Copyright © 1967 AmericanSocietyforMicrobiology Printed inU.S.A.
Attachment and
Eclipse of
Adenovirus
LENNART PHILIPSON
DivisionofCellBiology,DepartmentofMedicalMicrobiology,UppsalaUniversity,Uppsala, Sweden
Received forpublication26 June 1967
The attachment and eclipse of adenovirus have been studied with the aid of highly purified "4C-threonineand32P-labeledadenovirustype 2 in KBcells in suspen-sion cultures. Adenovirus
particles
andinfectivity
appear toattachat the same rate. The attachment rateappearstobe highly dependent on the cellconcentration and less dependent on virus concentration within the multiplicity range from 0.15 to 3 plaque-forming units per cell, probably corresponding to 4.5 to 90particles per cell. Subsequenttoattachment, 5to8% ofthe14Clabel iselutedfromthecellat a structure level, corresponding tofree hexon. The 3"2Pactivity is rapidly associated withthe cells andisconverted within20 to 30min to 65 to 85% deoxyribonuclease-susceptible material. This process isunaffectedby actinomycinandpuromycin. The deoxyribonuclease-sensitive material is, however, associated with 'IC label for an extended period after infection, and does not sediment as free deoxyribonucleic acidinsucrosegradients.Theimplications ofthesefindingson thepenetration mech-anismofanimalvirusesare discussed.It hasbeen assumedthat theviral genomewill notbe abletoreplicateand directprotein synthe-sis within susceptible cells unless it is uncoated from the protein envelope, but since vaccinia messenger ribonucleic acid(mRNA)isapparently synthesized in the absence of protein synthesis (19, 24) and since this virusrequiresprotein syn-thesis for complete uncoating
(15, 16),
this assumptionmay beerroneous. Beforegeneraliza-tions are made, however, additional viruses should be
investigated
withregard
to these properties.Although themechanism for
uncoating
of the genome has beenpartially
resolved for tailedbacteriophages (8,
12),
thesame eventsinanimalvirus infection have only recently been studied withbiochemical
techniques (15, 16).
Itisgenerally believed thatanimal virusesare
decoatedwithin,orinclosevicinityto,
phagocyto-tic vesicles subsequent to uptake of the intact virionsby pinocytosis
(for reviews,
see 4and17). From electronmicroscopic
studies, it has been concludedthatuncoating
ofadenovirusesoccursat or in close
vicinity
to the nuclear membrane afterprimary
pinocytosis
of thevirions(3).
Methods for obtaining highly purified
radio-actively
labeled adenovirus have recently beendeveloped (9).
Since such viruspreparations
constitute a
prerequisite
for biochemicalstudies
of attachment and
penetration
mechanisms,
adenovirus was selected as a model virus. An additional advantagewith adenovirus is that the structural
proteins
can be recovered ina solubleand essentially pure form (21), which may form the basis for evaluating the biological role of animal virus proteins, if any. The present study deals with attachment and eclipse of adenovirus and the role of cellular metabolism in these events.
MATERIALS AND METHODS
Cells. KBcellsoriginally obtainedfrom
Microbio-logicalAssociates,Inc.,Bethesda, Md.,weregrownin
spinner cultures in Eagle's spinner medium (5) with
5% horse serum,
2%0
calf serum, and antibiotics.HeLacells from the same source were grownin the
samemedium. KB cellsforplaqueassay weregrown in plastic petri dishes with Eagle's minimal essential
medium (5) with 15% calfserum and 4% tryptose
phosphatebroth.
Virus. Adenovirus type 2, the prototype strain
originally obtained from Dr. Huebner, National InstitutesofHealth, Bethesda,Md.,wasused
through-out.
