0022-538X/11/$12.00 doi:10.1128/JVI.01616-10
Copyright © 2011, American Society for Microbiology. All Rights Reserved.
North American Porcine Reproductive and Respiratory Syndrome
Viruses Inhibit Type I Interferon Production by
Plasmacytoid Dendritic Cells
䌤
Gabriela Calzada-Nova, William M. Schnitzlein, Robert J. Husmann, and Federico A. Zuckermann*
Department of Pathobiology, College of Veterinary Medicine, University of Illinois, Urbana, Illinois 61802
Received 2 August 2010/Accepted 20 December 2010
Although enveloped viruses typically trigger the prodigious secretion of alpha interferon (IFN-␣) by
plas-macytoid dendritic cells (pDC), porcine pDC remain quiescent when exposed to porcine reproductive and respiratory syndrome virus (PRRSV). This inactivity is likely due to virus-mediated interference since the
typical IFN-␣ response by either purified or nonsorted porcine pDC to transmissible gastroenteritis virus
(TGEV) or the Toll-like receptor 9 agonist, oligodeoxynucleotide (ODN) D19, was markedly reduced in the presence of PRRSV. Suppression occurred independently of virus viability and acidification of pDC early
endosomes but correlated with diminished levels of IFN-␣mRNA. This change was attributed to an abrogation
of transcription resulting from a decrease in the otherwise enhanced amounts of the requisite interferon regulatory factor 7 (IRF-7), whose gene expression in turn was limited as a consequence of a lessened availability of nuclear-localized signal transducer and activator of transcription 1 (STAT1). While PRRSV also
inhibited tumor necrosis factor alpha (TNF-␣) synthesis by pDC responding to either agent, only the
inter-leukin-2 (IL-2) and IL-6 production instigated by ODN D19 exposure was blocked. Likewise, PRRSV did not impact a specific TGEV-associated enhancement of IL-8 expression. Moreover, an augmented phosphorylation
of NF-B seen in activated pDC was not only unaffected by PRRSV but actually occurred in its presence. Thus,
as supported by a demonstrated resilience of pDC to PRRSV infection, this pathogen may interact with a cell surface protein(s) to selectively impede the completion of cascades involved in cytokine production by stimu-lated pDC.
Porcine reproductive and respiratory syndrome virus (PRRSV) is a small, enveloped virus with a single-stranded, positive-sense RNA genome. This arterivirus afflicts swine throughout the world and causes pregnant sows to abort or give birth to weak or dead piglets and younger pigs to experi-ence respiratory distress and grow slowly (1). Upon entry into a pig, PRRSV preferentially replicates in a subset of its alve-olar macrophages (AM) (15), produces a viremia for at least 4 to 5 weeks (3), and persistently infects the lungs, lymphoid organs, and tonsils for various periods of up to 150 days (4, 29, 36). The elicited nontypical, polarized host immune response is characterized by a rapid and robust production of nonneutral-izing antibodies (42, 51, 76) and a delayed and protracted generation of virus-specific gamma interferon (IFN-␥ )-secret-ing cells (46, 73). Moreover, unlike porcine respiratory coro-navirus, swine influenza virus, and transmissible gastroenteritis virus (TGEV) that also target the pig’s lungs and stimulate copious production of IFN-␣, PRRSV induces very little syn-thesis, if any, of this cytokine at this site (2, 7, 69). This differ-ence likely reflects an impaired function of susceptible AM as indicated by their in vitro mounting of an undetectable or relatively low IFN-␣response to PRRSV infection in compar-ison to that provoked by TGEV (2, 40). A similar disparity between the high serum concentrations of IFN-␣ in animals
infected with pseudorabies virus (PrV) and TGEV relative to the lesser amounts in pigs inoculated with PRRSV has also been noted (2). However, in this case, the PRRSV-associated deficiency might be attributed to the inactivity of plasmacytoid dendritic cells (pDC) that are present in the peripheral blood. pDC are an integral part of the host innate immune system and usually react to the presence of foreign entities such as viruses with a rapid and copious secretion of type I interferons in addition to lesser amounts of other cytokines including tu-mor necrosis factor alpha (TNF-␣) and interleukin-6 (IL-6) (13, 24). Consequently, pDC can quickly create an antiviral environment and subsequently impact the development of in-nate and acquired immunity. Their released IFN can incite NK cell activity (23) and induce the generation of regulatory T cells (11, 30, 45), the maturation of myeloid DC into antigen-pre-senting cells (20), and the transition of monocytes into DC (53, 61). Moreover, the IFN coupled with pDC-produced IL-6 can promote the differentiation of B cells into plasma cells (32, 55, 57).
This unique response of pDC is instigated by the interaction of pathogen-associated molecular patterns (PAMPS) present in virus genomes/replicative intermediates composed of single-stranded RNA or DNA molecules with endosome-bound Toll-like receptor 7 (TLR7) or TLR9, respectively (19, 24), and in other virus components such as glycoproteins with cell surface receptors (63). Although a variety of enveloped viruses have been shown to readily elicit IFN production by pDC (18), both measles virus (MV) and the A strain of respiratory syncytial virus (RSV) can infect human pDC without triggering the
* Corresponding author. Mailing address: Department of Pathobi-ology, College of Veterinary Medicine, University of Illinois, Urbana, IL 61802. Phone: (217) 333-7767. Fax: (217) 244-7421. E-mail: fazaaa @illinois.edu.
䌤Published ahead of print on 29 December 2010.
2703
on November 7, 2019 by guest
http://jvi.asm.org/
synthesis of IFN-␣and can also block stimulation by synthetic TLR7 and TLR9 (TLR7/9) agonists (62). Similarly, the non-enveloped, porcine circovirus type 2 (PCV2) does not activate porcine pDC (71) and reduces the extent of their IFN-␣ syn-thesis elicited by exposure to a TLR7 or TLR9 agonist as well as to the stimulatory pathogens classical swine fever virus (CSFV), PrV, and TGEV (70).
pDC from several species have been shown to be quiescent in the presence of other enveloped viruses although their im-pact on the IFN responsiveness of the cells to known stimuli was not evaluated. In addition to an apparent lack of recogni-tion of various clinical isolates of RSV by human pDC (62), a comparable indifference was afforded to murine gammaher-pesvirus 68 by pDC of mouse origin (72). Recently, this phe-nomenon was also observed in regard to the exposure of por-cine pDC to a North American PRRSV strain. While only a relatively small quantity of IL-2 was synthesized by the pDC in the presence of PRRSV, the production of IFN-␣, IL-2, IL-6, IL-8, and TNF-␣ was readily elicited by another enveloped virus, TGEV, and also a TLR9 ligand, oligodeoxynucleotide (ODN) D19. Moreover, although exposure to either stimulant resulted in the pDC upregulating their interferon regulatory factor 7 (IRF-7) expression and acquiring a dendritic cell-type morphology with an accompanying increase in the amount of CD80/CD86 maturation markers, such alterations were not noted during incubation with PRRSV (8).
This idleness of porcine pDC in the presence of PRRSVin
vitroas well as in a similarin vivoscenario as postulated above
could merely reflect an absence of triggering of one or more pathways leading to cytokine production and cell maturation. However, in view of the virion’s potential stimulatory elements, namely, its glycoproteins and single-stranded RNA genome, it is more likely that PRRSV actively suppresses a specific anti-viral response by pDC. If so, then by analogy to the inhibitory actions of measles virus, PCV2, and RSV A2 strain (62, 71), PRRSV’s repressive ability should be more universal and ex-tend to blockage of the IFN-␣response of porcine pDC ex-posed to known agonists, ODN D19 and TGEV. Indeed, as shown here, North American PRRSV does impede IFN-␣ production by pDC otherwise stimulated by either agent. Moreover, although the synthesis of TNF-␣by either type of activated pDC was similarly inhibited, only the ODN D19-induced expression of two other proinflammatory cytokines, IL-2 and IL-6, was reduced. Thus, PRRSV is the first arteri-virus shown to be capable of repressing an elicited IFN-␣ response by porcine pDC and also of selectively antagonizing other activated functions of these cells.
