JOURNAL OFVIROLOGY, Aug. 1984,p. 272-282 0022-538X/84/080272-11$02.00/0
Copyright C) 1984, American Society forMicrobiology
VOL. 51,No. 2
Monoclonal Antibodies
to
Rous
Sarcoma Virus pp6Osrc React with
Enzymatically Active Cellular pp6Osrc of Avian and Mammalian
Origin
SARAHJ. PARSONS,* DEBORAH J. McCARLEY, CONSTANCE M. ELY,DAVID C. BENJAMIN,AND J. THOMAS PARSONS
Department of Microbiology, University of VirginiaSchoolof Medicine, Charlottesville, Virginia22908 Received16 January 1984/Accepted 17 April 1984
The derivation and characterization of 22 hybridoma clones producing monoclonal antibodies (Mabs) specific for the transforming protein of Rous sarcoma virus, pp6Osrc, are described. AllMabs reacted with
pp6Ov-src encoded by Prague,Schmidt-Ruppin,and Bratislava 77 strains of Roussarcomavirus.Of theseMabs, 10efficiently immunoprecipitated pp60Ocsrc from chicken embryocells. Ofthese 10Mabs, 2(GD11 and EB8) readilydetected pp6O-src fromavarietyof rodent and human cultured cells and fromratbraintissue inan in
vitro immune complex kinase assay. Mapping experiments have tentatively localized the determinant(s) recognized by GD11 and EB8toaregionof thesrcprotein boundedbyaminoacid residues 82 to169, whereas
theremaining Mabs appearedtorecognize determinants residingwithinresidues1to82or169 to173. Most of
theMabs complexed denatured pp6O-srcinaWesternimmunoblot,and severalwereused tolocalize pp6Ov-src
inRoussarcoma virus-transformed chicken embryo cells by indirect immunofluorescence microscopy.
Functional expression ofpp60vsr", the transforming pro-tein encoded by Rous sarcoma virus (RSV), is required for
manifestation of the oncogenic events which are initiated upon RSV infection (1, 17). The initial identification of
pp60v-src and the subsequent studies which have led to its
partial characterization were carried out with sera from
rabbits bearing RSV-induced tumors (TBR sera) (1, 5). Theseantisera provided criticalreagents forthe determina-tion of many of the properties of pp6Osr, including its
intrinsic tyrosine-specific phosphotransferase activity (10,
23), its localizationtothe inner face ofthe plasma membrane (11, 20, 41), and its association with two cellular cytoplas-mic proteins, pp9Oandpp50(3, 4, 27). Theseantisera have
afso
been used to identify the normal cellular homolog ofpp60v-src,
termed pp6Oc-src (9, 28, 34). In addition, otherantisera reactive with pp6Osr' have been generated utilizing
smallpeptidesasimmunogens. Thisapproach has resulted in the production of rabbit antisera to a peptide containing tyrosine419(residues415 to424) (40),tothe
pentadecapep-tide corresponding to residues 498 to 512 (15), to the
carboxy-terminal 6 amino acids (residues 521 to 526) of
pp60v-src
(36), and to a variety of synthetic peptidescorre-sponding to sequences distributed throughout the
pp60v-src
molecule(38). Mostantipeptide seraimmunoprecipitated in
vivo-labeled
pp6ovsrc,
but thecross-reactivity ofthese serafor
pp60c-src
is uncertain. TBR antisera which detect c-srcarenotreproducibly obtained.
Several recent findings have accentuated the need for
antisera highly cross-reactive with c-src from a variety of
species. First is the observation that anti-c-src TBR
immunoglobulin G(IgG) becomes phosphorylated when it is
incubated with membranes of cells stimulated with epider-malgrowth factor, but not when it is incubated with unstimu-lated membranes (1, 7, 21). This result suggests that c-src may be involved in the response of the cell to
growth-stimulating factors, such as epidermal growth factor.
Sec-*Corresponding author.
ond, the observed association of the c-src protein with polyoma middle T antigen has provoked speculation that this interaction is necessary for transformation by polyomavirus (12). Monoclonal antibodies (Mabs) reactive with the c-src protein would provide important tools to probe the associa-tiveorsynergistic roleofc-srcinregulating growthin normal cells and inmediating neoplastic transformation.Inaddition,
Mabs to nonoverlapping epitopes within the src protein wouldprovideimportant reagents forcomparative studiesof the structureandfunction of normal cell andv-srcproteins.
Inthiscommunication, wereportthe isolationand
charac-terization of 22 mouse hybridoma clones producing Mabs which react with pp6Ov-sr' as specified by Prague subgroup A, Schmidt-Ruppin subgroup D, and Bratislava77subgroup C strains ofRSV (PrA-RSV, SRD-RSV, and B77 C-RSV, respectively).Tenof these Mabscross-reactedstronglywith
pp60c-sr, from chicken embryo (CE) cells, and 2 of these 10 Mabs (GD11 and EB8) detected enzymatically active pp6c-sr,,froma variety of rodent and human cells. Prelimi-nary mapping experiments suggested that GD11 and EB8
recognize an antigenic determinant(s) bounded (in part) by amino acid residues82 to169, whereas theremaining Mabs
appearedtorecognize determinants mappingwithin residues 1 to 82and 169 to 173 ofpp6Ovsr'. Most of the Mabs bound
pp60v-src inaWesternimmunoblot,although the
efficiency
of binding differed among the individual Mabs. Several Mabs wereused tolocalize pp6Ov-src inRSV-transformed CE cellsby indirectimmunofluorescence microscopy.
MATERIALSAND METHODS
Cells, viruses, andplasmids. Primarycultures of CE cells were preparedfrom 10-day-oldgram-negative, chf-negative, Marek's disease-negative embryos (SPAFAS, Norwich, Conn.) and maintained in culture as described previously (30). Secondaryculturesof CE cells were infected with the
following strains of RSV: PrA-RSV,SRD-RSV, B77C-RSV,
or mutants ofPrA-RSV, tsCHdlll9, CHdl121, or CHdl300 (6; J. T. Parsons, D. Bryant, V. Wilkerson, G. Gilmartin,
272
on November 10, 2019 by guest
http://jvi.asm.org/
Mabs TO RSV pp60src 273
and S. J. Parsons in A.
Levine,
W. Topp, and G. Vande Woude, ed., CancerCells,
Vol.II,
Oncogenes and Viral Genes, in press).SR-1T,
SRD-RSV-transformed field volecells;
3Y1, normal ratfibroblasts; BALB/c,
normal mousefibroblasts;
HeLa, a human carcinomaline;
normal minkfibroblasts;
J.L.M., a normal human fibroblast cell lineadaptedtoculture byT. Kelly
(University
ofVirginia);
and NHF, normal human foreskinfibroblasts, kindly
provided by John Herr (University of Virginia) were maintained inDulbecco modified Eagle medium (GIBCO
Laboratories,
Grand Island, N.Y.)
supplemented
with 10% calf serum.Escherichia coli cellsbearing thebacterial plasmid plac-src (16), which directs the synthesis of PrA-RSV
p60src,
ortheplasmid pLSZ
(29), which encodes thefusionproteinsrc-3-galactosidase containing
204 amino acids ofsrc fused toI-galactosidase,
werepropagated
aspreviously
described(16).Purification of src protein and immunization of mice.
BALB/c micewereimmunized with
biweekly
subcutaneousinjections
ofpurified
srcantigen
emulsified inFreundadju-vant.
pp60src
from SR-1T cells was prepared byimmunoaf-finity
chromatography of detergent extracts with TBR andtumor-bearing
mouseimmunoglobulins conjugated
toAffi-Gel10asdescribed
by
Eriksonetal.(13). Boundpp6Osr'
waseluted with 3 M
KSCN, dialyzed extensively
in 10 mMNH4HCO3
andlyophilized.