Preparationofradioactivelylabeledvirus.The
proce-dure outlined by Greenand Piiia (9) was followed
withslightmodifications. KBcells inspinnercultures
wereinfectedat acelldensityof3 )K 105to 5 X 105
cells/ml withamultiplicityof 10plaque-formingunits
(PFU)/cell. Two-liter batches were processed each
time.32P-labeled viruswasproducedincellssuspended
in minimal essential medium (MEM) with citrate
instead of phosphate and 10% calfserum dialyzed
against the citrate medium. Radioactive carrier-free
orthophosphate was added to aconcentration of2.5
mcperliter of medium.Thecellswereharvestedafter
72hr, and the cellpellet wascollectedby low-speed
centrifugation (1,000 X g for 10 min). Freon
treat-ment and sedimentation on acushion of RbCl were
868
on November 11, 2019 by guest
http://jvi.asm.org/
the same as theoriginal report. The finalpurification
was achieved by layering the virus band from the
RbCl gradient on top ofpreformed CsCl gradients
(1.2 to 1.5g/ml)andcentrifugingfor 4 hrat100,000X
g. The virus band was collected and first dialyzed
against 0.05 M tris(hydroxymethyl)aminomethane
(Tris)chloride (pH 7.4) with 5 X 10-4 M
ethylenedi-aminatetraacetate (EDTA) and storedat4 C. Itwas
repeatedly observed that purified adenovirus type 2
was comparatively labile and disintegrated if stored
at -60 or-20 C, and also afterprolonged periodsat
4 C. However, when 0.25 M sucrose-10-3 M MgCl2in
0.02 M Tris chloride (pH 7.4) was used as storage
solution,thedisintegrationwasminimalforperiodsof
)4
days at 4 C.Table 1 shows thecharacteristicsof thepurified 32P-labeled virus and alsoa comparison
be-tween Tris-EDTA and sucrose buffers as storage
solutions for purified virus. The sucrose buffer was
thereforeused in thisstudy.
Virus labeled with 14C-threonine was produced in the same manner except that the cell medium
con-tained Eagle's MEM with only 0.03 mm unlabeled
threonine. Theaddition of cold threoninewas
neces-sarytoachieve maximalyieldof virus in infectedcells.
Uniformally labeled '4C-threonine with aspecific
ac-tivityof 84mc/mmole(NewEnglandNuclearCorp.,
Boston,Mass.)wasadded inaconcentration of 10Ac
per107 cells. Thesameprocedurewasused for
purifica-tion of "GC-labeled virus as described for 32P-labeled
virus. Less than1%of theradioactive labelinthevirus
was acid-solubleand
>99%o
of thelabel sedimented withvirusinfectivityinsucrosegradients.Virusinfectivity. Infectivity ofviruswasassayed by
the plaque technique in KB-cells as previously
de-scribed(21).
Attachment oflabeled virus to cells. Attachment
oflabeledvirustocellswascarriedoutin MEM with
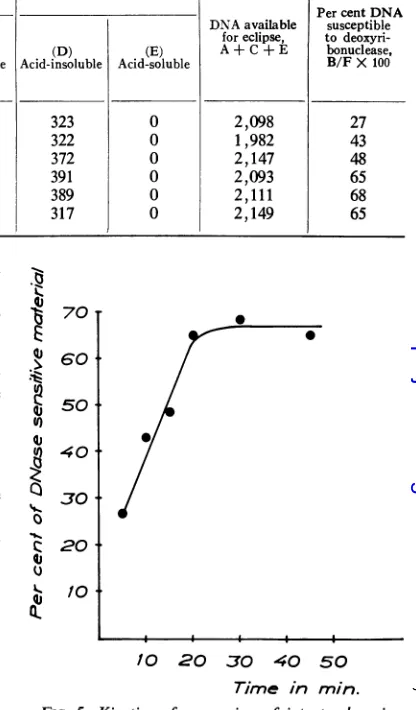
TABLE 1. Characteristics and spontaneous
disintegration of32P-labeledpurified
adenovirus type2in
differenit buffers
Percent- Percentage ODbat Countsper age of ofcounts
Buffer' Day 260mr/ min per 1010 counts susceptibleto
1010 PFU PFU acid- deoxyribonu-soluble cleasec
I 1 5.0 1.5 X 106 1.4 3.2
10 7.0 1.3 X 106 2.6 10.8
II 1 4.5 1.5 X 106 1.4 1.0
101 4.5 9.5 X 105 1.5 2.2
aThe composition of buffer I was 0.05 M Tris
(pH 7.4) with5 X 10-4M EDTA. Thecomposition
of buffer IIwas0.25 Msucrose-103 MMgCl2-0.02M
Tris (pH 7.4).
