Lack of Effect of Cytoplasmic Tail Truncations on Human
Immunodeficiency Virus Type 2 ROD Env
Particle Release Activity
STEPHAN P. BOUR,* CLAUDIA ABERHAM, CHRISTE`LE PERRIN, ANDKLAUS STREBEL
Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland 20892-0460
Received 15 June 1998/Accepted 30 September 1998
In addition to its role in receptor binding, the envelope glycoprotein of certain human immunodeficiency virus type 2 (2) isolates, including ROD10, exhibits a biological activity that enhances the release of HIV-2, HIV-1, and simian immunodeficiency virus particles from infected cells. The present study aims at better defining the functional domains involved in this biological activity. To this end, we have characterized the envelope protein of the ROD14 isolate of HIV-2, which, despite 95% homology with the ROD10 envelope at the amino acid level, is unable to enhance viral particle release. Site-directed mutagenesis showed that the presence of a truncation in the cytoplasmic tail of the ROD14 envelope was not responsible for the lack of activity, as previously reported for the HIV-2 ST isolate (G. D. Ritter, Jr., G. Yamshchikov, S. J. Cohen, and M. J. Mulligan, J. Virol. 70:2669–2673, 1996). Similarly, several modifications of the length of the ROD10 envelope cytoplasmic tail did not impair its ability to enhance particle release, suggesting that, in the case of the HIV-2 ROD isolate, particle release activity is not regulated by the length of the cytoplasmic tail.
The human immunodeficiency virus type 1 (HIV-1) encodes Vpu, a protein capable of enhancing the release of viral par-ticles from infected cells (7, 19–21). Although the vpu gene is absent in all known HIV-2 isolates, both the ROD10 (6, 14, 16) and ST (11, 12) molecular clones of HIV-2 express a functional homologue to Vpu. In both cases, the envelope glycoprotein has been shown to enhance the release of viral particles from infected cells in a manner indistinguishable from that of gen-uine Vpu (2, 15). Both Vpu and ROD10 Env augment the release of chimeric viruses bearing the gag-pol regions of re-lated retroviruses, including HIV-1, HIV-2, and simian immu-nodeficiency virus (SIV) (4, 8), suggesting a common mecha-nism of action. However, while functionally equivalent, Vpu and HIV-2 Env may differ in the location of their functional domains. While in the case of Vpu the transmembrane (TM) domain was shown to be crucial for particle release activity (18), the corresponding activity has been attributed to the cytoplasmic domain in the case of the HIV-2 envelope glyco-protein (15). Indeed, while the envelope glyco-protein of the HIV-2 ST isolate bearing a full length gp41 cytoplasmic tail enhanced the release of virus-like particles from vaccinia virus-infected SupT1 cells, no such activity was detected for the envelope protein of the closely related ST#2 isolate, for which the cy-toplasmic tail is truncated to 17 amino acids (15). Similar to the ST#2 isolate, the envelope glycoprotein of ROD10 naturally bears a stop codon truncating its cytoplasmic tail to 18 residues (4). However, in contrast to ST#2, the ROD10 isolate dis-played a particle release activity identical to that of Vpu de-spite its short cytoplasmic tail (2). The present work addresses the role of the HIV-2 Env cytoplasmic domain in the particle release efficiency of two closely related isolates of HIV-2: ROD10 and ROD14.
