a
good
match
of
predictedand
experimental
data.
Optimization
of
the
system
2.1.4
Block
Diagrams
and
SIMULINK
Interface
Up
until
this
point,
no
mention
has been
made
of
transfer
functions
or
block
diagrams.
Part
of
this
study includes
the
evaluation
of
desktop
software,
MATLAB
in
particular,
as a valid
tool to
be
used
in
research activities associated
withbiological
systems.MATLAB
includes
a
package
called
SIMULINK
which
allows
simulationof
dynamic
systems
by
defining
a
block
(simulation)
diagram
representation of
the
system
(See Figure
2-9).
s+1
Input
s2+ s +
1
^
Output
T
ransfer
Functic
>nFigure 2-9. Block diagram
representationof
a systemtransfer
function.
The
transfer
function
of anentire system can
be broken up into
smallersubsystems
that
are all
linked
together
in
the
block diagram.
Feedback
loops, integrators,
and
summing
junctions
can
all
be
extracted
from
the
system
transfer
function,
ordrawn right from
the
constitutive equations
for
the
particular system.
There
are
distinct
advantages
to
defining
asystem
in
this
manner.First,
the
block diagram
provides a
visualaid
in understanding
the
dynamics
and
various
pathways
associated
withthe
state
variables.Second,
the
block
diagram
makes
it easy
to
play "what
if..."
scenarios.
That
is,
additionalblocks
and pathways
could
be
added
in
a
very
short
time
without
having
to
derive
the
analyticexpressions
defining
the
state spaceof
the
model.One disadvantage
of
using SIMULINK
over
an
analytic
formulation
of
the
state-spaceis
that
computationtime
increases
as
MATLAB has
to
"convert"
beginning
of
this study, the
actual
cost
in
time
was
unclear,
but
the
results
of
this
study
should provide a good
benchmark
that
can
be
compared
to
otherforms
of
simulation.The
SIMULINK
package works
withinthe
MATLAB
workspacejust
as
any
otherm-file
or
function,
since
it is
saved as
lines
of code within
an m-file.Within
the
m-fileis
information pertaining
to the
state
variables
of
the
system,
and
in fact
the
state spacerepresentation of
the
system can
actually be
extracted
from
the
m-file
in
terms
of
the
A, B, C,
and
D
matrices mentioned
in Section 2.1.3.
Because
of
this,
the
sametype
ofRunge-Kutta
2.1.5
State Space
and
Block
Diagram
Formulation
of
Models
1,2,
& 3
SIMULINK
was used
in
the
solution and optimization of
the
models postulatedfor
predicting
blood flow
during
this
study.
SIMULINK
can
be
called
by
any
function,
such asLEASTSQ
(least
squares
optimization
routine,
see
Section
2.2),
making
custom sub-routinesvery
simple.
But
before
a
SIMULINK
representation
of
the
model can
be
written,
the
modelmust
first
be
converted
to
block diagram form
from
the
constitutive
equationsdefining
the
interaction
of
the
model parameters.
The
formulation
and conversion of each
modelis
as
follows.
Model
1
figure 2-10.
Model
1:
resistive-capacitive modelof
aortic arches and conotruncus withWindkessel
afterload model.By
continuity
we can write
the
constitutive equations:Qs=Qi
Qi
=Q2
+
Q3
Q3
=Q4
+
Q5
From
the
elemental equations we can
write:
dP
1
Q2
=cct-^Pa=3-jQ2dt
P
-PR
TaadP,
=
7^-jQ4dt
Q4
=Caa
~ =>?b
aa^
bc
ilil
P
-PRv,
dP
1
Q7
=R
(2.12)
(2.13)
(2.14)
(2.15)
(2.16)
(2.17)
v2A
block
diagram
can
be formulated
directly
from
the
constitutive and elemental
equations:
ITime.Qs]
From
Workspace
Ql
=HD- Q2
17s
3L
Pa-Pb-n*
fg]
^i/cj>
* l/sSL
l/Rvl Pb-Pc {35=1/Rv2
Q6
i=HD-
07 1/Cv * l/s PaPb
Pc
[image:29.562.75.482.357.593.2]Outport
Using
the
elemental
equations
(2.12-2.17)
in
the
continuity
equationswe arrive at
the
following
state
equations:
a
PR
a+
r
R
"b
+
p.Qs
^ctKTaa
*~ctKTaa
^ct
(2.18)
Pb
=^aa^Taa
P.-+
.^aa^-Taa
^aa^vl
Pb
+
CaaRvl
(2.19)
P.