MATERIALS AND METHODS
Cells.Porcine peripheral blood mononuclear cells (PBMC) were separated from the fresh, heparinized venous blood of cross-bred Yorkshire⫻Landrace pigs by density centrifugation through Ficoll-Hypaque 1077 gradients. pDC were then either enrichment sorted or purity sorted from PBMC preparations by using flow-activated cell sorting techniques. Both types of cells were maintained in RPMI complete medium (8). The porcine AM cell line, ZMAC-4, originated from fetal lung lavage samples (F. A. Zuckermann, unpublished data) and was propagated in RPMI complete medium (8) that lacked 2-mercaptoethanol but had its fetal bovine serum content doubled to 10%.
Viruses.PRRSV wild-type strains 37/McLean (25), 16244B (48), 1198, a de-rivative of 46448 (34), MN-99 (provided by K. Rossow, University of Minnesota), NADC-9 (47), NADC-20 (27), and NVSL 97-7895 (67) and a modified live virus (MLV) vaccine (Ingelvac PRRS MLV; Boehringer Ingelheim GmbH) were
propagated and titrated in MARC 145 cells (50% tissue culture infective dose [TCID50]) as previously described (46). Cell-free preparations of virus were prepared by centrifugation of the medium of infected cell monolayers showing ⱖ80% cytopathic effect (CPE) at 4°C and 350⫻gfor 10 min. Similar procedures for the Becker strain of PrV and the Purdue strain of TGEV were conducted in PK-15 and swine testes (ST) cell lines, respectively. A stock of green fluorescent protein (GFP)-expressing PRRSV (P129-GFP virus) (75) was kindly provided by D. Yoo (University of Illinois). For some experiments, PRRSV strain NADC-20 was further purified by ultracentrifugation of a cell-free virus preparation at 85,100⫻gand 4°C for 1.5 h, with the resulting pellet being resuspended in TNE buffer (10 mM Tris, pH 7.6, 100 mM NaCl, 1 mM EDTA). The associated supernatant was ascertained to be free of infectious virus since MARC 145 cells were unaffected by its presence. When required, the purified NADC-20 virus and TGEV were inactivated during exposure to short-wave (254 nm) UV light for 3 min. Loss of viability was verified by the inability of the UV light-exposed viruses to produce a cytopathic effect on monolayers of MARC 145 (NADC-20 virus) or ST (TGEV) cells.
Activation of porcine PBMC and pDC.Porcine PBMC and pDC were exposed at 37°C in a 5% CO2atmosphere to the minimum amounts of PrV or TGEV (multiplicity of infection [MOI] of 0.05) previously determined to elicit the maximum IFN-␣response from these two populations. As production of this cytokine was not induced by PRRSV, dosage of this virus was maintained at an MOI of 0.1 (8). For exposure to the PRRSV NADC-20-free supernatant, an amount corresponding to this value for the nonseparated virus stock was used. When required, the cells were incubated with either 2g/ml of ODN D19 (Qiagen, Valencia, CA) (33), a CpG-containing, type A oligodeoxynucleotide (ODN), or various concentrations of the lysosomotropic agent chloroquine (Sigma-Aldrich Corp., St. Louis, MO).
Quantitation of cytokine secretion by porcine PBMC and pDC.Medium used to culture porcine PBMC and pDC that had been left untreated or had been exposed to ODN D19, PrV, or TGEV in the presence/absence of PRRSV was examined for the presence of IFN-␣by using a specific enzyme-linked immu-nosorbent assay (ELISA) as described before (8). For the detection of other cytokines (IL-2, IL-6, IL-8, and TNF-␣), a Searchlight chemiluminescent multi-plex assay (Pierce Biotechnology, Thermo Fisher Scientific, Rockford, IL) was used.
Determination of PRRSV particle number.PRRSV genomic RNA was ex-tracted from pelleted virus preparations by using a QIAamp viral RNA minikit (Qiagen Inc., Valencia, CA), and the 3⬘ends were converted into cDNA by using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) in the presence of a complementary reverse primer, CACACGGTCGCCCTAA TTG. Real-time PCR for the amplification/detection of PRRSV genomes in the reaction mixtures was performed by using the TaqMan Universal PCR Master Mix, an ABI SDS 7000 machine (Applied Biosystems, Foster City, CA), forward primer TGGTGAATGGCACTGATTGAC, the above-mentioned reverse primer, and TaqMan probe, 6-FAM-TGTGCCTCTAAGTCACC (where FAM is 6-carboxyfluorescein). Primers and probe were designed with Primer Express, version 2.0, software (Applied Biosystems) and were purchased from Integrated DNA Technologies, Inc. (IDT, Coralville, IA), and Applied Biosystems, respec-tively. PRRSV RNA copy number was determined by comparison of the ob-tained threshold cycle (CT) values to a standard curve generated by using known amounts of RNA transcripts corresponding to approximately 9% of the 3⬘ -terminal region of the genome of PRRSV strain VR-2332.
Detection of PRRSV activity in porcine pDC and ZMAC-4 cells. Twenty thousand ZMAC-4 or purity-sorted porcine pDC were seeded into individual wells of a 96-well plate, previously coated with 50l of 0.01% poly-L-lysine (Sigma, St. Louis, MO) per well, and then incubated with P129-GFP virus (MOI of 5.0) for 20 h. Afterwards, the presence of GFP in the cells was visualized with an Olympus (Olympus America Inc., Center Valley, PA) fluorescent microscope with digital image capture capability. Magnifications of⫻400 and⫻600 were used for examining the ZMAC-4 cells and pDC, respectively, due to the rela-tively smaller size of the latter cells.
Analysis of gene expression by porcine pDC.After mock treatment or an initial exposure to PRRSV for 4 h and continued incubation in the presence/ absence of ODN D19 or TGEV for 10 h, each sample of 1⫻105purity-sorted, porcine pDC was lysed in buffer RLT, and cell RNAs were purified with an RNeasy minikit (Qiagen Inc., Valencia, CA). After DNase treatment with an Ambion Turbo DNA-free kit (Austin, TX), the RNAs were reverse transcribed in the presence of random hexamers (Invitrogen, Carlsbad, CA) and subjected to real-time PCR as described above. Primers and probes for the amplification/ detection of porcine IFNA1 (forward primer, GCATCTGCAAGGTTCCCAAT; reverse primer, AGATGGCATTGCAGCGTAG; and probe, 6-FAM-AGCCTT CCTCACGGCCCTGGTG-TAMRA, where TAMRA is
on November 7, 2019 by guest
http://jvi.asm.org/
rhodamine) and IFNB1 (forward primer, CTGCACACTCCTGAAGACTTC ACT; reverse primer, GGCATTGATGAAAACGGAAC; and probe, 6-FAM-C GGTTACTGGCTCCACTACTCAAGTGCTG-TAMRA) gene transcripts were designed by C. McGillivray (Glasgow, Scotland), except for the IFNA1 reverse primer that was derived with Primer Express, version 2.0, software. Both probes were synthesized by Applied Biosystems while the primers were purchased from IDT. The forward primer (GCGCACCCTGTCTGACTACA), reverse primer (AGATCTGCATCCCACCTCTGA), and TaqMan probe (TET-AGTCCACCC TGCACCTGGTCCTCC) for real-time PCR of ubiquitin C (UBC) gene tran-scripts were designed and provided by H. Dawson (USDA, Beltsville, MD). Changes in the extent of expression of the IFNA1 and IFNB1 genes were determined by using the comparativeCTmethod and the formula 2⫺⌬⌬Ct(41), where UBC was used as the reference gene.
Detection of IRF-7 and phosphorylated NF-B (pNF-B) in porcine pDC. After mock treatment or an initial exposure to PRRSV for 1 h and continued incubation in the presence/absence of ODN D19 or TGEV for 3 h, each sample of 3⫻105
enrichment-sorted porcine pDC was fixed, stained, and analyzed for the presence of intracellular IRF-7 as previously described (8). A comparable procedure was used for detecting intracellular pNF-B in similarly mock-treated or virus-exposed cells except that rabbit anti-human pNF-B p65 (Ser 536) polyclonal IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) replaced the anti-human IRF-7 antibodies. Gating of the histograms obtained for mock-treated cells and subsequent application of the acquired gates to the relevant remaining graphs were performed with FlowJo software (Tree Star, Inc., Ash-land, OR).