We first assessed the effect of cytoplasmic tail truncations in
the envelope protein of the ROD10 isolate. In the case of HIV-2 ST, truncation of the full-length 164-amino-acid enve-lope cytoplasmic domain to 17 residues (HIV-2 ST#2) was reported to inactivate its Vpu-like activity (15). In contrast, we previously noted that ROD10 Env fully supported viral particle release even though its cytoplasmic tail was truncated to 18 residues (4). A possible explanation for this apparent discrep-ancy is that the 18-residue cytoplasmic domain represented the minimal sequence necessary for Vpu-like activity. To test this hypothesis and to address the general influence of the cyto-plasmic tail of ROD10 Env on its particle release activity, we constructed variants bearing different-length cytoplasmic tails. The ROD10.17 (719QZ) variant has a 17-residue cytoplasmic tail and in that regard is equivalent to the ST#2 isolate, where-as the ROD10.FL (720ZQ) mutant encodes a full-length 157-residue tail. Both plasmids are full-length molecular clones and were obtained by site-directed mutagenesis of the ROD10 env gene with the Altered Sites mutagenesis system (Promega, Mad-ison, Wis.). Mutagenesis templates were constructed by clon-ing a 793-bp NcoI fragment (Env residues 608 to 858) from pROD10 into the pALTER.Ex1 vector. The presence of the proper mutations was verified by sequencing of the entire env gene.
The ability of the ROD10.17 and ROD10.FL mutants to support viral particle release, compared to wild-type ROD10 (2) and env-deficient mutant ROD10.env1 (2), was assessed by pulse-chase analysis of transfected HeLa cells, as recently de-scribed (2, 4). Briefly, calcium phosphate-precipitated plasmid DNA (25 to 30mg) was added to HeLa cells grown to near confluence in 25-cm2flasks (53106cells per flask). After 4 h
of incubation at 37°C, the cells were subjected to glycerol shock for 2.5 min. For pulse-chase analysis of particle release effi-ciency, HeLa cells were pulse labeled with 1 mCi of Tran35
S-label (ICN Biomedical, Inc., Costa Mesa, Calif.) per ml for 30 min. Cells were subjected to a chase at 37°C in 300 ml of prewarmed Dulbecco modified Eagle medium-fetal bovine se-rum for the indicated time periods. At each time point, cells * Corresponding author. Mailing address: NIH/NIAID, Building 4,
Room 312, 9000 Rockville Pike, Bethesda, MD 20892-0460. Phone: (301) 496-3132. Fax: (301) 402-0226. E-mail: sbour@nih.gov.
778
on November 9, 2019 by guest
http://jvi.asm.org/
were collected and lysed in NP40-DOC buffer (20 mM Tris-HCl [pH 8], 120 mM NaCl, 2 mM EDTA, 0.5% deoxycholate [DOC], 1% Nonidet P-40 [NP40]). The culture supernatants were filtered through 0.45-mm cellulose acetate filters, and virus particles were pelleted in a refrigerated Eppendorf microcen-trifuge (4°C, 90 min, 16,0003g). Cell and virus lysates were immunoprecipitated with a 1:1 mixture of serum from an asymptomatic HIV-1-seropositive patient (TP serum) and a pool of HIV-2 patient sera (contributed to the AIDS Research and Reference Reagent Program by Saladin Osmanov). Viral proteins were separated by sodium dodecyl sulfate-polyac-rylamide gel electrophoresis (SDS-PAGE) with 12% gels and visualized by fluorography with Bio-Max MR film (East-man Kodak, Rochester, N.Y.). As shown in Fig. 1A, the pres-ence or abspres-ence of cytoplasmic tail truncations had no appar-ent effect on the synthesis and maturation of Gag proteins in cells, nor did they change the composition of the virions re-leased in the culture supernatant. The synthesis and stability of the different envelope proteins was also similar during the 1-h chase (Fig. 1A). All envelope glycoproteins were incorporated