='RVIcv
P.-
l
+l
R.
vlR
ivv2
(2.20)
[image:30.562.57.508.116.487.2]Model
2
Figure 2-12.
Model
2:
seriesinertance
modelof
aortic arches and conotruncus withWindkessel
afterload model.By
continuity
we can write
the
constitutive
equations:Qs
=Qi
Qi
=Q2
+
Q3
From
the
elemental equations
we can write:
dP
1
q.-C-^p.-JLj
Q;dt
b -"-b
P.-R
dt
Q3=f
J(Pa-Pb)dt
Q3=^^R
TaadP
dP
1
dt
P
-PR
vl
HP
1
Q6=Cvl^=>Pd=^/Q6dt
*-(2.21)
(2.22)
(2.23)
(2.24)
(2.25)
(2.26)
(2.27)
v2
[image:31.562.72.497.75.628.2]02 Pa .m
[Time.Qs]
21
^1/Cct^
l/s-Hi_r
'LU
Outport From
Workspace
Q3
l/s
<M/Ib
. Pa-Pb pn**
LU*
Pb
Rtaa^>
03Rtaa tftl'trn_ 04
1/Caa>
C^/Rvl
1/C^>
Cj/Rv2
Pc
SLJ^
Q5 vc-vd r+>4
tm_os
LJ*
1Pd
wu
07
Using
the elemental
equations(2.21-2.27)
in
the
continuity
equationswe arrive at
the
following
state equations:
p-=iQ>+Q
Q,'^-p,-~Q1--^p,
*h
*-h
It.
1
Pc
=C~Q3-C
R
*~aa
^aaKvl
1
P.+-U>,
Pd
=1
CvRvl
P.-CaaRvl
1
1
+
CvRvl
CvRv2
(2.28)
(2.29)
(2.30)
(2.31)
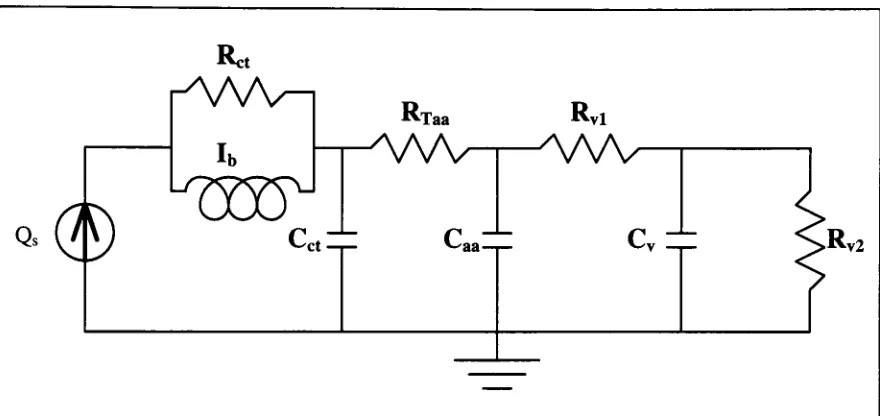
[image:32.562.58.501.115.516.2]Model
3
Figure 2-14.