Detection of nuclear-translocated STAT1 in porcine pDC.After mock treat-ment or an initial exposure to PRRSV for 1 h and continued incubation in the presence/absence of TGEV for 3 h, each sample of 5⫻105enrichment-sorted porcine pDC was fixed in PBS containing 1% paraformaldehyde for 20 min, washed with PBS containing 2% calf serum (Gibco, Invitrogen), and stored in the latter buffer at 4°C. After 18 h, the cells were permeabilized in PB buffer (PBS containing 0.1% Triton X-100 and 2% calf serum) for 15 min at 25°C, incubated with rabbit anti-human STAT1 polyclonal IgG (Southern Biotech, Birmingham, AL) or nonspecific polyclonal IgG in PB buffer for 30 min at 25°C, and washed twice in PB buffer. The cells were then incubated for 30 min at 25°C in PB buffer containing goat anti-rabbit IgG conjugated to Alexa Fluor 647 (Molecular Probes, Invitrogen), washed twice in PB buffer, again fixed in PBS containing 1% paraformaldehyde, and shipped overnight on ice to the Amnis Corp. (Seattle, WA). There, cell nuclei were stained with 100 ng/ml 4⬘,6⬘ -diamidino-2-phenylin-dole (DAPI) before an ImageStreamXmultispectral imaging flow cytometer was used to acquire image files of between 30,000 and 70,000 single CD4⫹CD172⫹ cells for each sample. These were analyzed with the IDEAS analysis software to provide similarity scores that in this case represent the log-transformed Pearson’s correlation coefficient of the pixel values of DAPI (cell nucleus) and Alexa Fluor 647 (STAT1) images within individual cells (22). Positive scores indicate colo-calization of the two images and nuclear translocation of STAT1 while negative scores signify disparate placement of the two images and a cytoplasmic localiza-tion for STAT1.
Statistical analysis.An unpairedttest was performed to determine if signif-icant differences existed in regard to various parameters exhibited by porcine PBMC and pDC exposed to stimulant ODN D19 or TGEV in the presence/ absence of PRRSV.
RESULTS
Both viable and UV light-irradiated PRRSVs suppress the
IFN-␣ response of ODN D19- and TGEV-activated porcine
PBMC. Previously, among swine PBMC subsets, pDC had
been shown to be the principal source of IFN-␣. However, despite readily secreting this cytokine after exposure to other viruses, such as PrV and TGEV, or to the TLR9 agonist, ODN D19, these cells as well as a nonfractionated PBMC population were found to be nonresponsive to PRRSV (8). To determine whether this quiescence was a consequence of suppressed pDC activity, swine PBMC were incubated separately with each of the three agonists in the presence/absence of PRRSV, and the amounts of released IFN-␣were compared (Fig. 1A). Regard-less of whether the PBMC were exposed to PRRSV at 4 h prior to or after the addition of a stimulant, IFN-␣production was
always less than that measured in the absence of this virus. The greatest interference (82 to 93%) occurred with the TGEV-stimulated cells while the least (30 to 56%) was obtained with ODN D19-treated PBMC. In both cases, the inhibition was more pronounced when PRSSV was added before introduc-tion of the agonist. Such temporal dependence was not
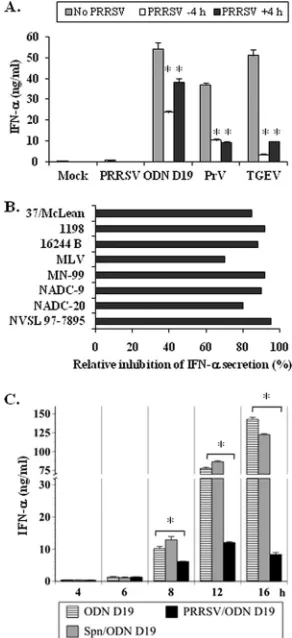
ob-FIG. 1. PRRSV inhibits IFN-␣ production by porcine PBMC ex-posed to other viruses or a TLR9 agonist. (A) One million porcine PBMC were cultured in medium alone (Mock) or in medium contain-ing PRRSV, ODN D19, PrV, or TGEV. Where indicated, PRRSV strain 1198 was also introduced 4 h before (⫺4 h) or after (⫹4 h) the addition of the stimulants. The quantities of IFN-␣ present in the medium at 16 h after inclusion of the agonists were determined by using a specific ELISA. Significant differences between the IFN-␣ responses of PBMC to the same stimulant in the absence and presence of PRRSV are indicated (ⴱ,Pⱕ0.05). Bars represent the mean⫾ standard error of the mean of a representative experiment (n⫽3). (B) One million porcine PBMC were cultured in medium alone or in medium containing the designated PRRSV isolate for 4 h prior to the inclusion of TGEV. After an additional 16 h, the quantities of IFN-␣ present in the medium were determined by using a specific ELISA. The reductions in the amount of IFN-␣released by PBMC exposed to both an individual PRRSV strain and TGEV compared to that se-creted by cells incubated with only TGEV (32.6 ng/ml) are shown. (C) One million porcine PBMC were cultured in medium alone or in medium containing either PRRSV strain NADC-20 that had been separated from the infected cell medium during ultracentrifugation or the corresponding supernatant (Spn) for 4 h prior to the inclusion of ODN D19. At the indicated times postexposure to ODN D19, the quantities of IFN-␣present in the medium were determined by using a specific ELISA. Significant differences between the IFN-␣responses of PBMC to ODN D19 in the absence and presence of PRRSV are indicated (ⴱ,Pⱕ0.05).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:3.585.347.493.70.389.2]served when PrV served as the inducer in that the level of repression caused by PRRSV remained nearly constant at 68 to 70%.
To establish whether the suppression was a unique property exhibited by the one tested isolate, six additional wild-type PRRSV strains and a commercial, attenuated live virus vaccine were assayed for their ability to influence IFN-␣synthesis by TGEV-stimulated PBMC (Fig. 1B). Like PRRSV strain 1198, the other field isolates caused severe reductions ranging from 80 to 95% in the IFN-␣responsiveness of the TGEV-activated cells while the attenuated virus was less effective, with a 65% inhibition. Similarly, as previously reported (8), none of these viruses elicited the secretion of quantities of IFN-␣ greater than the detection limit of the assay (0.04 ng/ml) (data not shown). As these assays had utilized cell-free virus prepara-tions containing cultured cell medium, PRRSV could be pro-moting the inhibition of pDC immune function via a soluble factor. Therefore, the independent capabilities of these two components were examined during the course of IFN-␣ pro-duction by ODN D19-stimulated pDC (Fig. 1C). Despite this precaution, the NADC-20 virus isolate was still repressive starting at 8 h postinduction, whereas the virus-free medium did not have a significant impact at any measured time throughout the assay period. Likewise, the purified virus still interfered with IFN-␣synthesis by TGEV-activated pDC (Fig. 2A) while the supernatant medium was noneffective against
induction by this agonist (data not shown). This inhibition of pDC activity appeared to be dose dependent, regardless of the stimulant, as it significantly dropped when the ratio of particles per PBMC was reduced from approximately 680 to 68 (Fig. 2A). Based on pDC having a frequency of 0.5% within the porcine PBMC population (8), a maximum of approximately 1.4⫻105purified PRRSV particles per pDC appeared to be
sufficient to suppress pDC IFN-␣ responsiveness. A similar value of 1.2⫻105particles per cell was obtained when we used
purity-sorted pDC and PRRSV strain 1198 retentate accumu-lated during filtration of infected cell culture medium through a 100,000-nominal-molecular-weight-limit membrane (data not shown).
Since RNA-containing viruses such as MV and RSV can produce proteins to impede the induction of IFN-␣synthesis in infected human pDC (62), the requirement for viable PRRSV to perform a similar function was investigated by evaluating the suppressive ability of inactivated virus. Despite a loss of detectable replication competency after a 3-min exposure to UV light (data not shown), the treated PRRSV retained the ability to block ODN D19- and TGEV-induced IFN-␣ expres-sion by porcine PBMC (Fig. 2B) at levels comparable to those exerted by the untreated virus (Fig. 2A). Moreover, the extent of their suppressive activity similarly declined as a function of particle number per cell.