at similar levels into secreted virions (data not shown). To estimate the ability of the different envelope proteins to pro-mote viral particle release, radioactive bands corresponding to p58gag, p26-27CA, and p16MAwere quantified in a Fujix BAS
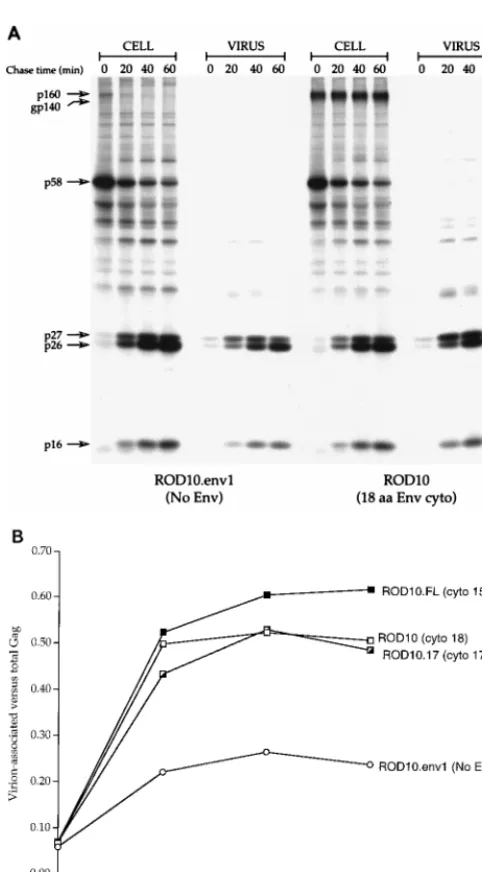
2000 Bio-Image Analyzer and the ratio of Gag proteins in virions to the total (cell and virus) was calculated and plotted as a function of time (Fig. 1B). Consistent with our previous reports (2, 4), the presence of the truncated ROD10 envelope protein led to a more-than-twofold increase in the rate of viral particle release in as little as 20 min (Fig. 1B; compare ROD10 and the env-deficient ROD10.env1). We next addressed whether, similarly to the situation observed with the HIV-2 ST isolate, removal of the ROD10 Env premature stop codon could lead to enhanced activity on viral particle release. As shown in Fig. 1B for ROD10.FL, the presence of a full-length Env cytoplasmic tail had no effect on the efficiency of particle release during the first 20 min of chase. We did, however, observe a modest but reproducible 10% positive effect at later time points (Fig. 1B, ROD10.FL). To further demonstrate the lack of effect of cytoplasmic tail truncations in ROD10 Env particle release activity, the 18-residue cytoplasmic tail was further truncated to 17 residues. Although such truncation led to a loss of activity in the case of HIV-2 ST, it had no effect on ROD10 particle release activity (Fig. 1B, ROD10.17). Taken together, these data suggest that the Vpu-like activity of the ROD10 envelope glycoprotein is not modulated by the length of its cytoplasmic tail in transfected HeLa cells.
[image:2.612.53.294.63.499.2]We next asked whether this lack of effect of cytoplasmic tail truncations was a ROD10-specific phenomenon. To address
FIG. 1. Effect of cytoplasmic tail truncations on HIV-2 ROD10 particle re-lease efficiency. (A) Kinetic analysis of viral particle rere-lease by ROD10 Env cytoplasmic tail mutants. HeLa cells were transfected with a wild-type pROD10 molecular clone or mutants bearing 17-amino-acid (pROD10.17) or full-length 157-amino-acid (pROD10.FL) Env cytoplasmic tails. The pROD10.env1 Env-deficient vector was used as a negative control. Cells were pulse-chased, and viral proteins recovered by immunoprecipitation were separated by SDS–12% PAGE. The HIV-2 major Gag proteins p58gag, p26-27CA, and p16MA, as well as the
HIV-2 envelope glycoprotein precursor gp140, are identified on the left. The p160 Gag-Pol precursor protein is also visible in the absence of envelope proteins on the ROD10.env1 panel. (B) Bands corresponding to the HIV-2 major Gag proteins in panel A were quantified, and the efficiency of particle release at each time point was calculated and plotted as a function of time. cyto, cytoplasmic tail length (in amino acids).