bOptical density (OD) withalight pathof 1 cm
corrected forlight scattering. The ratio of OD at
260m,MtoODat280mrn was 1.31.
cCounts converted to acid-soluble form by
treatmentwithdeoxyribonuclease (100
,.g/ml)
for 30minat 37C.2% calf serum. Whendifferent media were tested for
attachment, theefficiency wassignificantly lower in all
media tested, including phosphate-buffered saline
(PBS) with or without 10-2M MgCl2 and with and
without the addition of serum. Attachment was
carried out inround-bottom tubes vigorously shaken
in a water bath at 37 Cwith a celldensity of 2.5 X 107
to 5 X 107 cells/ml. In some experiments, the cell
density was varied.
Elution of labeled virus from cells. 32p_- and 14C.
labeled virus was attached to cells for 10 to 20 min at
37 C, according to the procedure described above.
Thecellsweresubsequentlydiluted 10-fold in ice-cold
MEMandcentrifugedat500 X gfor5min; the cells
were resuspended in prewarmed medium at a cell
density of 107cells/ml andatvariousintervalssamples
were removed and the supernatant fraction was
as-sayed for radioactivity. In some experiments, the
elutedradioactivitywas analyzed by sucrose gradient
centrifugation.
Eclipse of labeledvirus.After attachmentfor5 to20
min the cells were diluted 10-fold inice-cold MEM
andcentrifugedat 500 X gfor 5 min. Thevirus-cell
complexes were again resuspended in prewarmed
MEM with 2% calf serum at acell concentration of
107cells/mlandincubated in thewaterbath at37 C.
At different time intervals, samples were taken for
analysis of the distribution of radioactivity in the
virus-cell complexes. Time-zero was assigned to the
instant ofreincubationof thecells in the water bath.
Analysis ofdistribution of radioactivity in virus-cell
complexes. Each sample of I ml of32P-labeled virus
removedfrom theincubationmixturewascentrifuged
at 500 X g for5min,and 0.3 ml of the supernatant
fluidwasassayedforradioactivity.Another 0.3 ml of
supernatantfluidwasaddedto 5mlof cold
trichloro-acetic acid (5%), and the mixture was filtered on
membrane filters (Millipore Corp.,Bedford, Mass.),
which subsequently were assayed for radioactivity.
Thecellpelletwassuspendedin2mlof0.01 M sodium
phosphate buffer (pH 7.0) with 0.01 M MgCl2 and
sonicallytreated for 15 sec;two 0.9-mlsamples were
removed for determination of cell-associated
acid-soluble and acid-insoluble material and for material
brought into solution by deoxyribonuclease
(Worth-ington Biochemical Corp., Freehold,N.J.;crystalline,
ribonuclease-free). Deoxyribonuclease treatment was
carried out with 100 ,g/ml for 30 min at 37 C.
Trichloroacetic acidwasadded inafinalconcentration
of
8.5c,,,
and all samplesprecipitatedwithtrichloro-aceticacidwerestoredat4 Cfor 18 hr beforecounting
the supernatant fluid and the sediment (the latter
taken upin 0.1 MNaOH).This procedurefor
deoxy-ribonuclease treatment rendered more than 90% of
purified 32P-labeled adenovirus deoxyribonucleic acid
(DNA) acid-soluble. The procedureis essentiallythe
same asthatdescribedby Joklik (15).