Model
3:
parallelinertance
modelof
aortic arches andconotruncus
withWindkessel
afterload model.By
continuity
we can write
the
constitutive
equations:Qs
=Qi
Qi
=Q2
+
Q3
Q2
+
Q3
=Q4
+
Q5
Q5
=Q6
+
Q7
From
the elemental
equationswe can
write:
Pa-Pb=Q2Rct
bxb
dt
Q3=-^-J(Pa-Pb)dt
HP
1
Q4=Cct^^Pb=-i-j-Q4dt
dt
PaTP"
R-Q5=^^
LTaa
Q6=Ca^=>R
Q7
=dt
P
-Pc
rd
=7^JQ6dtR
Q8
=cv
vldt
Pd=-l-jQ8dt
Q9=^R
v2Figure 2-15.
Model 3
block diagram.
(2.32)
(2.33)
(2.34)
(2.35)
(2.36)
(2.37)
(2.38)
(2.39)
K2-^
0
OutportITinie.Qs]
Ql Q2fc Ret ^ Pa-Pb
1
^
>
H3
From Workspace
Q3
zfcFl 04 f
^
\
\
PbRU
Q5 -<^j/Rtaa Pb-PcPTf-Llfl^
-trn1/C^>
Pc -RLl f Q7 -<1/Rvl Pc-Pd t+m^Ifl1^
-fcFl
Q
8 ,1/Cv^>
Pd::RU
Q9
"*k
Using
the
elemental
equations
(2.32-2.39)
in
the
continuity
equations we arrive at
the
following
state equations:
Q3=^LQ3+^LQs
(2.40)
^b
=rrT~Pb+FlT-p<+^-Qs
(2-41>
*-ctKTaa
<~ctKTaa
<-ct
P=
l
P-c
r
r
b^aa^Taa
P=-J_P-d
CR
<1
1
+
Ca^Taa
^"aa^vl
Pc+TT-^Pd
C
R
(2-42)
1
1
+
v"-vl
L
v*^vl*-v^-v2
Pd
(2.43)
2.1.6
Initial
Conditions
In
all simulation
methods,
there
exist
initial
conditions on
the
state
variablesof
the
system(s).
If
asystem
is starting
out
from
rest, the
initial
conditions can remain at
zero,
as
this
would
be
the
true
condition,
but
the
systems
in
this
study
are
already dynamic
and
have
been
so
for
along
time
(cardiovascular
activity;
the
heart is
the
first
functioning
organ).
Regardless
of
the
values given
to the
initial
conditions,
the
states should
eventually steady
out
to
the
conditions
dictated
by
the
model
in
a
simulation,
and given a
long
data
series, this
may
not
be
a problem
if
some of
the
data
during
the
start-up
periodof
the
simulation can
be
sacrificed.
The data in
this
study,
however,
is broken up
to
singlebeats
orcycles of cardiac
flow,
allowing virtually
no
time
for
"start-up"of
the
simulation.In
other
words, the
initial
conditions on
the
state
variablesin
the
simulationmust
representthe
values of
the
states at a
point
midway (or
thereabouts)
in
a
single cardiac cycle.Assigning
the
correct values
to the
initial
conditionson
the
states
willallow
the
model simulationto
behave
as
if it had been
Fortunately,
the
initial
conditions can
be
computed
from
the
analytic state
equations.If
we
use
the
first
few
values
of
input
flow, Qs,
(as
we
will
for
the simulations)
andexperimental
Pressure, Pa,
(this
is
the
output
ofthe
simulations)
we
canback-calculate
to
determine
what
the
state values should
be
at
the
beginning
of
the
simulation.The
calculatedvalues
will
be
only
approximations since
differentiation
of
the
data
series(finite
difference)
is
required
to
accommodate
the
analytic
expressions,
but
the
method
has
provedto
be
sufficientin
all
test
cases.
It
should also
be
noted
that the
initial
conditions on
the
states willneed
to
be
calculated
at each optimization
step
since
the
system parameter
values willbe
changing
from
2.2
Optimization
Techniques
2.2.1
Design Variables
In
any
optimization
problem
it is
necessary
to
specify
a set of
design
variables whichare used
to
find
the
solution
to the
problem.
The design
variables are used
in
the
calculationof
the
objective
function,
and
in
some cases are
limited
by
constraint equations
(i.e.
equality,
inequality,
side).