About 25 ,ug ofpp6OSrc
wasobtained from
109
SR-1T cells.Theantigen
wasresuspended
in
phosphate-buffered
saline(PBS)andemulsifiedin Freundadjuvant,
and5to25 ,ugofprotein
wasadministeredtoeachmouse per
injection.
Based on Coomassie blue and silver nitratestaining
ofpolyacrylamide
gels, this material wasjudged
to be ca. 85 to 90% pure. Mice immunized for 4months with
pp60src
antigen purified
in thismannerexhibiteda weak
anti-pp60sr'
response.Subsequently,
these micewerechallenged with
p6Osrc
purified fromextractsofE. colicontaining
theplasmid plac-src.
Bacterialp6Osr(
waspre-pared
as follows.Spheroplasts,
obtainedby
treatment ofcells in 50 mM
Tris-hydrochloride (pH 8.0)-2
mg oflyso-zyme
(Sigma
ChemicalCo.,
St.Louis,
Mo.) perml-10 mMEDTA-25% sucrosefor 15min at
4°C,
were incubated withDNase
(Sigma)
(0.25mg/ml
in5 mMMgCl2)
andlysed
in 10 mM Tris-hydrochloride (pH 7.2) containing 1% NonidetP-40, 0.5% sodium deoxycholate, 100 mM
NaCl,
and 1 mM EDTA. Extracts were clarified at 10,000 x g, and theinsoluble
fraction,
which contained about 95% ofthep6Osrc,
waswashed one time in 10 mMTris-hydrochloride (pH7.2)
containing
1MNaCl
and1 mMEDTAand threetimes with1M ureain RIPA buffer. The washed
pellet
was dissolved insample
buffer(22)
withoutboiling
andapplied
to a 9%preparative polyacrylamide
gel.p60srs
was localized bystaining
withacid-free,
water-solubilized Coomassie bluedye
and excised from the gel.p60
was eluted in PBSovernight
andfiltered beforeinjection. Approximately0.5 to1.0 mg of
p6Osrc
was obtained from 1 liter offully induced bacterialculture andwasjudged
tobegreater than90%pureby polyacrylamide
gel electrophoresis. Mice were boostedwith
biweekly
injections of 50 to 100 ,ug ofp6Osr(
for anadditional 2 months. Sera from such mice exhibited
anti-pp6src
titers in a standard enzyme-linked immunosorbent assay (ELISA), 300- to 1,000-fold greater than those frommice immunized with live or irradiated RSV-transformed
cells (30).
Fusion and screening of hybridomas for
anti-pp6Osrc
pro-duction.Splenocytes
from immunized mice were fused in the presence of37%polyethyleneglycol 1000 (Koch-Light) withSP2/O BALB/c myeloma cells,and theresulting hybridomas
wereportioned intomicrotiter wells and cultured in
conven-tional mediaaspreviously described (26, 31). Culture
super-natants weretested forthe presenceofsrc-specific
antibod-ies bytwomethods.Initial screeningof thesupernatants was
accomplished with an ELISA with purified bacterial src as
antigen (appliedto microwellsat concentrations of 2 ,ug/ml) and alkaline phosphatase-conjugated goat anti-mouse IgG (Zymed) as the secondary antibody. Specific binding of antibody was detected by addition ofthe substrate
p-nitro-phenyl phosphate (Sigma)as described elsewhere (19). Cul-turesupernatantsoftheclone,R2D2(29),producinganti-src
Mab and SP2/O myeloma cells were used as positive and
negative controls, respectively. All culture supernatants
exhibitingapositive ELISA reactionwerefurther testedfor
theirability to immunoprecipitate pp60-sr( fromextracts of PrA-RSV-transformed CE cells labeled with
32p,.
Hybri-doma cultures yielding antibodies whichimmunoprecipitat-ed
pp6Ov-src
wereimmediately
subclonedby limiting
dilution(2 to 3 times) and injected into Pristane-primed mice for production of ascites fluid.
Purification of immunoglobulin. Immunoglobulins were
purified from mouse ascites fluid as follows. Lipid was removedbydextran sulfateprecipitation, and immunoglob-ulinwas precipitated by addition of 50% ammonium sulfate
(31).After extensivedialysis, theimmunoglobulinwas
chro-matographedonprotein A-Sepharose bythemethodof Eyet al. (14). Immunoglobulin preparedinthismannerwas >95%
pure asjudged by Coomassie blue staining. Alternatively, immunoglobulin was partially purified and rendered
prote-ase-free by chromatography on DEAE Affi-Gel Blue
(Bio-Rad Laboratories, Richmond, Calif.) by a modification of
the method ofBruck et al. (2). Briefly, 2ml of ascites fluid
was dialyzed against 20 mMTris-hydrochloride(pH 7.2) and
appliedto acolumncontaining7mlofDEAEAffi-GelBlue
equilibrated in the same buffer. The column was washed with the starting buffer to remove unadsorbed material. Stepwise elutionwith 20 mM NaCl removed the majority of bound transferrin, after which the immunoglobulin was
eluted was 45 mM NaCl. Albumin and proteases remained boundtothe columnatthis salt concentration.
Immunoglob-ulinpurifiedinthis manner wasjudgedtobe about 75 to80%
pure.
Isotyping. Theisotypes ofindividual Mabs(obtained from either culture supernatants or diluted ascites fluid) were
determined with a commercial isotyping kit
(Boehringer
Mannheim Corp., New York, N.Y.). Assignment of the
isotypeswasconfirmed by double diffusion and chromatog-raphyon protein A-Sepharose.
Radiolabeling of cells and immunoprecipitation.Cellswere
labeledfor2to 3 h inphosphate-free medium 199(GIBCO) containing 5% dialyzed calfserum and 500 ,uCi of
carrier-free
32p;
(ICN Pharmaceuticals, Irvine, Calif.) per ml. Ex-tracts were prepared as described previously (30). Forscreening experiments, samples of labeled extract were
immunoprecipitatedwith 500
VI
ofculture supernatant and 3,u1
of rabbit anti-mouse IgG as secondary antibody. Forsubsequent
experiments, extracts were immunoprecipitatedwith 1 to 10 ,ul of ascites fluid or 2 to 25 ,ug of purified
immunoglobulin.Theamountsof eachimmunoglobulinused were determined to be in antibody excess for v-src in an extractderived from 2 x
105
RSV-infected CE cells and for c-srcinanextractderived from1 x 106uninfected CE cells. Immune complexes were collected on formaldehyde-fixed Staphylococcus aureus Cowan I, washed, and subjected toelectrophoresison9%polyacrylamide gels. Gels were dried and exposed to X-Omat film (Eastman Kodak Co., Roches-ter,N.Y.), with Cronex intensifying screens.
VOL.51, 1984
on November 10, 2019 by guest
http://jvi.asm.org/
274 PARSONS ET AL.
B4
.-P60- _
A B C D E F G H J K L M N O P A 8 C D E F
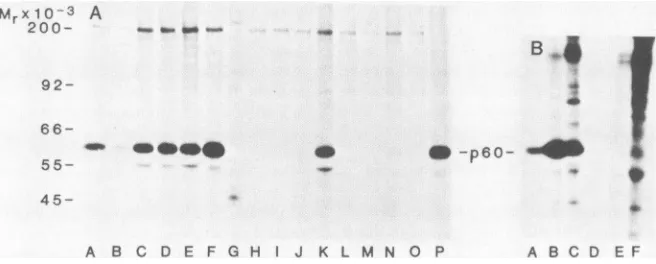
FIG. 1. Screening of hybridoma culturesupernatantsfor RSV src-specific antibody. PrA-RSV-infectedCE cellswerelabeled with32Pifor
2h, andextractswerepreparedasdescribed inthetext.Samples,correspondingto2x 105cells,wereimmunoprecipitatedwith 500p.lof
cul-turesupernatant(A,lanesA through0;B,lanes A through F)or3 p.lof rabbit anti-p60srC (A,lane P), and the labeled proteinswereanalyzed
by polyacrylamidegel electrophoresisand autoradiography. Molecular weights of labeled proteinsweredetermined relativetothe migration
of known molecular weight standards(200,000,myosin; 92,000, phosphorylaseA; 66,000,BSA;55,000,human immunoglobulinheavychain; and 45,000, ovalbumin). The autoradiogram wasexposedfor 12 h.