PRRSV-associated suppression of the IFN-␣ response of
TGEV-activated porcine PBMC is not impaired by
chloro-quine. Although viable PRRSV was not required to inhibit
IFN-␣synthesis by pDC activated within a PBMC population, the question still remained whether this blockage originated on the cell surface or internally. In this regard, PRRSV has been shown to become compartmentalized in the early endosomes of susceptible cells (68), where a reduction in pH (50) and the involvement of the host’s aspartic protease cathepsin E, an unidentified serine protease (49), and the virus’s small enve-lope protein E (39) enable PRRSV uncoating and replication. To determine whether these events enabled subsequent sup-pression of IFN-␣production by stimulated pDC, the acidifi-cation of endosomes in pDC during the cells’ exposure to TGEV in the presence/absence of PRRSV was prevented by prior incubation with chloroquine, a weak base that increases intralysosomal pH (56). Selection of the minimum (2.5M) and maximum (10.0M) concentrations of this compound to be tested was based on these amounts being nonlethal to por-cine pDC but sufficient to elevate endosomal pH in mouse peritoneal macrophages (56) and prevent PRRSV replication in porcine AM (50), respectively. The effectiveness of the greater quantity was verified by the demonstration that it, as well as an intermediate dose (5.0M), was ample for elimi-nating the IFN-␣response of ODN D19-treated pDC (Fig. 3). Although TGEV stimulation of pDC activity has been shown to occur via interaction of the virus’s M and E proteins with cell surface molecules (5, 38), this coronavirus was inactivated with UV light before use to ensure that any induction due tode novo TGEV RNA or protein synthesis should not transpire and be disrupted by the chloroquine treatment. At the three tested concentrations of chloroquine, inhibition of the IFN-␣ responsiveness of the pDC to TGEV was found to be dose dependent (Fig. 3). In contrast, the relative degree of PRRSV suppression of pDC activity nearly paralleled the loss in IFN-␣
FIG. 2. UV light inactivation of PRRSV viability does not alter the virus’s ability to inhibit IFN-␣production by porcine PBMC exposed to TGEV or a TLR9 agonist. One million porcine PBMC were cul-tured for 4 h in the absence or presence of the indicated relative amounts of PRRSV strain NADC-20 that had been separated from the infected cell medium during ultracentrifugation and was either un-treated (A) or UV light-inactivated (B) prior to the inclusion of ODN D19 or TGEV. After an additional 16 h, the quantities of IFN-␣ present in the medium were determined by using a specific ELISA. The results are presented as the percent reduction of IFN-␣secretion by ODN D19 (90.0 ng/ml)- and TGEV (8.1 ng/ml)-stimulated PBMC in the presence of 10-fold dilutions of PRRSV where a value of 1.0 is
equivalent to 6.8⫻103virus particles/cell.
on November 7, 2019 by guest
http://jvi.asm.org/
production by the TGEV-stimulated cells at each dose of chlo-roquine. These drops were not due to a loss of cell viability as the frequency of live cells remained between 85 and 90% regardless of the type of treatment and was comparable to the 90% survival rate of the mock-treated cells (data not shown). Thus, disassembly of PRRSV in an intracellular, acidified en-vironment does not seem to be a prerequisite for subsequent inhibition of the cells’ IFN-␣ response to TGEV. Moreover, the effectiveness of PRRSV in impairing this function of pDC, even after their purification from the general PBMC popula-tion, indicates that this virus can act directly without a contri-bution from some other type of blood lymphocyte.
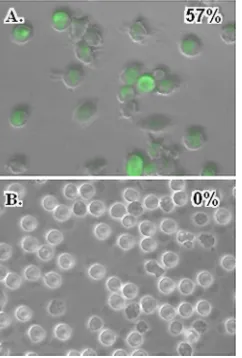
Porcine pDC are resilient to PRRSV infection.As specified
above, prolonged incubation of porcine pDC with PRRSV did not significantly alter cell viability. Moreover, the chloroquine-resistant nature of PRRSV suppression of the pDC IFN-␣ response indicated that this event probably began on the cell surface. Consequently, it was not clear whether PRRSV could reproduce in porcine pDC or even undergo an abortive repli-cation in them as can foot-and-mouth disease virus (FMDV) after its introduction as a virus-IgG complex (26). To evaluate the susceptibility of porcine pDC to PRRSV, these cells as well as the ZMAC-4 cell line known to support PRRSV infection (F. A. Zuckermann, unpublished data) were exposed to P129-GFP virus for 16 h before being screened for the presence of green fluorescence. Although GFP was readily found in ZMAC-4 cells (Fig. 4A), this protein was not detected in the cytoplasm of any examined pDC (Fig. 4B). That porcine pDC were indeed resilient to PRRSV infection was further sup-ported by our inability to measure a change in the amount of virus open reading frame 7 (ORF7) mRNA present in pDC after an 8-h exposure to PRRSV even though we observed an approximately 800-fold increase in the relative quantity of this transcript in virus-infected ZMAC-4 cells after the same time interval (data not shown).
PRRSV suppresses enhanced type I IFN gene expression by
ODN D19- and TGEV-activated porcine pDC. As observed
here with pDC, incubation of porcine AM with PRRSV usually failed to elicit the secretion of IFN-␣(2, 40). Examinations of such virus-exposed cells have revealed a strong induction of either IFNA1 (40) or IFNB1 (21) gene transcription. To es-tablish whether PRRSV influenced porcine pDC in either manner, the type I IFN gene expression profiles of mock- and virus-treated pDC were compared. In contrast to the AM, pDC did not respond to the presence of PRRSV with any significant changes in the relative amounts of their IFNA1 (1.8-fold) or IFNB1 (0.7-fold) gene transcripts (Fig. 5A and B). However, activation of the pDC with either TGEV or ODN D19 did result in a tremendous increase in the relative quantities of IFN-␣ (approximately 25,000-fold and 78,000-fold, respec-tively) and IFN-ß (approximately 290-fold and 750-fold, re-spectively) transcripts. These augmentations in type I gene expression were severely reduced (91 to 93%) by exposure of the cells to PRRSV before stimulation. Such treatment also resulted in a corresponding diminution in the amounts of IFN-␣(91 to 98%) secreted by the activated pDC (Fig. 5C). Determinations of IFN-ß release were not conducted due to the unavailability of a specific immunoassay for this cytokine.
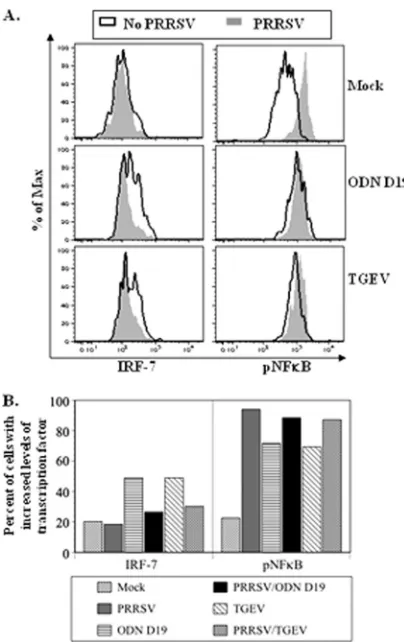
PRRSV impedes IRF-7 production but promotes NF-B
phosphorylation by ODN D19- and TGEV-activated porcine pDC.Activation by either ODN D19 or TGEV of porcine pDC has been shown to result in a rapid increase in the cells’ intracellular IRF-7 concentration, whereas no significant change in this parameter was afforded by exposure to PRRSV alone (8). Since this factor is required for transcription of the IFN genes in pDC (28, 35), the effect of PRRSV on its pres-ence in stimulated cells was examined. As seen in the histo-grams in the left-hand panels of Fig. 6A, activation of the pDC increased the frequency of cells whose IRF-7 amounts were similar to or greater than the amounts in cells in the top quintile of the mock-treated population, and the extent of
[image:5.585.363.484.70.249.2]FIG. 3. Chloroquine does not block PRRSV’s ability to inhibit IFN-␣production by porcine pDC after exposure to TGEV. A total of 10,000 purity-sorted porcine pDC were cultured for 30 min in the absence or presence of chloroquine at the indicated concentrations prior to the addition of PRRSV strain 1198 where indicated. After an additional 4 h, the cells were exposed to UV light-inactivated TGEV or ODN D19 for 16 h, and then the quantities of IFN-␣present in the medium were determined by using a specific ELISA. Significant dif-ferences between the IFN-␣responses of pDC to TGEV in the ab-sence and preab-sence of PRRSV at the same concentration of chloro-quine are indicated (ⴱ,Pⱕ0.05). The dotted line indicates the limit of detection for IFN-␣(0.04 ng) afforded by this assay.