on November 9, 2019 by guest
http://jvi.asm.org/
this question, we examined the particle release activity of sev-eral different molecular clones of HIV-2 ROD obtained from the original chronically infected CEM cells (5, 6, 14). One such clone, pROD14, is identical to pROD10 except for an HindIII-BsmI fragment (from the vpr gene to residue 810 in the enve-lope cytoplasmic tail) that is derived from the pROD2 clone (16). To assess the level of divergence between the ROD10 and ROD14 clones, the env genes from both isolates were
FIG. 2. Characterization of the ROD14 envelope protein. (A) Alignment of the ROD10, ROD10.FL, and ROD14 Env amino acid sequences with the orig-inal HIV-2 ROD sequence (GenBank accession no. X05291). Amino acid num-bering starts at the Env initiation methionine. The positions of amino acid substitutions between the original HIV-2 ROD sequence and the ROD10 and ROD14 molecular clones are indicated in boldface type. Asterisks denote the presence of premature stop codons. (B) The particle release efficiency of the molecular clones identified on the figure was assessed by pulse-chase analysis of HeLa cells as described in the legend to Fig. 1 (not shown). The efficiency of particle release at each time point was calculated as described in the legend to Fig. 1, and the ratio of virion-associated to total Gag proteins was plotted as a function of time.
on November 9, 2019 by guest
http://jvi.asm.org/
sequenced and their translation products were compared to the original HIV-2 ROD amino acid sequence registered in GenBank (6, 9). We found that the sequence of ROD10 Env is virtually identical to that of the published ROD sequence (HIV-2 ROD [Fig. 2A]) with the exception of a stop codon at amino acid position 720 and two substitutions at positions 312 and 536 which are shared by the ROD14 clone (Fig. 2A). ROD14 Env contained an additional five specific substitutions at positions 40, 59, 211, 422, and 598 compared with ROD10 Env. ROD14 Env also bears a stop codon at position 750 that truncates its cytoplasmic tail to 48 residues (Fig. 2A). Similar truncations were previously reported for the HIV-2 UC1mc and GH1 molecular clones (1, 10).
Our conclusion that envelope truncations to 17 and 18 res-idues did not influence the particle release activity of ROD10 (Fig. 1) did not formally rule out the possible contribution of a truncation at 48 residues. We therefore examined the particle release efficiency of ROD14 in pulse-chase experiments, as described in the legend to Fig. 1. The Gag proteins immuno-precipitated at each time point were separated by SDS-PAGE (data not shown) and quantified as described above. As shown in Fig. 2B, less than 20% of the total Gag proteins produced by ROD14 during pulse labeling were secreted as virions during the 1-h chase. This low efficiency of particle release is similar to that observed for the envelope-deficient ROD10 mutant (Fig. 1) and suggests that the ROD14 envelope does not have the ability to enhance viral particle release. Although the gag and pol genes of ROD10 and ROD14 are identical, we verified that the low rate of particle release by ROD14 was indeed due to a lack of activity of the envelope. The entire ROD14 env sequence was introduced into ROD10 by cloning a 2,397-bp PCR-amplified BsaAI-BsmI fragment from pROD14 into the corresponding sites in pROD10. The particle release efficiency of the resulting ROD1014 chimeric virus was identical to that of ROD14 (Fig. 2B), showing that the inability of ROD14 to support efficient viral particle release maps to the envelope gene. These data suggested that truncation of HIV-2 ROD Env to 48 cytoplasmic residues might lead to the inactivation of its particle release activity in a process similar to that observed for the HIV-2 ST#2 isolate (15). To test this hypothesis, we examined the effect of either removing the premature stop codon at position 750 in ROD1014 (ROD1014.FL) or intro-ducing a stop codon in the ROD10.FL envelope protein at the same location (ROD10.48). Plasmids ROD1014.FL and ROD10.48 were generated by site-directed mutagenesis, as described above. Consistent with data presented in Fig. 1, the presence of a premature stop codon in the ROD10 envelope cytoplasmic tail did not alter its ability to support efficient virus release (Fig. 2B, ROD10.48). Similarly, removal of the ROD14 Env stop codon did not confer a particle release activity on the inactive envelope protein (Fig. 2B, ROD1014.FL). A modest 10% enhancement observed for ROD1014.FL is similar to that shown in Fig. 1 in the case of ROD10.FL, suggesting that full-length cytoplasmic tails have a slight positive effect on viral secretion. However, since this effect was observed both in the context of the active ROD10 and the inactive ROD14 enve-lope proteins, it likely represents a nonspecific effect, distinct from the particle release activity described in this work. Taken together, these data indicate that the inability of ROD14 Env to enhance viral particle release was not due to the presence of a truncated cytoplasmic tail.