The idea is
to
vary
the
design
variables
in
such
a
way
as
to
produceminimization of
the
objective
function.
For
the
lumped
parameter circuit models presented
earlier, the
design
variables arethe
parameter values
(i.e.
Cct,
Rtaa,
Caa
etc.).
Two
scenarios will
be
entertainedwith
regardsto
these
parameter values: constant parameter models and
linearly
varying
parameter models.Constant
parameter models consist of a single
valuerepresenting
the
parameter.Linearly
varying
parameters consist of a constant
term
and a
term that
varies
linearly
withthe
input
flow,
Qs.
Constant Parameters
Linearly
Varying
Parameters
Rtaa
Rtaa
=Rtaao
+
Rtaai*Qs
Cct
Cct
=Ccto
+
Ccti*Qs
Caa
Caa
=Caao
+
Caai
*Qs
Cv
Cv
=Cv0
+
Cv,*Qs
Rvl
Rvl
=Rvl0
+
Rvli*Qs
Rv2
Rv2
=Rv20
+
Rv2,
*Qs
lb
Ib
=Ib0
+
Ibi*Qs
Ret
Rct
=Rcto
+
Rcti*Qs
The
linearly
varying
parameter modelsactually have
twice the
number
of
design
variables
as
do
the
constant
parametermodels
by
way
of
the
constantterm
in
addition
to the
coefficient on
the
linear
term.
This
"doubling"of
design
variablessignificantly
increases
the
2.2.2
The Method
of
Least
Squares
In
least
squares
optimization,
the objective
function
becomes
the
sum
ofthe
squared
differences between
the
model predicted and
experimental
data,
which wouldbe
the
pressureat node
(a),
Pa,
for
all
three
models postulated
to
predict
blood flow.
If
we
refer
to
the
objective
function
as
F(x),
then the
optimization problem appears as:
/(x)
=|Pa_experimentai-Pa
.predicted](differencing
equation)
(2.44)
m
min:
F(x)
= f(\)2 = /(x)r/(x)
(objective
function)
(2.45)
i=\
Where
x
is
a vector of
the
design
variables of
length
n.Numerous
optimization routines exist
for minimizing
the
sum-of-the-squares
in
such
an
application.
Most
methods
(i.e.
Steepest
Descent)
will require
the
computation of
the
gradient at
discrete
time
points with respect
to the
variables
in
order
to
determine
a search
direction
at a particular point.
The
gradient
is
typically
found
by determining
the
Jacobian
matrix with respect
to
the
design
variables and
incorporating
it in
the
following
expression:
VF(x)
=27(x)r/(x)
(2.46)
The
Jacobian, J(x),
is
simply
a
matrix
of
the
first
partialderivatives
of
the
differencing
equation(2.43)
with
respectto the
design
variables:7(x)
=[/(x)l
(Jacobian)
(2.47)
dxt
To
solve
for
the
gradientof
F(x)
analytically, the
objectivefunction
must
be
written
in
a
closedform in
terms
of
the
system parameters(design
variables)
and
the
state variables.
Since
we
are
looking
at
multiple
time
points
in calculating
the
sum
of
the
squares,
the
function,
f(x),
is
not a single
value,
but
actually
an
array
of numbers.
When
the
difference is
squared,
we are
actually
performing
a
dot
product,
so
the
result
is
a
single valued objectivefunction
(see
equation
2.45).
The fact
that the
differencing
function, f(x),
contains multiplepoints
implies
that the
Jacobian
matrix
is actually
vectorized.
That
is,
the
elementsof
the
Jacobian
matrix represent
the
first
partial
derivatives
with respect
to the
design
variablesat
multiple
time
points.
JT[f(*)]
=<ti\_
#2.
<!f3_
<m
\dxx
dxx dxx
dx
\J
<%J_
%2_
j^3_
%jn
y
dx2 dx2 dx2
dx2
j
<%\
tfl
#3
ft
dx'dx/dx' '
dx,
n n n nJ
(n
design
variables,
mtime
points)
(2.48)
In
optimization
by
means
of
the
Steepest Descent
method, the
search
direction
is
calculated as
the
negative of
the
gradient.By
equation2.48
the
gradientends
up
being
a
vector with
nelements,
the
same as
the
number ofdesign
variables.