FIG. 4. PRRSV infects porcine macrophages but not pDC. ZMAC-4 cells (A) or purity-sorted pDC (B) were cultured with P129-GFP virus for 20 h. Cells were then visualized with bright-field and fluorescent microscopy (magnifications of⫻400X and⫻600X for panels A and B, respectively), and the resulting images were merged. Numbers in the upper right-hand corner of the figures indicate the percentage of cells exhibiting fluorescence from a total of 120 cells in four examined fields for each sample.
on November 7, 2019 by guest
http://jvi.asm.org/
these two transitions was diminished by prior incubation of the stimulated cells with PRRSV. Thus, while the percentage of pDC deemed to be highly IRF-7 expressive increased approx-imately 2.4-fold after a 3-h exposure to either ODN D19 or TGEV, these boosts were reduced 78 and 65%, respectively, if the cells were initially cultured with PRRSV (Fig. 6B, left-hand panel). In contrast, the inclusion of PRRSV did not diminish the augmented phosphorylation of NF-B, an event necessary for the expression of the proinflammatory cytokines, detected
in either type of stimulated pDC (Fig. 6A, right-hand panels). Instead, while ODN D19 or TGEV treatment resulted in ap-proximately a 3-fold increase in the frequencies of pDC de-fined as possessing a maximal amount of pNF-B, these values were surpassed by the approximately 4-fold enhancement re-sulting from exposure of the pDC to PRRSV alone (Fig. 6B, right-hand panel).
PRRSV blocks the nuclear translocation of STAT1 in
TGEV-activated porcine pDC. Early, IFN-␣ independent,
[image:6.585.318.520.66.387.2]TLR9 agonist-induced IRF-7 production in human pDC re-quires the activation and nuclear translocation of STAT1 (66). To ascertain if PRRSV repressed a similar event in stimulated porcine pDC, the occurrence of nuclear-localized STAT1 in pDC that had been mock treated or TGEV activated for 3 h in the presence/absence of PRRSV was compared (Fig. 7). Whereas there was an approximately 3.5-fold increase in the frequency of nuclear STAT1-positive cells during stimulation
FIG. 5. PRRSV suppresses IFN gene transcription and IFN-␣ pro-duction by porcine pDC exposed to TGEV or a TLR9 agonist. A total of 100,000 purity-sorted pDC were cultured for 4 h in the absence or presence of PRRSV strain 1198 before the inclusion of ODN D19 or TGEV. After an additional 10 h, RNA was isolated from each sample and utilized in real-time PCR assays for the detection of IFNA1 (A) and IFNB1 (B) gene transcripts. Relative fold differences in the extent of expression of these two genes between treated and mock-treated pDC was determined by using the comparativeCTmethod and
the formula 2⫺⌬⌬CT, where UBC was used as the reference gene.
Significant differences between the amounts of transcript encoding IFN-␣or IFN-ß in pDC exposed to the same stimulant in the absence and presence of PRRSV are indicated (ⴱ,Pⱕ0.05). (C) The quantities of IFN-␣present in the medium were determined by using a specific ELISA. Significant differences between the IFN-␣responses of pDC to the same stimulant in the absence and presence of PRRSV are indi-cated (ⴱ,Pⱕ0.05).
FIG. 6. PRRSV inhibits an enhancement of IRF-7 but not of pNF-B quantity in porcine pDC exposed to TGEV or a TLR9 ago-nist. A total of 300,000 enrichment-sorted porcine pDC were cultured for 1 h in the absence or presence of PRRSV strain 1198 and then mock treated or exposed to either TGEV or ODN D19 for 3 h before being stained for the intracellular presence of either IRF-7 or pNF-B. (A) Histograms of stained pDC. (B) Frequencies of pDC populations expressing maximal quantities of IRF-7 or pNF-B. Values represent the percentages of cell samples determined to be within the positive signal range in the histograms of IRF-7- or pNF-B-stained cells shown in panel A. This range is defined by the limits of fluorescent intensity representing the top quintile of mock-treated cells in regard to their individual amounts of the respective transcription factor.
on November 7, 2019 by guest
http://jvi.asm.org/
by TGEV, this augmentation was reduced 62% in the presence of PRRSV. A similar reduction (45%) in this parameter was also observed in regard to nonactivated pDC when PRRSV was present. Therefore, PRRSV appeared to partially inhibit
the migration of STAT1 into pDC nuclei, even in the absence of external stimulation.
PRRSV selectively suppresses some cytokine responses of
TGEV-activated porcine pDC.In addition to IFN-␣, PRRSV
has been shown to be incapable of eliciting the secretion of detectable amounts of IL-6, IL-8, and TNF-␣by porcine pDC. However, PRRSV did provoke the release of a small quantity of IL-2. Since all four cytokines had been produced by pDC activated with ODN D19 or TGEV (8), the potential selectivity of PRRSV regarding inhibition of these pDC responses was evaluated (Fig. 8). Only for TNF-␣was the relative decrease in synthesis comparable to that observed for IFN-␣(Fig. 5C), and this PRRSV-mediated effect was independent of whether ODN D19 (99% reduction) or TGEV (91% reduction) acted as the stimulant. Lesser, but still significant, drops of 48 and 62% in the fold increase of IL-2 and IL-6 production, respec-tively, by pDC stimulated by ODN D19 were noted in the presence of PRRSV. Exposure to this agonist did not elicit a detectable IL-8 response from the cells, a result compatible with the previously observed, very limited secretion of this cytokine by porcine pDC incubated with ODN D19 (8). No significant changes in the enhanced IL-2, IL-6, and IL-8 pro-duction by pDC in response to TGEV were incurred by the inclusion of PRRSV.
DISCUSSION
[image:7.585.61.266.67.503.2]As an extension of our previous study indicating a relative indifference of porcine pDC to PRRSV compared to known stimulants that induced cytokine secretion and cell maturation (8), we now show that PRRSV strongly represses pDC IFN-␣ production otherwise elicited by TGEV or the TLR9 agonist ODN D19. This ability was not due to a blockage of binding sites on the pDC since inhibition could still be achieved if the cells were exposed to either stimulatory agent 4 h prior to the addition of PRRSV. However, it was replication independent, as indicated by the comparable effectiveness of nonviable
FIG. 7. PRRSV inhibits STAT1 translocation into the nuclei of porcine pDC exposed to TGEV. A total of 300,000 enrichment-sorted porcine pDC were cultured for 1 h in the absence or presence of PRRSV strain 1198 and then either mock treated or exposed to TGEV for 3 h. Afterwards, the cells were fixed, and their STAT1 proteins and nuclei were separately stained with Alexa Fluor 647 and DAPI, re-spectively. STAT1 localization in the pDC was determined by using imaging flow cytometry and IDEAS software at Amnis Corp. (A) His-tograms of the similarity scores (similarity STAT1/DAPI) of individual pDC. Horizontal bars define the region of positive correlation for the scores as determined by nonparametric analysis. The percentage of cells having nuclear translocated STAT1 in each sample is shown in the upper right-hand corner. (B) Bright-field and fluorescent microscopic images (nucleus, red; STAT1, green; merge, red and green) of a rep-resentative cell within the regions of negative (cytosolic) and positive (nuclear) similarity as defined in the histograms in panel A are shown.