Immune complex protein kinaseassay. Tomeasurepp6oArc
protein kinase activity, cells were lysed in RIPA buffer
lacking sodium dodecyl sulfate, andextracts were
immuno-precipitated with Mabs as described above. Immune
com-plexeswerecollected, washed in PBS, suspended in 30 .I1 of
kinase buffer(20 mM PIPES [pH 7.2], 10 mM MnCl2, 10 pLCi of[_y-32P]ATP [5,000 Ci/mmol; ICN]), and incubated at22°C for 30min.The reactionswerestopped by the addition of 15 p.l of sample buffer concentrated three times. Labeled sam-ples wereheated at 100°C and subjectedto electrophoresis on9%polyacrylamide gels, and the phosphorylated proteins
were visualized byautoradiography.
Phosphoamino acid analysis.pp6Owaseluted fromcrushed
gel slices in 50 mM ammonium bicarbonate-0. 1% sodium dodecyl sulfate-5% 3-mercaptoethanol overnight at room
temperature,precipitatedincoldacetone,andhydrolyzedat
110°C for 2 h in 6 N HCI-0.1 M phenol. Phospho-amino acids wereresolved by electrophoresis intwo dimen-sions on cellulose thin-layer plates as described by Hunter
and Sefton (18). Labeled amino acids were visualized by autoradiography, and the position of phosphoamino acid standardswas determined by ninhydrin staining.
Western immunoblot analysis. pp6O-SrC in whole-cell ex-tracts was detected on nitrocellulose paper by the method outlined by Towbin et al. (39). Briefly, RSV-infected CE cells from a 100-mm tissue culture dish were harvested,
washed, and solubilized in 400 p.l of sample buffer with boiling for 4 min. Theextractwasclarifiedby centrifugation at100,000x gfor 30min and appliedtoa9%polyacrylamide
gel. The separated proteins were electrophoretically
trans-ferredto0.2-p.mnitrocellulose paper(Schleicher&Schuell, Inc., Keene, N.H.). The unbound sites on the paperwere
blockedby incubation in 3% bovineserumalbumin(BSA)in
PBS for1 h at 43°C orovernight at 4°C. Excess BSA was
removedby washing the paperinPBSat room temperature for 5 min. Thepaper was cut in strips, and each strip was
incubated inasealed bag withan individualMab(about 0.1
p.g/ml) overnight at 4°C. The strips were removed from the
bags and washed for 5 min in PBS three times at room temperaturewithgentle agitation. The primary antibodywas
detectedby incubation of the strips with horseradish peroxi-dase-conjugatedgoatanti-mouse IgG (Tago, Inc.) for 2 hat room temperature, followed by three additional washes in PBS for 5 min each and subsequent incubation with the substrates dianisidine and hydrogen peroxide for 10 to 20
min. The strips were then washed in distilled water for several minutes, allowedto airdry, andphotographed.
Indirect immunofluorescence staining of cells. RSV-infect-ed and uninfected CE cells were seededon coverslips and allowed togrow overnight. Cells were fixed in 3%
parafor-maldehyde, permeabilized in 0.4% Triton X-100 (25), and incubated with 100 pL.of anti-src Mab (at concentrations of 5 to20 pLg/ml) for 30 minat roomtemperature.Thebinding of the Mabs waslocalized by incubation of the cells with goat F(ab')2 anti-mouse IgG (Jackson Immuno Research) (10 p.g/ ml) for 30 min, followed by incubation with fluorescein-conjugated rabbit F(ab')2 anti-goat F(ab')2 (Jackson Immuno Research) (10 p.g/ml) for an additional 30 min. Between
incubations with antibody, the cover slips were washed three times with PBS by addition of the buffer, followed by gentle swirling and aspiration. The stained cellswere exam-ined with a Leitz fluorescence microscope equipped with
epi-illumination.
RESULTS
Isolationandidentification of Mabstov-srcprotein.
Hybri-doma cell lines producing anti-pp60src antibodies were ob-tained by fusing splenocytes from immune BALB/c mice withSP2/Omousemyeloma cellsasdescribed above. Tento 14days after fusion, growth of hybrid cells wasobservedin 64%(246/384) of the microtiterwells seeded. Culture
super-natants wereassayed by ELISAto determinespecific anti-body production. Of the wells, 82% (201/246) contained antibody which bound to bacterial p605rc in the ELISA. Culture supernatants which exhibited a positive binding in
the ELISAwere furthertested for theirability to immuno-precipitate pp6Ov-src from extracts of
32Pi-labeled
PrA-RSV-infected CE cells. In total, 21% (42/201) of the culture supernatants tested in this manner immunoprecipitated aphosphoprotein of 60,000 Mr (Fig. 1A). The majorityof these supernatants precipitatedasingle phosphoprotein of60,000
Mr; however, foursupernatants precipitated multiple phos-phoproteins ranging in molecular weight from 25,000 to >200,000 in additiontothe60,000-Mrprotein (Fig.1B,lanes C and F).
Toverify that the Mabs immunoprecipitated pp6Ovsrc, the Mabswereusedtoimmunoprecipitate astructurallyaltered srcproteinencoded bythemutantofPrA-RSV, tsCHdlll9. tsCHdlll9 encodes a src protein, pp53Sr containing a
deletion of aminoacids 173 to227 and isdescribed in detail MrX10 ...3 A
200
- 92- 66-
55-_ _*_
45-Q0
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.152.480.78.210.2]Mabs TO RSV pp6src 275
4'
pp6O
-pp 5
3src
rcA B C D E F G H I J K L M N O
FIG. 2. Immunoprecipitation ofpp53S"C byculturesupernatants containing src-specific antibodies. Samples of an extract of32p,_
labeledtsCHdIll9RSV-infected CE cells were immunoprecipitated with500p.1of src-specific culturesupernatants(lanes A through M), 3 p.1 of normal rabbit serum(lane N), or 3 ,ul ofrabbitanti-p60src serum(lane0). Polyacrylamidegelelectrophoresis and autoradiog-raphy were performed as described in the legend to Fig. 1. pp6O denotes the position in thegelofwild-typepp6Osrc.
elsewhere(6).Culture supernatantsfrom13 individual
hybri-domas, all of whichprecipitateda60,000-Mr phosphoprotein from wild-type RSV-infected CE cells,
readily
immunopre-cipitated
pp53src
from extracts of32Pi-labeled
tsCHdlll9-infected CE cells (Fig. 2). Culture supernatants which
im-munoprecipitated
diminished amounts ofpp53src
(e.g., Fig.2, lanes H, I, K, and L) exhibited
marked increases
in antibody titer upon subcloning and continuedpropagation.
Inadditiontothe
immunoprecipitation
ofpp53src
the speci-ficity oftheMabsforv-srcproteinwasverifiedby
S.aureus V-8 proteasedigestion
and phosphoamino acid analysis of the immunoprecipitated 60,000-Mrphosphoprotein
andby
immunoprecipitation ofa60,000-Mrprotein fromextractsof
[35S]methionine-labeled
CE cells infected withPrA-RSV,
SRD-RSV,and
B77C-RSV (data
notshown).