SD
=-VF(x)
(Search
Direction)
(2.49)
The
problem
is
then
turned
into
a
one-dimensional optimization problem withthe
introduction
of a
step
size,
a.
min:
F
(a)
= F(x'+
a'SD')
(2.50)
The
goal
is
to then
determine
a
step
size,
a*,
which will minimizethe
objective
function
at
the
current
values ofthe
design
variables.A
good method
for
determining
an
appropriate
step
size
is
the
Golden Section
search,
which usesthe
Golden
Ratio
to
reduce
the
interval
ofuncertainty
after
the
initial
bracketing
ofthe
minimumis
achieved.The
interval
is
whole process
is iterated
untilconvergence criteria
are
met,
or
untilsome minimum
step
sizeis
reached.
2.2.3
Numerical Optimization
Techniques
The
method
just described
requires analytic expressions
for
the
objectivefunction in
order
to
compute
the
search
directions.
For
simple
models,
this
is
a
relatively
simple
task,
assuming
we
have only
a
few design
variables.
MATLAB
contains a
"canned"
optimization
routine
specifically
catered
towards
least
squares minimization.
LEASTSQ
requiresa
callto
an m-file
that
computes
the
objective
function based
uponthe
design
variables,
and
initial
conditions or
starting
values
for
these
variables.The
computation ofthe
searchdirections
and
step
sizes are
allcontained within
the
canned routine.
While
the
LEASTSQ
routinemay
not
be specifically designed for
use
withan objective
function
whichis
computed
through
the
simulation of a
dynamic linear
system,
it is
a numericalmethod,
implying
that the
routineshould
be
general enough
to
handle
most optimizations problems
withouthaving
analyticexpressions
to
compute
the
gradients.2.2.4
Verification
of
Optimization Techniques
To
test the
validity
and
performancebehind both
the
analytic
least
squaresmethod
and
the
canned
LEASTSQ
routine, the
circuit ofModel
1
wasused
asa
test
case.The
modelwas
bread-boarded in
the
laboratory
and
the
circuitwas
driven
witha
sinusoidalcurrent.
The
L
Rvi
AAA-y
U
c
v
16
Cct=10^f
RT=11.76kQ
Caa=10|if
Rv,=17.58kI2
C^lOnf
Rv2=H.78kfl
Cv:
Figure
2-16.
Benchtop
test
case
ofModel 1 using
electrical circuit.Since
the
solving
of
the
state variables at each
iteration
ofthe
optimization processwas
independent
of
the
optimization
technique
used,
both
tests
usedthe
state
space
formulation
and
the
function LSIM
to
simulatethe
model.This,
as
mentionedbefore,
requiresthat
we
develop
the
state
equationsfor Model 1
.By
continuity
we
can writethe
constitutive equations:Is=ii
ii
=b
+
i3
i3
=i4
+
is
is
=ie
+
h
From
the
elemental
equationswe can
write:=
Ca^-=>v.=J-fi2dt
ctdt
C
J
h=-
Va"vb
R
i-i
=Ca
Taadvv
dt
-F-JMt
vb-vc
[image:40.562.66.507.51.279.2]dv
?"/'.*
1-,
=7"r
v2Using
the
elemental
equations
in
the
continuity
equations we arrive at
the
following
stateequations:
1
1
1
C,RT
'C,RT,a
C
cti,LTaa '
ctixTaa
Vb
=^-aa^-Taa
V,
-+
^aa^Taa
^aaRvl
vb
+
CaaRvl
V
=Rv,cv
Vb
n
l
l
+
R.i
vlR
x>-v2
To simplify
matters,
we will
only
use
Cct, C^,
and
RTaa
as
the
design
variable.
Letting
va
be
the
desired
output,
the