FIG. 8. PRRSV selectively inhibits cytokine production by porcine pDC exposed to TGEV or a TLR9 agonist. A total of 100,000 purity-sorted pDC were cultured for 4 h in the absence or presence of PRRSV strain 1198 before the inclusion of ODN D19 or TGEV. After an additional 10 h, the quantities of secreted IL-2, IL-6, IL-8, and TNF-␣were determined by using a Searchlight chemiluminescent as-say. Significant differences between the individual cytokine responses of pDC to the same stimulant in the absence and presence of PRRSV are indicated (ⴱ,Pⱕ0.05). Bars represent the mean⫾standard error of the mean of a representative experiment (n⫽2).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:7.585.334.507.70.197.2]PRRSV and the nonsusceptibility of pDC to infection by this virus. This novel antagonism can be attributed to a PRRSV-influenced reduction of IFNA gene transcription resulting in diminished levels of the relevant mRNA. Moreover, PRRSV also blocked IFNB1 gene transcription in stimulated pDC and by itself did not elicit a significant increase in the amounts of either type of IFN mRNA. The mode of action for rendering pDC nonresponsive is probably different from that used for silencing porcine AM. Depending on the strain, these cells have reacted to PRRSV by releasing IFN-␣in undetectable or minor quantities that were disproportionate to strongly up-regulated IFNA gene expression (40) or by increasing IFNB1 but not IFNA1 gene transcription (21). Although for the latter no correlation between the extent of protein production and transcript amount was established, a similar response to PRRSV has also been reported for monocyte-derived DC and, to a lesser extent, for lung DC. In these cases, the possibility of the transcripts being translated was considered unlikely (43). Therefore, in modulating the host IFN response, PRRSV in-tervenes at a pre- or posttranscriptional event occurring in activated porcine pDC or AM and certain DC populations, respectively.
Viruses have been shown to block type I IFN synthesis by activated/infected cells through either a general repression of host transcriptional/translational processes or a specific ob-struction of a step(s) in pathways leading to the production of these proteins (58). In regard to its interactions with porcine pDC, PRRSV utilizes the second approach as it targets par-ticular, agonist-dependent, cytokine responses. Although IFN-␣, TNF-␣, IL-2, and IL-6 production by pDC stimulated with ODN D19 was inhibited in the presence of PRRSV, synthesis of the last two proteins as well as IL-8 by TGEV-activated pDC was not significantly affected. This selectivity precludes the possibility of a single mechanism whereby PRRSV would interfere with the pathways leading to type I IFN, proinflammatory cytokine, and chemokine synthesis and is a strategy used by other viruses. Both the A2 strain of RSV and the Schwarz strain of measles virus have been shown to obstruct self-induced and TLR7/9-mediated induction of IFN-␣ synthesis but to have no effect on IL-8 production by mock-treated human pDC (62). Moreover, although PCV2 severely inhibited various cytokine responses by porcine pDC to CSFV, PrV, TGEV, and a TLR9 agonist, only the cells’ TLR7-mediated synthesis of IFN-␣and IL-12 but not of IL-6 or TNF-␣was affected (70).
In considering potential sites for PRRSV intervention, it should be noted that pDC sense natural or synthetic agonists in various ways and respond by activating intricate cascades that culminate in the transcription of primarily type I IFN genes and also of ones encoding various other cytokines (19, 24). Recognition of an exogenous, type A CpG-DNA such as the ODN D19 used in this study is considered to be a two-step process (61). The foreign DNA first associates with plasma membrane-bound receptors and after internalization into en-dosomes joins with TLR9 that has translocated from the pDC endoplasmic reticulum (37). The ligand-bound TLR recruits and forms complexes with various proteins for participation in multiple signaling pathways (19, 24). For the expression of proinflammatory cytokines such as IL-6 and TNF-␣, these complexes are involved in the activation of transcription
fac-tors IRF-5 (65) and NF-B as well as the mitogen-activated protein kinases (MAPK) (24). While IRF-5 may also contrib-ute in directing the transcription of IFN-␣genes, the prereq-uisite protein for this function is IRF-7 (28, 35). Unlike ODN D19, TGEV can induce a pDC IFN response via a nucleic acid-independent mechanism as the triggering can be caused by multimeric structures composed of the virus’s M and E glycoproteins (5). Despite the apparent TLR7/9 independency of this induction, it is conceivable that the responsible, cur-rently uncharacterized, cascade would utilize at least some components, especially IRF-7, involved in TLR9-directed cy-tokine production by pDC.
IRF-7 is constitutively expressed at high levels in pDC (14, 31) due to the involvement of constitutively activated NF-B (52) and the relatively limited amounts of intracellular 4E-BP1 and 4E-BP-2, two inhibitors of IRF-7 mRNA translation (12). However, its availability may not be sufficient to maximize the IFN production that occurs in response to type A CpG-DNA exposure and independently of autocrine IFN-␣ action (28). Instead, additionalde novoexpression of IRF-7 resulting from the activation of NF-B and p38 MAPK through the TLR9 signaling pathway may be necessary (52, 66). In this case, one consequence of the MAPK being activated would be the down-stream, dual phosphorylation of STAT1 that in turn would enable it to coalesce with STAT2 and IRF-9 and form IFN-stimulated gene factor 3 (ISGF3). Subsequently, ISGF3 would migrate to the nucleus and, in conjunction with pNF-B, pro-mote transcription of the IRF-7 gene. Part of this model was based on an overall increase in the quantity of translocated STAT1 in human pDC after a 3-h exposure to CpG-DNA compared to mock-treated cells (66). Here, a similar enhance-ment occurred in porcine pDC that had been stimulated with TGEV for the same period of time. However, whereas the former study utilized a Western blotting procedure that en-compassed the intracellular contents of the entire pDC sample, our current examination relied on flow cytometry-based meth-odology that could differentiate individual cells based on the extent of their nuclear-localized STAT1. This approach was chosen to eliminate a potential background of nonreactive cells as previously only 7 to 10% of sorted porcine pDC had been found to secrete IFN-␣in response to TGEV (8). With this type of analysis, exposure to TGEV was shown to increase the frequency of pDC having demonstrable STAT1 nuclear migra-tion⬎3-fold to approximately 10%. Since this augmentation was almost halved in the presence of PRRSV, it is likely that this agent intercedes at a step prior to the formation of ISGF3 and, in doing so, diminishes induced IRF-7 synthesis and sub-sequent IFN production. In this regard, the early upregulation of pDC IRF-7 expression induced by ODN D19 or TGEV was nearly abolished by PRRSV. However, since PRRSV’s nega-tive impact on the temporal expression of IFN-␣by pDC was detected starting at only 8 h but not at 6 h after exposure to ODN D19 (Fig. 1C), it can be envisioned that this virus did not affect the activation of constitutively expressed IRF-7 that, in turn, was primarily responsible for the initial, elicited IFN-␣ response.
Previously, the only observed response of porcine pDC to PRRSV was a meager secretion of IL-2. Other cytokines, in-cluding IFN-␣, IL-6, IL-8, and TNF-␣, whose synthesis can be induced by enveloped viruses, were not detected (8). Even
on November 7, 2019 by guest
http://jvi.asm.org/
though PRRSV likely repressed the generation of these cyto-kines where applicable, this virus did enhance NF-B phos-phorylation in exposed cells to a level comparable to that observed in both ODN D19- and TGEV-stimulated cells. Whether this surplus of pNF-B promotes the expression of an inhibitor of the TLR signaling pathway is unknown. However, like PRRSV, hepatitis B virus (HBV) does not productively infect pDC and thus is unable to directly generate inhibitory proteins. Instead, HBV soluble antigen (HBsAg) circulating in the blood of infected people may interact with pDC and indi-rectly impair their functionality by causing increased transcrip-tion of the gene encoding the suppressor of cytokine signaling 1 (SOCS-1), a negative regulator of the TLR9 signaling path-way (74). Such a course of action contrasts with that employed by the replication-competent MV, whose newly synthesized V protein binds to both the cellular inhibitor of B kinase ␣ (IKK␣) and IRF-7 to prevent IRF-7 nuclear translocation and subsequent IFN production (54).