Immunoprecipitation of
pp60C-src
from uninfected CE cells. To test the cross-reactivity of the Mabs withpp60csrc,
purified immunoglobulin from a representative panel of22
A
src
pp60 -
_m_-
-hybridomas was used to immunoprecipitate pp6O-crc from
32P_-labeled, uninfected CE cells. Concurrently,
equal
amounts ofpurified Mab were used to precipitate pp6O-src from extracts of 32P_-labeled PrA-RSV-infected CE cells. Figure 3A (lanes D through I) shows the
immunoprecipita-tion of pp6Ov-src by six representative Mabs, and Fig. 3B shows theimmunoprecipitationofpp6Oc-srcfrom uninfected
CE cells bythe same sixMabs.Three Mabs(Fig. 3B,lanes D through F) immunoprecipitated significant levels of
pp6Ocsrc, whereas the remaining three Mabs (lanes G
through I) precipitated pp6Oc-src with lowefficiency, in spite
of their apparently high reactivity with pp60v sr. Such differences in reactivity could be due to affinity differences
forthe twoproteins which might reflect thepresence ofan altered determinant on pp6ocsrc. Figure 3C shows the
immunoprecipitationofpp6Oc-srcby nine additional Mabs. In total, 10of 22Mabs immunoprecipitated readily detectable
levelsof pp60c`rs'.
Toconfirmthat the 60,000-Mrphosphoprotein
precipitat-edfrom CE cellswaspp6oc-src, anS. aureusV-8proteolytic
digestion of theimmunoprecipitated proteinwascarriedout. Figure 4 shows the V-8 protease map of pp6Oc-src immuno-precipitated with Mabs EB8 and GD11 and rabbit anti-p60src. Two major c-src-specific peptides of 34 and 26 kilodaltonsas well as two smaller peptides (28) were observed. In addition,
phosphoamino acid analysis of the immunoprecipitated
60,000-Mr protein revealed the presence of bothp-tyrosine
and p-serine (data not shown). These results, therefore,
support the conclusion that the 60,000-Mr phosphoprotein
precipitated by the Mabs from uninfected CE cells is pp-fcsrc
In vitro tyrosine protein kinase activity of Mab immune complexes. To determine whether pp6Osrc in immune
com-plexes formed with Mabs exhibited protein kinase activity, kinase assays were carried out asdescribed above, andthe in vitro-labeled products were resolved by polyacrylamide gel electrophoresis and visualized by
autoradiography.
Fig-ure 5 showsthatboth pp60-src (Fig. 5A, lanes C through
J)
andpp6Oc-src (Fig. SB,lanesCthrough J)were
phosphorylat-edinvitro, presumably by the intrinsicautophosphorylation activity of
pp60fsr,.
A further analysis of individual Mabs showed that all 22 Mabs immunoprecipitatedpp60-sr'
asC
or 0- I
11
1I.
a
A B C D E F G H A B C D E F G H J K L M N 0 P Q R
FIG. 3. Cross-reactivityofpp6vOsrc_specific Mabs withpp6c-src.Samplesof extracts from
32Pi-labeled
PrA-RSV-infectedCE cells (A) or uninfected CEcells (B and C) were immunoprecipitated with ascites fluid or protein A-Sepharose-purified Mab, and the resulting immune complexeswereanalyzed by polyacrylamide gel electrophoresis and autoradiography as in Fig. 1. For the experiments shown in A, individual samples containedextract fromabout 2x 105cells, whereas in B and C, each sample contained extract from about 1x 106 cells. In A andB, labeledextractswere immunoprecipitated with 10pJ
of rabbitanti-p605"(laneA), 10pd
ofnormal rabbit serum (lane B), 17 ,ug of mouse anti-BSAimmunoglobulin(lane C),or the following anti-src Mabs: lane D, FG1; E, GD11; F, EB8; G,EC10;H,GD1; andI,
EB7.In C, labeledex-tractswereimmunoprecipitatedwith the following Mabs: lane J, GE7; K, FC6; L, GB6; M, FG8; N, FH2;0,FD7; P, GF3;Q,GAl;and R, EE10.
VOL.51, 1984
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.55.295.77.208.2] [image:4.612.134.488.522.653.2]276 PARSONS ET AL.
Mrxl 3 55-
45-..*
-pp6O
* 'u- -pp34
* -pp26
22-40 1
2-A B C D E F
FIG. 4. Partial V-8 protease digestion patterns of
pp6Ocsrc
im-munoprecipitated by Mabs GD11 and EB8 and rabbit anti-p605'. Extracts of32P-labeled, uninfected CE cells were immunoprecipi-tatedwith Mabs GD11, EB8, or rabbit anti-p605", and the immune complexes were subjected to polyacrylamide gel electrophoresis andautoradiography. Radioactive proteins migrating with an appar-entMrof 60,000 were recovered from the gel and digested with S. aureus V-8 protease (lanes A through C, undigested control; lanes D through F, 50 ng of V-8 protease), and the products were analyzed bypolyacrylamidegelelectrophoresis by the method of Cleveland et al.(8). Lanes Aand D, Mab EB8; lanes B and E, Mab GD11; lanes C and F, rabbit anti-p60src. Molecular weights were determined as in thelegend to Fig. 1 with the additional molecular weight standards of human immunoglobulin light chain, 22,000, and cytochrome c, 12,000.detectedinthe immune complex kinaseassay. The 10Mabs which reproducibly immunoprecipitated in vivo
32Pi-labeled
c-src also immunoprecipitated pp6Oc-src from normal CE
cells as detected by in vitro
phosphorylation
(Table 1). Invitro phosphorylation of c-src wasweak orundetectablein
immune complexes formed with the 12 remaining Mabs, even when Mab concentrations were increased three- to
fivefold. In noinstance did we observe phosphorylation of mouse immunoglobulin heavy chain. The 60,000-Mr
phos-phorylated protein labeled in immune complexes derived
from both transformed andnormal CE cells contained
only
phosphotyrosine (datanot
shown).
Immunoprecipitation ofpp6Oc-src from rodent and human cells. Mabs to pp6Osrc were also tested for their
cross-reactivitywithsrcproteinsfrom normal cells ofanumber of
species, including
mouse, rat,mink,
and humanspecies.
With the invitro immunecomplex kinase assay to identify pp60csr two Mabs, GD11 and EB8, were found to cross-reactwiththe c-src protein from normal mouse, rat,
mink,
and human fibroblasts (Fig. 6Athrough D andF) butfailed to detectc-srcfromHeLacells,atumorofepithelial
origin
(Fig. 6E). Thislatter result could be duetoexpressionof c-src in these cells which contain an altered epitope not
recognized by Mabs GD11 and EB8, or it could be due to expression of c-src at a level below thelimits of detectionby
the assays. Roughlyequal amounts of cell protein were used in all assays. In all cell lines tested, the amount of srcprotein
detected by the Mab-immune complex kinase assay correlat-ed well with the amount of pp6Ocsrc immunoprecipitated
from
32P,-labeled
cell extracts (data notshown).Thesamepanel of Mabswasalsousedto
immunoprecipi-tatepp6Osrc fromextracts ofrat braintissue (Fig. 7A).With the in vitro kinaseassay, Mabs GD11 and EB8were again
found to be strongly cross-reactive, whereas rabbit anti-p6Osrc and Mabs GB6, FG1, FG8, HB7, and FB7 appearedto be weakly cross-reactive, and Mabs EE10, FC6, and FH2 were non-cross-reactive. The detection of a weak cross-reactivity with five Mabs and rabbit anti-p60src which had previously been shownto be non-cross-reactivewas
proba-bly due to the higher concentrations of c-src in rat brain extracts (H. Kim and J. T. Parsons, unpublished data) as compared withtissue culture cellextracts,thereby augment-ing the ability of low-affinity Mabs to bind pp6Ocsrc. As before, the authenticity of the pp6Oc-src was established by
its phosphotyrosine content (Fig. 7B) and its typical V-8 peptide profile (Fig. 7C), which shows the predominant
tyrosine-specific phosphorylation of the 26-kilodalton car-boxy-terminal peptide.
