Analytical Method Development and Validation of Teriflunomide by RP- HPLC
Full text
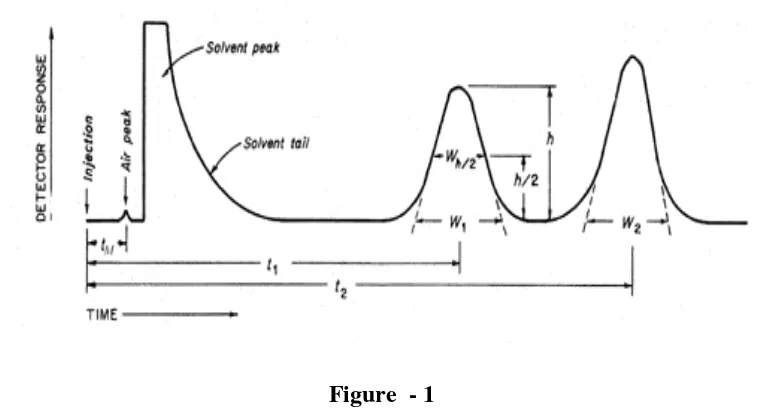
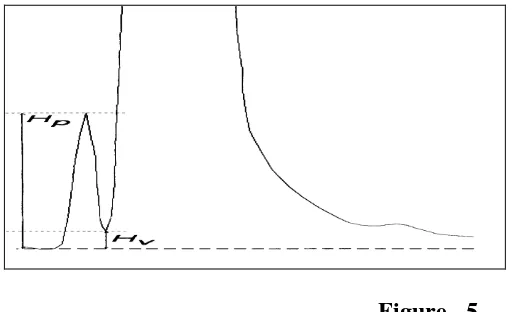
Figure



Related documents
hydrochloride in bulk and pharmaceutical dosage forms used mobile phase. combination of phosphate buffer
DEVELOPMENT AND VALIDATION FOR THE ESTIMATION OF CLOTRIMAZOLE IN HUMAN PLASMA BY RP-HPLC METHOD” being submitted for the partial fulfillment of Master of Pharmacy
Research Article DEVELOPMENT AND VALIDATION OF RP HPLC METHOD FOR DETERMINATION OF LEVAMISOLE IN BULK AND DOSAGE FORM *1Department of Pharmaceutical Analysis and Quality Assurance,
Saiful Islam, et al, (2016), Development and validation of RP-HPLC method for determination of saxagliptin hcl in bulk and tablet dosage form, World journal of pharmacy and
Patil Amol, PG Student, Department of Quality Assurance, Gangamai College of Pharmacy, North Maharashtra University, Jalgaon (M.S.) India. Warude Kapil:
The proposed method when used for extraction and subsequent estimation of ivabradine hydrochloride from pharmaceutical dosage forms after spiking with 12, 24 and 36 μ g/ml
G College of Pharmacy, Nallajerla, West Godavari, A.P, India 3 Department of Pharmaceutical Analysis and Quality Assurance, Yalamarty College of Pharmacy, Tarluwada Visakhapatnam,
A simple and accurate RP-HPLC method has been developed for the estimation of Neverapine in bulk and pharmaceutical dosage forms, using C-18 column 150 x 4.6 mm i.d, 5μm particle size