The demonstrated chloroquine insensitivity of the PRRSV-mediated inhibition of the IFN-␣response of porcine pDC to TGEV indicated that uncoating of PRRSV within an intracel-lular compartment was not required for its repressive ability. Since this drug acts as a TLR9 antagonist (60), a similar eval-uation regarding the impact of PRRSV on the pDC response to ODN D19 could not be made. However, as prior inactiva-tion of PRRSV did not diminish its suppressive nature, it is unlikely thatde novovirus products were involved. Rather, the interaction of PRRSV proteins with porcine pDC surface re-ceptors may have triggered a pathway designed to negatively regulate the pDC IFN-␣response, as proposed for the binding of the HBsAg to the human pDC C-type lectin receptor, blood dendritic cell antigen 2 (BDCA-2) (74), and of human immu-nodeficiency virus (HIV-1) gp120 to both BDCA-2 and CD4 (44). Interestingly, in both of these cases, the interference was specific as the induction due to exposure to TLR9 but not to TLR7 agonists was affected. In contrast, this selectivity is not maintained by PRRSV because this virus has been found to suppress IFN-␣ production by porcine pDC exposed to the TLR7 agonist, imiquimod (data not shown). Moreover, it is likely that recognition of this virus’s single-stranded RNA ge-nome via TLR7 is repressed. Otherwise, as with HIV-1 (6), the presence of PRRSV would spur on pDC IFN-␣ production. However, control of this activity may be ligand specific since monoclonal antibody (MAb) cross-linking of BDCA-2 enabled a generalized inhibition of both TLR7- and TLR9-mediated IFN-␣responses (10, 16).
When the effects of cross-linked BDCA-2 and PRRSV on pDC activity induced by CpG DNA are compared, several parallels and dissimilarities can be discerned. First, both BDCA-2 and PRRSV imparted a rapid disruption of the in-duced pDC IFN-␣response. Exposure to either anti-BDCA-2 MAb (10) or PRRSV resulted in an effective inhibition of IFN-␣production by stimulated pDC even when the cells were first incubated with CpG DNA for 4 h, an interval only 2 h shorter than when IFN-␣secretion by similarly treated human pDC was initially detected (66). Second, both MAb-bound BDCA-2 (10, 59) and PRRSV reduced the upregulated IFNA1 and IFNB1 gene transcription in stimulated pDC. Based on a characterization of the impact of cross-linked BDCA-2 on her-pes simplex virus-1-activated pDC, this suppression may be the
consequence of a decreased nuclear presence of IRF-7 that occurred despite a stable, intracellular IRF-7 content (17). While the extent of IRF-7 nuclear migration in pDC was not determined in the current study, it is possible that this param-eter would proportionately correlate with the observed inhibi-tion of enhanced IRF-7 producinhibi-tion in activated pDC previ-ously exposed to PRRSV. Third, in addition to IFN-␣, both ligated BDCA-2 (10) and PRRSV inhibited the induced pDC expression of the proinflammatory cytokines IL-6 and TNF-␣. All of these actions by cross-linked BDCA-2 are considered to proceed through a B cell receptor (BCR)-like cascade involv-ing intracellular tyrosine-based activation motif (ITAM) sig-naling (10, 59). One predicted fulfillment of this pathway is NF-B activation (10), as was exemplified by the enhanced presence of pNF-B in pDC exposed to PRRSV. However, ligation of BDCA-2 had no apparent effect on the NF-B canonical pathway in nonstimulated pDC but did result in decreased activation of this pathway in CpG-DNA-stimulated pDC, as evidenced by the swift repression of an otherwise induced enhancement of the phosphorylation of IKK␣ (59). Thus, based on the different outcomes regarding the apparent availability of activated NF-B required for the transcription of genes encoding proteins such as IL-6 and TNF-␣, PRRSV may interact with a receptor distinct from a porcine analog of BDCA-2. In this regard, other proteins that signal through the ITAM or the distinct intracellular tyrosine-based inhibitory motif (ITIM) pathway to negatively regulate cytokine produc-tion by stimulated human or murine pDC have been described (9, 64).
In closing, PRRSV is the first arterivirus demonstrated to repress the ability of porcine pDC to mount a type I IFN and proinflammatory cytokine response to itself as well as to a known stimulatory virus, TGEV, and a TLR9 agonist, ODN D19. As this subjugation could have important ramifications regarding the host’s innate and adaptive immune responses against PRRSV and even secondary invaders, an evaluation of pDC functionality in pigs during acute virus infection is being conducted.
ACKNOWLEDGMENTS
We thank Mauricio Villamar and Janie Frye (University of Illinois) for their excellent technical support and Tad George and Raymond Kong (Amnis Corp.) for the ImageStream analysis.
This study was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service Coordinated Agricultural Project (number Q6706392373/2004-35605-14197) and by the National Research Initiative, Competitive Grants Program (project number 2007-35204-18419).
REFERENCES
1.Albina, E.1997. Epidemiology of porcine reproductive and respiratory syn-drome (PRRS): an overview. Vet. Microbiol.55:309–316.
2.Albina, E., C. Carrat, and B. Charley.1998. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syn-drome virus. J. Interferon Cytokine Res.18:485–490.
3.Albina, E., L. Piriou, E. Hutet, R. Cariolet, and R. L’Hospitalier.1998. Immune responses in pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Immunol. Immunopathol.61:49–66. 4.Allende, R., et al.2000. Porcine reproductive and respiratory syndrome virus:
description of persistence in individual pigs upon experimental infection. J. Virol.74:10834–10837.
5.Baudoux, P., C. Carrat, L. Besnardeau, B. Charley, and H. Laude.1998. Coronavirus pseudoparticles formed with recombinant M and E proteins induce alpha interferon synthesis by leukocytes. J. Virol.72:8636–8643. 6.Beignon, A. S., et al.2005. Endocytosis of HIV-1 activates plasmacytoid
on November 7, 2019 by guest
http://jvi.asm.org/
dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115:3265–3275.
7.Buddaert, W., K. Van Reeth, and M. Pensaert.1998. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syn-drome virus (PRRSV). Adv. Exp. Med. Biol.440:461–467.
8.Calzada-Nova, G., W. Schnitzlein, R. Husmann, and F. A. Zuckermann. 2010. Characterization of the cytokine and maturation responses of pure populations of porcine plasmacytoid dendritic cells to porcine viruses and Toll-like receptor agonists. Vet. Immunol. Immunopathol.135:20–33. 9.Cao, W., and L. Bover.2010. Signaling and ligand interaction of ILT7:
receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol. Rev.234:163–176.
10.Cao, W., et al.2007. BDCA2/FcεRI␥complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol.5:e248. 11.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna.2000.
Plasma-cytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol.1:305–310.
12.Colina, R., et al.2008. Translational control of the innate immune response through IRF-7. Nature452:323–328.
13.Colonna, M., G. Trinchieri, and Y. J. Liu.2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol.5:1219–1226.
14.Dai, J., N. J. Megjugorac, S. B. Amrute, and P. Fitzgerald-Bocarsly.2004. Regulation of IFN regulatory factor-7 and IFN-alpha production by enve-loped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J. Immunol.173:1535–1548.
15.Duan, X., H. J. Nauwynck, and M. B. Pensaert.1997. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV). Arch. Virol.142:2483–2497.
16.Dzionek, A., et al.2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med.194:1823–1834.
17.Fanning, S. L., et al.2006. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-alpha production. J. Immunol. 177:5829–5839.
18.Feldman, S. B., et al.1994. Viral induction of low frequency interferon-alpha producing cells. Virology204:1–7.
19.Fitzgerald-Bocarsly, P., J. Dai, and S. Singh.2008. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev.19:3–19.
20.Fonteneau, J. F., et al.2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander mat-uration of myeloid dendritic cells. J. Virol.78:5223–5232.
21.Genini, S., et al.2008. Genome-wide transcriptional response of primary alveolar macrophages following infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol.89:2550–2564.
22.George, T. C., et al.2006. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J. Immunol. Methods311:117–129.
23.Gerosa, F., et al.2005. The reciprocal interaction of NK cells with plasma-cytoid or myeloid dendritic cells profoundly affects innate resistance func-tions. J. Immunol.174:727–734.
24.Gilliet, M., W. Cao, and Y. J. Liu.2008. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8:594–606.
25.Goldberg, T. L., E. C. Hahn, R. M. Weigel, and G. Scherba.2000. Genetic, geographical and temporal variation of porcine reproductive and respiratory syndrome virus in Illinois. J. Gen. Virol.81:171–179.