Previous experiments suggesting a role for the HIV-2 Env cytoplasmic domain in enhancing viral particle release were performed in CD41SupT1 cells (15). It is therefore
conceiv-able that our contrasting results were at least in part due to the absence of the CD4 receptor in HeLa cells used in the present
study. We addressed whether the effect of cytoplasmic tail truncations on the envelope protein’s fusion activity could lead to enhanced viral particle release in the context of a spreading infection in CD41 cells. Infections were initiated by direct
electroporation of A3.01 CD41 T cells with plasmid DNAs
encoding viruses bearing full-length or truncated envelope cy-toplasmic tails. Briefly, 5mg of plasmid DNA was added to 53
106cells and subjected to an electric pulse of 0.3 kV at 950mF
with 0.4-cm cuvettes. The electroporated cells were immedi-ately transferred to a T25 culture flask containing 106 fresh
A3.01 cells in 5 ml of complete RPMI 1640. All electropora-tions were performed in duplicate. Eighty percent of the me-dium was replaced at 12 h postelectroporation, and the infec-tion was allowed to proceed for an addiinfec-tional 2 days. At that time, and every 2 days thereafter, the medium was replaced and an aliquot was collected to monitor the progression of the infection by reverse transcriptase assay, as described previously (23). The cultures were also assessed for cytopathic effects and formation of syncytia by microscopic examination. Represen-tative data from three separate experiments are shown in Fig. 3. The amount of virus produced at peak infection for each molecular clone closely paralleled the data obtained by pulse-chase analysis of transfected HeLa cells (Fig. 1 and 2B). In-deed, viruses bearing full-length or truncated ROD10 enve-lope proteins were released at an equivalent rate. Viruses bearing less than 48 cytoplasmic residues in their envelope were less infectious, as demonstrated by the 8-day delay of peak virus production (Fig. 3, ROD10 and ROD10.17). These results are in agreement with previous findings with similarly truncated strains of the SIVmacisolate (14). The delay in
es-tablishment of a productive infection by ROD10 and ROD 10.17 was not correlated with differences in cell death or levels of cell fusion (data not shown). The ROD1014 chimeric virus bearing the env gene from ROD14 showed a defect in particle release similar to that observed in HeLa cells. This low particle release efficiency was not due to lesser infectivity of that vari-ant, since it exhibited infection kinetics similar to that of wild-type ROD10 as well as the similarly truncated ROD10.48 (Fig. 3). We therefore conclude that our inability to demonstrate a role for the Env cytoplasmic domain in HeLa cells was not due to the absence of the CD4 receptor in that assay system.
[image:4.612.313.550.68.236.2]Our data indicate that, in contrast to the situation observed for HIV-2 ST, the length of the cytoplasmic tail does not
FIG. 3. Effect of Env cytoplasmic tail truncations on particle release during productive A3.01 infection. A3.01 cells were transfected by electroporation with the indicated molecular clone DNAs, mixed with fresh A3.01 cells, and assessed for de novo virus production by monitoring the reverse transcriptase activity released in the culture medium over time.
on November 9, 2019 by guest
http://jvi.asm.org/
modulate the particle release activity of the ROD10 envelope glycoprotein. Although it cannot be ruled out that residues important for particle release activity differ among different isolates of HIV-2, it is more likely that the increased fusion activity and cytopathic effects that accompany HIV-2 Env cy-toplasmic truncations (1, 13) are responsible for the low virus production observed by Ritter et al. (15). The HIV-2 ST#2 TM subunit also differs from that of the original ST clone at three amino acid positions in addition to the premature stop codon (13). It is therefore conceivable that these mutations are, at least in part, responsible for the particle release defect observed in ST#2.