Mapping of epitopes recognized by Mabs. The epitopes recognized by individual Mabs were localized within the
primary amino acid sequence ofpp6Osrc bydeterminingthe
pP60Sr
c
40t
.00
Bv'
-BW
-_u v9,t _s.
So-
+&&g
si
I
A B C D E F G H I J A B C D E F G H I J
FIG. 5. Invitro kinaseactivity ofpp60v-srcandpp60-srcinimmunecomplexes formedwith Mabs.Unlabeledextractsof uninfectedor PrA-RSV-infected CE cellswereimmunoprecipitated withMabs, and theimmunecomplexeswerecollected,washed,andincubated inakinase reaction mixture containing [-y-32P]ATP as described in the text. The products ofthe reaction were subjected to electrophoresis on 9% polyacrylamide gels and autoradiographed. RSV-infected CE cells (A)oruninfected CE cells(B)wereimmunoprecipitated with
anti-p60src
(lane A)orwith thefollowingMabs: laneB,anti-BSAMab; C, EB7; D, EE10; E,FC6; F,GD11; G,FG1; H, GB6;I, EB8;andJ,GF3. Auto-radiogram exposures: A,12h and B, 48h.4
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.90.278.78.246.2] [image:5.612.165.454.530.673.2]Mabs TO RSV pp6O3r( 277 TABLE 1. c-srccross-reactivitya
Cross-reactivitywith cells from: Western blot
Anti-v-src Immunoglobulin Ratbrain pattem for
clone isotype CEs Mice Rats
tissue
Minks Humansv-src
EB7 IgG2a + - - - ++
EB8 IgG1 + + + + + +
EC10 IgG2b + - - + - - ++
R2D2 IgG3 - - NTb -+ (MB)'
EE10 IgGl + - - - +
FB7 IgG2a + - - + - - +(MB)
FC6 IgG2a + - - - +
FD7 IgG2a + NT NT - - NT NT
FF10 IgG2a + - NT - - NT +
FG1 IgG2a + - - + - - ±(MB)
FG8 IgG2a + - - + - - ±(MB)
FH2 IgGl + - NT - - NT +
GAl IgGl + - - + - - +
GB6 IgGl + + + + + + +
GC6 IgGl - NT NT NT NT NT +
GD1 IgGl + - - + - - +
GD11 IgGl + + + + + +
GE7 IgG2b + + - + - + NT
GF3 IgGl - NT NT NT NT NT +
HB7 IgG2a + - - + - - NT
HC5 IgGl - NT NT - NT NT +
HC6 IgGl + - NT + - NT +
HD4 IgGl + NT NT + NT NT +
a Determined by in vitro immune complex kinase assay. Results are a consensus ofaminimumofthreeexperiments withtwoor moredifferentsourcesof
antibody, i.e., culture supernatant, ascites fluid, or purified immunoglobulin.
bNT, Not tested. C MB, Multiple bands.
capability ofeach of the Mabs to
immunoprecipitate
srcproteins encoded by src deletion mutants, as well as a
,B-galactosidase fusion protein (p130) containing the amino-terminal204amino acids ofsrcfusedto
,B-galactosidase
(29)(Fig.
8). All 22 Mabsrecognized epitopes
contained withinthe amino-terminal 204 residues of the src
molecule,
asjudged by their ability to
immunoprecipitate
bacterial p130 (Fig. 8A)andtheir failuretoimmunoprecipitate wild-type,B-galactosidase (data not shown). Further mapping was
ac-complished by analysis
ofimmunoprecipitations
oftwosrcdeletionmutants, one
containing
adeletionspanning
amino acidresidues173 to 227(tsCHdlll9)
andasecondcontaining
adeletion of residues 82to169(CHdl121)
(Parsons etal.,
in press). Aspreviously
shown inFig.
2, all Mabsprecipitated
pp53src
from tsCHdlll9-infected CEcells. Asimilaranalysis
of CHdl121 showed that all but two Mabs(EB8and
GD11)
were able to immunoprecipitate
pp53sr,
from CHdI121-in-fected CE cells (Fig. 8B). The assignment of the epitope recognized byGD11
and EB8 to that region bounded by amino acid residues 82 to 169 cannot be definitively madesince thedeletion of CHdl121 may
disrupt
the confirmation ofepitopes outsidethatregion. However, residues criticaltothe correct formation ofthe epitope(s) recognized by EB8 andGD11reside,atleast inpart, withinamino acid residues
82 to169. Byinference,then, theremaining epitopescanbe
placedwithin amino acid residues 1 to 82 and 169 to 173. The
placement ofseveraloftheepitopesintheamino third of the molecule has beenverifiedby the bindingof 10 Mabs to the
amino-terminalp34 V-8 proteasefragment ofsrc in a West-ern immunoblot analysis (data not shown). The
amino-terminal designation of the major epitopes was further
documentedby testingtheabilityof the Mabs toprecipitate an altered src protein encoded by mutant
CHdI300.
This mutant encodes an enzymatically inactive src protein(pp58sr) containing a deletion ofresidues 504 to 506 and terminates with 24aminoacidsunrelated to pp6OsrC (Parsons et al., in press). Figure 8C shows that all 22 Mabs
immuno-precipitated pp58src, indicatingthat carboxy-terminalamino acids were notinvolvedinepitoperecognition by any of the
Mabs.
Western blot analysis with src Mabs. The binding ofthe Mabs to denatured, immobilized pp60-sr( was tested in a Western immunoblot, as described above. Figure 9 shows
several patterns of binding. Mabs EC10 and EB7 (Fig. 9, lanesC andD) bound strongly to asingle protein of 60,000 molecular weight (i.e., pp6Osrc), whereas Mabs FG1, FG8,
andFB7 (lanes F,G, and N) exhibited weakbindingto v-src
and to multiple cellular proteins. Interestingly, these three Mabs also immunoprecipitated multiple proteins from nor-mal andtransformedcells (Fig. 1B), supporting the argument
thatthesecellularproteins sharedeterminants with pp6osr. Themajority ofMabs bound poorly to
pp6Osrc,
and the MabsEB8and GD11 appeared not tobind at all(Fig. 9, lanes B and K). This observation suggests that the epitopes
recog-nized by GD11and EB8 are denaturation sensitive. Detectionofpp6OSc by indirect immunofluorescence stain-ing with Mabs. The bindstain-ing of the Mabs topp60src in
PrA-RSV-infectedCE cells was tested in anindirect
immunofluo-rescencestaining assay. Thepp6ov-srcstaining pattern of one
Mab,EB7,isshown in Fig. 10. Incubation of
formaldehyde-fixed PrA-RSV-infected cells with EB7 revealed a diffuse
staining of the cytoplasm, with a more intense staining apparent in the perinuclear region, at the plasma membrane, and at cell-to-cell junctures. Such a pattern has previously been described by Rohrschneider (32, 33) with TBR antisera.
Background staining of uninfected cells is shown in Fig. 10D. Similarstaining of transformed cells was observed with Mabs EB8 andGD11.