26.Guzylack-Piriou, L., F. Bergamin, M. Gerber, K. C. McCullough, and A. Summerfield.2006. Plasmacytoid dendritic cell activation by foot-and-mouth disease virus requires immune complexes. Eur. J. Immunol.36:1674–1683. 27.Harms, P. A., et al.2001. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet. Pathol.38:528–539. 28.Honda, K., et al.2005. IRF-7 is the master regulator of type-I
interferon-dependent immune responses. Nature434:772–777.
29.Horter, D. C., et al.2002. Characterization of the carrier state in porcine reproductive and respiratory syndrome virus infection. Vet. Microbiol.86: 213–228.
30.Ito, T., et al.2007. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med.204:105–115. 31.Izaguirre, A., et al.2003. Comparative analysis of IRF and IFN-alpha ex-pression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol.74:1125–1138.
32.Jego, G., et al.2003. Plasmacytoid dendritic cells induce plasma cell differ-entiation through type I interferon and interleukin 6. Immunity19:225–234. 33.Kamstrup, S., D. Verthelyi, and D. M. Klinman.2001. Response of porcine peripheral blood mononuclear cells to CpG-containing oligodeoxynucleo-tides. Vet. Microbiol.78:353–362.
34.Katz, J. B., A. L. Shafer, K. A. Eernisse, J. G. Landgraf, and E. A. Nelson. 1995. Antigenic differences between European and American isolates of porcine reproductive and respiratory syndrome virus (PRRSV) are encoded
by the carboxyterminal portion of viral open reading frame 3. Vet. Microbiol. 44:65–76.
35.Kawai, T., et al.2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immu-nol.5:1061–1068.
36.Lamontagne, L., C. Page, R. Larochelle, and R. Magar.2003. Porcine re-productive and respiratory syndrome virus persistence in blood, spleen, lymph nodes, and tonsils of experimentally infected pigs depends on the level of CD8high
T cells. Viral Immunol.16:395–406.
37.Latz, E., et al.2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol.5:190–198.
38.Laude, H., J. Gelfi, L. Lavenant, and B. Charley.1992. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J. Virol.66:743–749. 39.Lee, C., and D. Yoo.2006. The small envelope protein of porcine
reproduc-tive and respiratory syndrome virus possesses ion channel protein-like prop-erties. Virology355:30–43.
40.Lee, S. M., S. K. Schommer, and S. B. Kleiboeker.2004. Porcine reproduc-tive and respiratory syndrome virus field isolates differ inin vitrointerferon phenotypes. Vet. Immunol. Immunopathol.102:217–231.
41.Livak, K. J., and T. D. Schmittgen.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2⫺⌬⌬CTmethod. Methods 25:402–408.
42.Loemba, H. D., S. Mounir, H. Mardassi, D. Archambault, and S. Dea.1996. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch. Virol.141:751– 761.
43.Loving, C. L., S. L. Brockmeier, and R. E. Sacco.2007. Differential type I interferon activation and susceptibility of dendritic cell populations to por-cine arterivirus. Immunology120:217–229.
44.Martinelli, E., et al.2007. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-␣secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A.104:3396–3401.
45.McKenna, K., A. S. Beignon, and N. Bhardwaj.2005. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol.79:17–27.
46.Meier, W. A., et al.2003. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology309:18–31.
47.Mengeling, W. L., K. M. Lager, A. C. Vorwald, and K. J. Koehler.2003. Strain specificity of the immune response of pigs following vaccination with various strains of porcine reproductive and respiratory syndrome virus. Vet. Microbiol.93:13–24.
48.Miller, L. C., W. W. Laegreid, J. L. Bono, C. G. Chitko-McKown, and J. M. Fox.2004. Interferon type I response in porcine reproductive and respiratory syndrome virus-infected MARC-145 cells. Arch. Virol.149:2453–2463. 49.Misinzo, G. M., P. L. Delputte, and H. J. Nauwynck.2008. Involvement of
proteases in porcine reproductive and respiratory syndrome virus uncoating upon internalization in primary macrophages. Vet. Res.39:55.
50.Nauwynck, H. J., X. Duan, H. W. Favoreel, P. Van Oostveldt, and M. B. Pensaert. 1999. Entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages via receptor-mediated endocytosis. J. Gen. Virol.80:297–305.
51.Nelson, E. A., J. Christopher-Hennings, and D. A. Benfield.1994. Serum immune responses to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J. Vet. Diagn. Invest.6:410–415.
52.Osawa, Y., et al.2006. Collaborative action of NF-B and p38 MAPK is involved in CpG DNA-induced IFN-alpha and chemokine production in human plasmacytoid dendritic cells. J. Immunol.177:4841–4852. 53.Paquette, R. L., et al.1998. Interferon-alpha and granulocyte-macrophage
colony-stimulating factor differentiate peripheral blood monocytes into po-tent antigen-presenting cells. J. Leukoc. Biol.64:358–367.
54.Pfaller, C. K., and K. Conzelmann.2008. Measles virus V protein is a decoy substrate for IB kinase␣ and prevents Toll-like receptor 7/9-mediated interferon induction. J. Virol.82:12365–12373.
55.Poeck, H., et al.2004. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood103:3058–3064.
56.Poole, B., and S. Ohkuma.1981. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J. Cell Biol.90:665–669.
57.Proietti, E., et al.2002. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J. Immunol. 169:375–383.
58.Randall, R. E., and S. Goodbourn.2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol.89:1–47.
59.Rock, J., et al.2007. CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLC␥2. Eur. J. Immu-nol.37:3564–3575.
60.Rutz, M., et al.2004. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol.34:2541–2550. 61.Sanjuan, M. A., et al.2006. CpG-induced tyrosine phosphorylation occurs
on November 7, 2019 by guest
http://jvi.asm.org/
via a TLR9-independent mechanism and is required for cytokine secretion. J. Cell Biol.172:1057–1068.
62.Schlender, J., et al.2005. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol.79:5507–5515. 63.Seeds, R. E., S. Gordon, and J. L. Miller. 2006. Receptors and ligands
involved in viral induction of type I interferon production by plasmacytoid dendritic cells. Immunobiology211:525–535.
64.Swiecki, M., and M. Colonna.2010. Unraveling the functions of plasma-cytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev.234:142–162.
65.Takaoka, A., et al.2005. Integral role of IRF-5 in the gene induction pro-gramme activated by Toll-like receptors. Nature434:243–249.
66.Takauji, R., et al.2002. CpG-DNA-induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid den-dritic cell precursors. J. Leukoc. Biol.72:1011–1019.
67.Truong, H. M., et al.2004. A highly pathogenic porcine reproductive and respiratory syndrome virus generated from an infectious cDNA clone retains the in vivo virulence and transmissibility properties of the parental virus. Virology325:308–319.
68.Van Gorp, H., W. Van Breedam, P. L. Delputte, and H. J. Nauwynck.2009. The porcine reproductive and respiratory syndrome virus requires trafficking through CD163-positive early endosomes, but not late endosomes, for pro-ductive infection. Arch. Virol.154:1939–1943.
69.Van Reeth, K., G. Labarque, H. Nauwynck, and M. Pensaert.1999. Differ-ential production of proinflammatory cytokines in the pig lung during dif-ferent respiratory virus infections: correlations with pathogenicity. Res. Vet. Sci.67:47–52.
70.Vincent, I. E., et al.2007. Silencing of natural interferon producing cell activation by porcine circovirus type 2 DNA. Immunology120:47–56. 71.Vincent, I. E., et al.2005. Subset-dependent modulation of dendritic cell
activity by circovirus type 2. Immunology115:388–398.
72.Weslow-Schmidt, J. L., et al.2007. Type I interferon inhibition and dendritic cell activation during gammaherpesvirus respiratory infection. J. Virol.81: 9778–9789.
73.Xiao, Z., L. Batista, S. Dee, P. Halbur, and M. P. Murtaugh.2004. The level of virus-specific T-cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J. Virol.78:5923–5933.
74.Xu, Y., et al.2009. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol. Immunol.46:2640–2646. 75.Yoo, D., S. W. Welch, C. Lee, and J. G. Calvert.2004. Infectious cDNA
clones of porcine reproductive and respiratory syndrome virus and their potential as vaccine vectors. Vet. Immunol. Immunopathol.102:143–154. 76.Yoon, K. J., et al.1995. Characterization of the humoral immune response to
porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Invest.7:305–312.