We did observe a modest positive effect on particle release upon removal of the ROD10 premature stop condon (Fig. 1). However, the fact that this phenomenon was observed to a similar extent for ROD14 Env, which does not exhibit a Vpu-like activity (Fig. 2), suggests that this effect is unrelated to the Vpu-like activity reported for ROD10 Env (2, 4). Also, this positive effect of full-length cytoplasmic tail on virus release is in its magnitude significantly lower than the Vpu-like activity of ROD10 Env. It is nevertheless possible that the positive influence of full-length Env cytoplasmic tails on virus release reported by Ritter et al. (15) is related to the nonspecific effect observed in our system but was magnified in the vaccinia virus expression system. Nonspecific effects of cytoplasmic Env se-quences on virus release have been reported for other systems. For example, addition of nonspecific sequences to the cyto-plasmic tail of the vesicular stomatitis virus (VSV) G protein was found to increase viral budding, and it was speculated that this could be the result of local bending of the plasma mem-brane, induced by the assembly of VSV G at the cell surface in tight arrays (17). Finally, it should be pointed out that in the case of SIVmac239, only truncated envelopes have the ability
to promote the release of virus-like particles from vaccinia virus-infected cells (22).
We conclude that the Vpu-like effect of ROD10 Env on virus release is not modulated by its cytoplasmic domain. It is more likely that differences in the amino acid sequences of the ectodomains of ROD10 and ROD14 Env (Fig. 2A) account for the differential activities of these Env variants with respect to virus release. Indeed, preliminary data obtained in our labo-ratory suggest that changes at amino acid positions 422 and 598 (Fig. 2A) account for the inability of the ROD14 envelope glycoprotein to promote viral particle release (3).
We thank Ronald Willey for discussions.
Part of this work was supported by a grant from the Intramural AIDS Targeted Antiviral Program to K.S.
REFERENCES
1. Barnett, S. W., M. Quiroga, A. Werner, D. Dina, and J. A. Levy. 1993. Distinguishing features of an infectious molecular clone of the highly diver-gent and noncytopathic human immunodeficiency virus type 2 UC1 strain. J. Virol. 67:1006–1014.
2. Bour, S., U. Schubert, K. Peden, and K. Strebel. 1996. The envelope glyco-protein of human immunodeficiency virus type 2 enhances viral particle release: a Vpu-like factor? J. Virol. 70:820–829.
3. Bour, S., and K. Strebel. Unpublished data.
4. Bour, S., and K. Strebel. 1996. The human immunodeficiency virus (HIV)
type 2 envelope protein is a functional complement to HIV Type 1 Vpu that enhances particle release of heterologous retroviruses. J. Virol. 70:8285– 8300.
5. Clavel, F., D. Gue´tard, F. Brun-Ve´zinet, S. Chamaret, M. A. Rey, M. O.
Santos-Ferreira, A. G. Laurent, C. Dauguet, C. Katlama, C. Rouzioux, D. Klatzmann, J. L. Champalimaud, and L. Montagnier.1986. Isolation of a new human retrovirus from West African patients with AIDS. Science 233: 343–346.
6. Clavel, F., M. Guyader, D. Gue´tard, M. Salle, L. Montagnier, and M. Alizon. 1986. Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature 324:691–695.
7. Cohen, E. A., E. F. Terwilliger, J. G. Sodroski, and W. A. Haseltine. 1988. Identification of a protein encoded by the vpu gene of HIV-1. Nature 334: 532–534.