VOL. 51, 1984
on November 10, 2019 by guest
http://jvi.asm.org/
278 PARSONS ET AL.
DISCUSSION
We have previously reported that a bacterially expressed oncogene product,
p6Osrc,
encoded by the plasmidplac-src, could beused to hyperimmunize mice for the production of Mabs to pp6Osrc (29). This method of immunization has proven tobe moreeffective in inducing an immune response to the src protein than immunization with liveRSV-trans-formed mouse tumor cells or with small amounts of src protein purified from tissue culture cells. Lipsich et al. have
alsoemployed asimilar immunizationprotocolwith bacteri-al src protein to obtain 13 Mabs to pp6OsrU (24). In an
extensionof our original results, we report here the isolation
andcharacterization of 22 additional src-specific Mabs. All were found to react with src proteins as encoded by
PrA-RSV,SRD-RSV, and B77C-RSV. Ten of the Mabs efficiently
immunoprecipitated the chicken cellular homolog of src,
pp6cc-src. The specificity of the Mabs for pp60src and the
utility of the Mabs were demonstrated by S. aureus V-8
proteolytic digestionpattern andphosphoamino acid analy-sisof the phosphorylated p60 proteinimmunoprecipitated by
the Mabs, the autophosphorylation of
pp6Osrc
in immunecomplexes, the immunoprecipitation of structurally altered src proteins containing defined amino acid deletions, the
bindingof certain Mabs topp6Osrcin a Western immunoblot,
A
w~~~~~o
U E
p rc
pp66O
- _S
_ ~
~~~~
_"M...
A BC D E F G H J K L M
_- pH1 9
I
BI
4 -p- tyr
-,p thf
--p ser
Mrx 10- C w 5 5
-422- kl
C
12-pp60src
A B C D
D
s60src
A B C
FIG. 7. Detectionofpp6O-srC inratbrain tissue.A,
Immunopre-cipitation
by
Mabs ofpp60c-src
from rat brain tissue. Extracts of_m
_ _ _^cerebellumtissue ofa12-day-oldratwerepreparedandimmunopre-w
*
w_
~~~~~cipitated
with Mabs as described in the text. Immunecomplexes
were incubated with [y-32P]ATP, and the labeled proteins were
analyzed by polyacrylamide gel electrophoresis. Mabs: lane A,
GD11; B, anti-BSA Mab; C, HB7; D, EE10; E, EB8; F, FG1; G, FG8; H, GB6; K, FB7; L, FC6; and M, FH2.LaneI,10,ul of rabbit anti-p60src and lane J, 10 plI of normal rabbit serum. B,
Two-A B C D A B C D dimensionalphosphoamino acid analysis of in vitro-labeledratbrain pp6Oc-srcimmunoprecipitatedby Mab GD11. C, Partial V-8protease digestion pattern of pp6csrc phosphorylated in vitro. pp60c-src
immunoprecipitated fromratbrainextractswith MabGD11 (lane A)
orEB8(lanes Band C)waslabeled inanimmune complexkinase
E F assay. The labeled protein was recovered from apolyacrylamide
gel, subjectedtopartial proteolysis byS.aureusV-8protease(5 ng) bythemethod of Clevelandetal.(8),andanalyzedasdescribedin
thelegendtoFig. 4.
andthebinding of Mabs topp6Osrc byimmunocytochemical techniques.
Two Mabs were of particular interest. GD11 and EB8
readily immunoprecipitated enzymatically active pp60csrc
A B C D A B C D A B C D from extracts of uninfected cultured cells, including CE,
CD,from Aodent Bnd CumaD mouse,rat, mink,andhumanfibroblasts, and fromratbrain
FIG. 6. Immunoprecipitationofpp60c-srcfrom rodent and human tissue. Theepitope(s) specified by GD11 and EB8appearsto
celllineswithMabs. Samples ofextractsfrom cellswereimmuno- behighlyconservedandwastentatively localizedtoaregion precipitatedwithindividualMabs,and theimmunecomplexeswere of the src amino acid sequence bounded by amino acid
incubated inakinase reaction mixture containing [-y- P]ATPand residues 82to169.However,since the deletion ofsrcusedto
analyzed as described inthe legendto Fig. 5. A, normal BALB/c
mouse fibroblasts (samples contained extracts from about 2 x 106 map this epitope(s) could also result in the perturbation of cells);B,3Y1,normalratfibroblasts(3 x 106 cells); C,normal mink epitopes outsidethe deleted region, a definitive placementof fibroblasts (2 x 106 cells); D, NHF, normal humanforeskin fibro- the epitope(s) cannot be made. These Mabsappeared tobind blasts (1 x 106 cells); E,HeLa(2 x 106 cells);andF,JLM,normal native pp6Osrc more efficiently than denatured pp6OSr, as humanfibroblast(2x 106cells).LanesA,MabEB8;B,MabGD11; judged by theirpoorreactivityin aWestern immunoblot. It C, anti-BSA Mab; and D, Mab FG1. is also not known if EB8 and GD11 recognize the same
A B
pp60
pp34
-pp26
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.612.324.561.76.380.2] [image:7.612.60.298.351.629.2]Mabs TO RSV pp6O'rc 279
- ..
--S.;
_
__&.EmI
-"WAmb~0- & m&
4~-m4 -m w 4
_2 2_
A B C D E F G H I J K L M N 0 P Q R
B
a
-. C
.-Ipp6}srk_
PP6 src ._
p p5s3
A B C D E F G H I J K L M N O
ABC_
A B C D E F G H I J K L M N 0
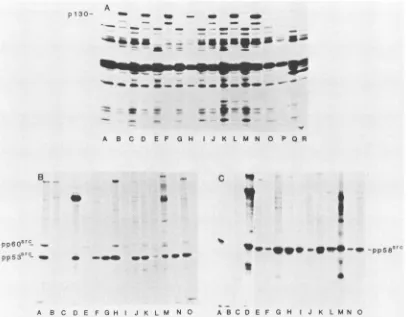
FIG. 8. Immunoprecipitation ofsrc-,-galactosidasefusion protein and structurally alteredsrcproteins encoded byRSV deletionmutants. A, Cultures ofE. colicarrying plasmid pLSZ and expressingan src-,B-galactosidase hybrid protein ofMr 130,000were labeledfor 2h in
sulfate-depletedgrowth mediumwith 100,uCiof[35S]sulfateperml. Extractswereprepared bythe methodofGilmeretal. (16). Asanegative
control, cultures ofE.coli containingaplasmid with the src-3-galactosidasecodingsequencebut lacking thelactoseoperator-promoter(pSZ)
were grownandlabeled in thesame manner. Alternatelanesbeginning withAdenoteimmunoprecipitation ofextractsofE. coli(pSZ) cells;
alternatelanesbeginning with B denote immunoprecipitation of extracts of pLSZ cells.Immunoprecipitationswerecarried out with 500p.lof
culturesupernatantsof Mabs FG8 (lanes A and B), EB8 (C and D), GD11 (E andF),HC5 (Gand H), GD1 (Iand J), and EB7 (Kand L) and 5 ,ulofrabbitanti-p60src(M and N), 5,ulof normal rabbitserum(Oand P), andanti-BSA (Q and R). B andC,ImmunoprecipitationwithMabsof srcproteinencoded by RSVmutantCHdll21orCHdl300. Samples ofextracts preparedfromCE cells infected withCHd/121 RSV (B)or
CHdl300 RSV (C)wereimmunoprecipitatedwithindividual Mabs, and the immune complexeswereanalyzedasin the legendtoFig. 1. Lanes A,3 of rabbitanti-p60sr;B, 3 p.1of normalrabbitserum;C,anti-BSA Mab;D,R2D2;E,GD11;F,FG1; G,EC10;H, EB7;I,EB8; J,EE10; K, FC6;L. FH2; M,FG8; N,GAL;and0,GD1.
epitope, because competitive binding forp6O,r"hasnotbeen tested.