8. Go¨ttlinger, H. G., T. Dorfman, E. A. Cohen, and W. A. Haseltine. 1993. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc. Natl. Acad. Sci. USA 90:7381–7385.
9. Guyader, M., M. Emerman, P. Sonigo, F. Clavel, L. Montagnier, and M.
Alizon.1987. Genome organization and transactivation of the human immu-nodeficiency virus type 2. Nature 326:662–669.
10. Ishikawa, K., H. Tsujimoto, M. Nakai, J. A. Mingle, M. Osei-Kwasi, S. E.
Aggrey, V. B. Nettey, S. N. Afoakwa, M. Fukasawa, T. Kodama, et al.1988. Isolation and characterization of HIV-2 from an AIDS patient in Ghana. AIDS 2:383–388.
11. Kong, L. I., S. W. Lee, J. C. Kappes, J. S. Parkin, D. Decker, J. A. Hoxie,
B. H. Hahn, and G. M. Shaw.1988. West African HIV-2-related human retrovirus with attenuated cytopathicity. Science 240:1525–1529. 12. Kumar, P., H. Hui, J. C. Kappes, B. S. Haggarty, J. A. Hoxie, S. K. Arya,
G. M. Shaw, and B. H. Hahn.1990. Molecular characterization of an atten-uated human immunodeficiency virus type 2 isolate. J. Virol. 64:890–901. 13. Mulligan, M. J., G. V. Yamshchikov, G. Ritter, Jr., F. Gao, M. J. Jin, C. D.
Nail, C. P. Spies, B. H. Hahn, and R. W. Compans.1992. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein com-plex of human immunodeficiency virus type 2 in selected cell types. J. Virol.
66:3971–3975.
14. Naidu, Y. M., H. W. Kestler III, Y. Li, C. V. Butler, D. P. Silva, D. K.
Schmidt, C. D. Troup, P. K. Sehgal, P. Sonigo, M. D. Daniel, and R. C. Desrosiers.1988. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2:
persistent infection of rhesus monkeys with molecularly cloned SIVmac. J.
Vi-rol. 62:4691–4696.
15. Ritter, G. D., Jr., G. Yamshchikov, S. J. Cohen, and M. J. Mulligan. 1996. Human immunodeficiency virus type 2 glycoprotein enhancement of particle budding: role of the cytoplasmic domain. J. Virol. 70:2669–2673. 16. Ryan-Graham, M. A., and W. C. K. Peden. 1995. Both virus and host
components are important for the manifestation of a Nef-minus phenotype in HIV-1 and HIV-2. Virology 213:158–168.
17. Schnell, M. J., L. Buonocore, E. Boritz, H. P. Ghosh, R. Chernish, and J. K.
Rose.1998. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J.
17:1289–1296.
18. Schubert, U., S. Bour, A. V. Ferrer-Montiel, M. Montal, F. Maldarelli, and
K. Strebel.1996. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J. Virol.
70:809–819.
19. Strebel, K., T. Klimkait, F. Maldarelli, and M. A. Martin. 1989. Molecular and biochemical analyses of human immunodeficiency virus type 1 vpu pro-tein. J. Virol. 63:3784–3791.
20. Strebel, K., T. Klimkait, and M. A. Martin. 1988. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science 241:1221–1223.
21. Terwilliger, E. F., E. A. Cohen, Y. C. Lu, J. G. Sodroski, and W. A. Haseltine. 1989. Functional role of human immunodeficiency virus type 1 vpu. Proc. Natl. Acad. Sci. USA 86:5163–5167.
22. Vzorov, A. N., and R. W. Compans. 1996. Assembly and release of SIV Env proteins with full-length or truncated cytoplasmic domains. Virology 221: 22–33.
23. Willey, R. L., D. H. Smith, L. A. Lasky, T. S. Theodore, P. L. Earl, B. Moss,
D. J. Capon, and M. A. Martin.1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62:139–147.