The epitopes defined by the remaining 20 Mabs appeared tomapwithin theamino acid residues 1 to82or169to 173. Assignment of these epitopes (presumably more than one; seebelow) is based on theimmunoprecipitation of structur-ally alteredsrcproteins encodedby deletionmutantsof RSV orsrc-f-galactosidase fusion proteins containingthe first204
6p s r c amino acids of
pp6Osrc
(Fig.
8A)
orthe first 110 amino acidsof src (unpublished results). Most of these Mabs bound
[image:8.612.109.517.66.383.2]A B C D E F G H I J K L M N
FIG. 9. Binding of Mabstopp6o0-sc inaWestern immunoblot. Extracts were prepared, subjected to polyacrylamide gel electro-phoresis,andelectrophoretically transferredtonitrocellulosepaper asdescribed inthetext.Thepaper wascutin strips,and eachstrip
wasincubatedwithanindividualMab (about 0.1 p.gof antibodyper
ml) overnight at 4°C. The strips were stained for specific Mab
bindingas described inthe text. Mabs: lane A, HC5; B, EB8; C, EC10;D, EB7; E, FC6; F, FG1; G, FG8; H, FH2; I,GA1;J, GD1;
K, GD11;L.GC6; M, GF3; andN,FB7. The molecularweightof pp605src was determined from the migration of standard proteins which hadbeen transferredtonitrocellulosepaperand stainedwith
0.1%amido black in 50% methanoland10%aceticacid.
A
p130- q
-ma.
I
VOL. 51,1984
40P
41W 40
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.612.57.298.524.661.2]280 PARSONS ET AL.
FIG. 10. Indirectimmunofluorescence staining of cells with Mab EB7. Cells were grown on coverslips,fixed,andstained asdescribed in the text. Athrough C,PrA-RSV-infected CE cells; andD,uninfected CE cells.
pp6O-sr( from unfractionated extracts of RSV-transformed cells in a Western immunoblot, although with various
de-grees ofefficiency. Such variations in binding could readily represent differences in either titers or affinity for various
epitopes. The total number of different epitopes recognized by this panel of Mabs is unknown at this time, since competitive binding studies for p60os1 have not been
com-pleted. However, based on reactivity profiles ofthe Mabs
withc-src fromdifferent species, withcellular
phosphopro-teins other than src, and with tyrosine kinase-transforming
proteins of other retroviruses, a minimum of four to five epitopescanbeidentified. One such profile is represented by MabsFG1, FG8, HB7, and FB7, whichimmunoprecipitated pp60srcaswellas amixtureofphosphoproteins rangingin Mr from <25,000to 200,000 fromextractsof normal and
trans-formedcells. These Mabs alsoboundtomultiple proteins in Western immunoblots of RSV-transformed cell extracts
(Fig. 9). Thepatternsof bindingamongtheseMabsappeared
virtually identical, suggesting that each ofthe Mabs
recog-nized a determinant common to multiple cellular proteins. Whethersuchacommonepitope(s) reflectstheidentification ofproteinswith similarfunction remains amatterfor
specu-lation. Additional determinants may be defined by those
Mabs which immunoprecipitated CE pp60csrc poorly (e.g., GC6 and GF3; Table 1) and by those Mabs which readily immunoprecipitated CE pp6Oc-,r' but reacted poorly with
pp60c-s1(
from rodentorhumancells (e.g., FC6andGA1). Acomparison of the deduced amino acid sequences of
PrA-RSV
pp6Os''
(35) andCEpp60c-sr'
(37) reveals7 amino aciddifferences within the first 100 amino acids and 10
differ-ences within thefirst 200residues. Thus it is not
surprising
thatsomeMabsdirectedtowardepitopes withinthe first 100
amino acid residues of
pp6O-src
might exhibit a decreasedaffinity for an altered sequence in
pp6Oc-src. Similarly,
the lackofstrongreactivity withpp6oc-sr
ofotherspecies
likely
reflects an alteration of the primary sequence of these
epitopes.
Finally, three observations suggest that the
previously
described Mab R2D2 (29) appearsto be directed
against
anamino-terminal determinant distinct from those described
above. First, R2D2
immunoprecipitates
thetransforming
proteins of Y73 sarcoma virus, p90gagYes, and Gardner Rasheedfelinesarcomavirus,
p70'agf'gr
(S.J. Parsons,D. J.McCarley, C. M. Ely, D. C.
Benjamin,
and J. T.Parsons,
manuscript in preparation). Secondly, R2D2 does not
effi-ciently immunoprecipitate
pp6Oc-src
from either avian orrodent cells. Third,
pp6O.rc immunoprecipitated
with R2D2 is not readilyautophosphorylated
in the immunecomplex
kinase assay. In summary, the above data suggest that the Mabsdescribed in thisstudyrecognizeaminimumoffourto five epitopes, each of which appearsto be localized to the amino-terminal third ofthe src protein.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.612.131.486.77.434.2]Mabs TO RSV pp60-"' 281 The utility of specific Mabs in studying structural and
functional aspects of srw-mediated transformation is
exem-plified by the demonstration of their reactivities in Western immunoblot assays and in immunofluorescence studies
briefly described here. Theuseofspecific Mabsto efficiently detect the cellular homolog ofsrc, as shown by the in vitro
immune complex kinase assays, may lead to a greater
understanding of its function in nonneoplastic cells. ACKNOWLEDGMENTS
We thank B. Creasy for excellent technical assistance. We are
indebtedtoourcolleagues D. Bryant and V. Wilkerson for providing
RSV mutants and to Tona Gilmer and Ray Erikson for providing
many helpful comments during the course of this work. We also
thank H. Kimfor providing therat brain tissuefor thec-src kinase assaysandJ. Croft forgivingunrelenting and valuable assistancein
preparation of themanuscript.
J.T.P. isarecipientofa Faculty ResearchAward, and S.J.P. isa
recipient of a Junior Faculty Research Award, both from the
American Cancer Society. This work was supported by Public
Health Service grants CA29243 and CA27578 from the National
Cancer Institute and grant MV-29 from the American Cancer Society.
LITERATURECITED
1. Bishop, J.M. 1983. Cellularoncogenesand retroviruses. Annu.
Rev. Biochem. 53:301-354.
2. Bruck, C.,D. Portetelle,C. Glineur, and A. Bollen. 1982.
One-steppurification ofmouse monoclonal antibodies from ascitic
fluid by DEAE Affi-gel Blue chromatography. J. Immunol.
Methods53:313-319.
3. Brugge, J. S., and D. Darrow. 1982. Rous sarcoma
virus-induced phosphorylation ofa 50,000 molecular weight cellular
protein. Nature(London) 295:250-253.
4. Brugge, J. S.,E. Erikson, andR. L.Erikson. 1981. The specific interaction of the Rous sarcoma virus transforming protein.
pp605'',withtwocellularproteins.Cell 25:363-372.
5. Brugge, J. S., and R. L. Erikson. 1977. Identification of a
transformation-specific antigen induced by an avian sarcoma
virus. Nature (London) 269:346-348.
6. Bryant, D., and J. T. Parsons. 1982. Site-directedmutagenesis
of the src gene of Rous sarcoma virus: construction and characterization ofadeletionmutanttemperature sensitive for
transformation.J. Virol. 44:683-691.
7. Chinkers,M.,and S.Cohen. 1981. Purified EGFreceptor-kinase
interacts specifically with antibodies to Rous sarcoma virus
transformingprotein. Nature(London) 290:516-519.
8. Cleveland, D., S. G. Fischer, M. W. Kirschner, and U. K. Laemmli. 1978. Peptidemappingby limited proteolysis in sodi-umdodecyl sulfate and analysis by gel electrophoresis.J. Biol.
Chem.252:1102-1106.
9. Collett, M.,E. Erikson, A. F. Purchio,J. S. Brugge, andR. L. Erikson. 1979. A normal cell protein similar in structure and
functiontothe aviansarcoma virustransforminggeneproduct.
Proc. Natl. Acad. Sci. U.S.A. 76:3159-3163.
10. Collett, M.S., and R. L. Erikson. 1978. Protein kinase activity
associated withthe aviansarcomavirussrcgeneproduct. Proc.
Natl. Acad. Sci. U.S.A. 75:2021-2024.
11. Courtneidge,S.A.,A.D.Levinson,andJ. M.Bishop. 1980.The protein encoded by the transforming gene of avian sarcoma virus (pp60"') and a homologous protein in normal cells
(pp60Y'ro9"'-"r ) areassociated with the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 77:3783-3787.
12. Courtneidge, S. A., and A. E. Smith. 1983. Polyoma virus transforming protein associated with the product ofthe c-src
cellulargene. Nature(London)303:435-439.
13. Erikson, R. L., M. S. Collett, E. Erikson, and A. F. Purchio. 1979. Evidence that theavian sarcoma virustransforminggene
productisacyclic AMP-independentproteinkinase. Proc. Natl.
Acad. Sci. U.S.A. 76:6260-6264.
14. Ey,P.L.,S.J.Prowse, and C. R. Jenkin. 1978.Isolation ofpure
IgG, IgG2aandIgG'- immunoglobulins frommouse serumusing
protein A-Sepharose. Immunochemistry 15:429-436.
15. Gentry, L. E., L. R. Rohrschneider, J. E. Casnellie, and E. G. Krebs. 1983.Antibodies to adefined region of pp6O"' neutralize the tyrosine-specific kinase activity. J. Biol. Chem. 258:11219-11228.
16. Gilmer, T. M., J. T. Parsons, and R. L. Erikson. 1982. Construc-tion of plasmids for expression of Rous sarcoma virus trans-formingprotein,p60"'',inE.coli. Proc. Nati. Acad.Sci. U.S.A. 79:2151-2156.
17. Hanafusa, H. 1977.Celltransformation byRNAtumorviruses.
p. 401-483. In H. Fraenkel-Conrat and R. R. Wagner (ed.). Comprehensive virology, vol. 10. Plenum Publishing Corp.. New York.
18. Hunter, T., and B. M. Sefton. 1980. Transforming geneproduct of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl. Acad. Sci. U.S.A.77:1311-1315.
19. Kearney, J. F., A. Radbruch, B. Liesegang, and K. Rajewskey. 1979. A new mouse myelomacellline that has lost immunoglob-ulin expression butpermits the construction of antibody-secret-inghybrid cell line. J. Immunol. 123:1548-1550.
20. Krueger, J. G., E. Wang, and A. R. Goldberg. 1980. Evidence that the src gene productof Rous sarcoma virus is membrane associated. Virology 101:25-40.
21. Kudlow, J. E., J. E. Buss, and G. N. Gill. 1981. Anti-pp6O"'' antibodiesare substrates for EGF-stimulatedprotein kinase. Na-ture (London)290:519-521.
22. Laemmli, U. K. 1970. Cleavage of structural proteinsduring the assembly of the head of bacteriophage T4. Nature (London) 227:680-685.
23. Levinson, A. D., H. Oppermann, L. Levintow, H. E. Varmus, and J. M. Bishop. 1978. Evidence that thetransforming gene of avian sarcoma virus encodes a protein kinase associated witha
phosphoprotein. Cell 15:561-572.
24. Lipsich, L. A., A. J. Lewis, and J. S. Brugge. 1983. Isolationof monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J. Virol. 48:352-360.
25. Little, C. D., and W. T. Chen. 1982. Masking of extracellular collagen and the co-distribution of collagen and fibronectin during matrix formation by cultured embryonic fibroblasts. J. Cell. Sci. 55:35-50.
26. Oi, V. T., and L. A. Herzenberg. 1980. Immunoglobulin-produc-ing hybrid cell lines, p. 351-372. In B. B. Mishell and S. M. Shiigi (ed.), Selected methods in cellular immunology. W. H. Freeman and Co., Publishers, SanFrancisco.
27. Oppermann, H., A. D. Levinson, L. Levintow, H. E. Varmus, J. M.Bishop, and S. Kawai. 1981. Two proteins that immuno-precipitate with the transforming protein of Rous sarcoma virus. Virology 113:736-751.
28. Oppermann, H., A. D. Levinson, H. E. Varmus, L. Levintow, and J. M. Bishop. 1979. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcomavirus transforming gene (src). Proc. Natl. Acad. Sci. U.S.A. 76:1804-1808.
29. Parsons, S. J., D. J. McCarley, C. M. Ely, D. C. Benjamin, and J. T.Parsons. 1983. Isolation and partial characterization of a monoclonal antibody to the Rous sarcoma virus transforming protein, pp6Or''. J. Virol. 45:1190-1194.
30. Parsons, S. J., S. C. Riley, E. E. Mullen, E. J. Brock, D. C. Benjamin, W. M. Kuehl, and J. T. Parsons. 1979. Immune response to the src geneproduct in mice bearing tumors induced by injection of avian sarcoma virus-transformed mouse cells. J. Virol. 32:40-46.
31. Parsons, S. J., L. D. Wilson, C. M. Ely, J. T. Parsons, and D. C. Benjamin. 1984. Monoclonal antibodies specific for the virion polypeptide, p27, of avian retroviruses. Hybridoma3:25-32. 32. Rohrschneider, L. R. 1979.Immunofluorescence on avian
sarco-mavirus-transformed cells: localization of the src gene product. Cell 16:11-24.
33. Rohrschneider, L. R. 1980. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc. NatI. Acad. Sci. U.S.A. 77:3514-3518.
34. Rohrschneider, L. R., R. N. Eisemann, and C. R. Leitch. 1979. Identification of a Rous sarcoma virus transformation-related protein innormal avian and mammalian cells. Proc.Natl. Acad. VOL.51, 1984
on November 10, 2019 by guest
http://jvi.asm.org/
282 PARSONS ET AL. Sci. U.S.A. 76:4479-4483.
35. Schwartz, D. E., R. Tizard, and W. Gilbert. 1983. Nucleotide
sequenceof Rous sarcomavirus. Cell 32:853-869.
36. Sefton,B. M., andG. Walter. 1982. Antiserum specific for the carboxy terminus of the transforming protein of Rous sarcoma
virus. J. Virol. 44:467-474.
37. Takeya,T.,and H.Hanafusa. 1983. Structure andsequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell 32:881-890.
38. Tamura, T., H. Bauer, C. Birr, and R. Pipkorn. 1983. Antibod-iesagainst synthetic peptidesasatool forfunctional analysis of
thetransforming protein pp6osrc. Cell34:587-596.
39. Towbin, H., T.Stahelin,andJ. Gordon. 1979. Electrophoretic transfer ofproteinsfrom polyacrylamide gelstonitrocellulose sheets. Proc. Natl. Acad. Sci.U.S.A. 76:4350-4354.
40. Wang, T. W., and A. R. Goldberg. 1981. Synthetic peptide fragmentofsrcgeneproduct inhibits thesrcproteinkinase and
crossreactsimmunologicallywithavianonckinasesandcellular
phosphoproteins. Proc. Natl. Acad. Sci. U.S.A. 78:7412-7416. 41. Willingham,M.C., G.Jay,andI.Pastan. 1979.Localizationof
the ASV src gene product tothe plasma membraneof
trans-formed cells by electron microscopy immunocytochemistry. Cell 18:125-134.
J. VIROL.