Samples of 1mlof '4C-threonine-labeledviruswere
removed from the incubation mixture, and the total
and acid-insoluble radioactivity were determined on
the supernatant fluid as described above.
Further-more,thesupernatantfluidwas plated after
centrifu-gation at 45,000 X g for 45 min, which sediments
intactvirusparticles.Thecellpellet was assayed inthe
869
on November 11, 2019 by guest
http://jvi.asm.org/
[image:2.462.32.226.424.563.2]same way after resuspensioninPBS and sonic
treat-mentfor 15sec.
Sucrose gradient centrifugation. Sucrose gradient
centrifugation was carried out in preformed linear gradients from5to25and 20to40% sucrose in 0.01
MTris (pH 7.4)-0.1 M NaCl.Thesampleswereapplied
atthetopof the
gradients,
andcentrifugationwasfor60 or 180 minat 100,000 X g. The sucrosegradients
weremadeonacushion ofRbCl (p = 1.40g/ml) to
preventpelletingof intactvirions.
Sonic treatment. Sonic treatment was done in an
MSE60-wultrasonicdisintegrator.
Radioactivity. Radioactivity was determined in a
Tracerlab FDI gas-flow counter equipped with a Monomol window. No corrections were made with
32P-labeledmaterial but 14Cradioactivity wascorrected
forself-absorption.
RESULTS
Attachment of32P-labeledadenovirus.32P-labeled adenovirus
containing
5 X 107PFU correspond-ingto 15,000 counts/minwasmixed witha total of2.5 X 107 KB cells(multiplicity -2)
both at0 and at 37 C, as described in Materials and Methods. At various intervals, samples were
re-moved,and the supernatantfluidwas
assayed
forradioactivity
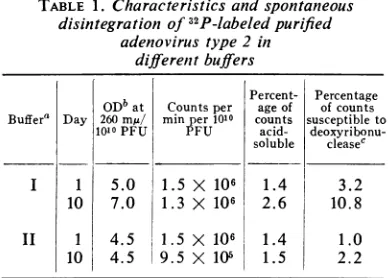
andinfectivity. Figure
1 showsthatattachmentofadenovirusis
relatively
slowat0C,
with an adsorbtion rate constant of1.8 x 10-3 per mlpermin.At 37
C,
85% oftheradioactivity
isattachedin 50min,withan
adsorption
ratecon-stant of 1.2 X 10-2per ml per min. The
radio-activity
and theinfectivity
are adsorbedatalmostidenticalrates at 37 C. Because of the
inaccuracy
of the
plaque
technique,
nocomparison
couldbemade at 0
C,
but nosignificant
reduction in1.0
U)
0 L.
0.5
0.2
0.1
't 0
U,
:3
I..
J7-C
10 20 30 60
Time in min. FIG. 1. Attachmentof'2P-labeledadenovirus type 2 to KB cells in suspension at different temperatures.
Symbols: 0,radioactivity; 0,infectivity.
infectivity was observed in the supernatant fluid after 30minatthat temperature.
Effect of multiplicity upon attachment of 32p_ labeled virus. The effect ofvirusmultiplicityupon attachment was tested by two different proce-dures.
(i) The cell density was varied in five different concentrations from 2 X 108 to 1 X 107 cells/ml, and the dose of32P-labeledvirus was constant.
(ii) The dose of32P-labeled virus was varied in five different concentrations, with multiplicities ranging from 3 to 0.15, with the samecell con-centration of 5 X 107 cells/ml. Atdifferenttime intervals after incubation at 37 C, samples
wer4
analyzed for radioactivity in the supernatant fraction. The details of the experimental tech-nique are described in Materials and Methods. Figure 2A shows that when the cellconcentration isvaried the attachment rate is stronglydependent onmultiplicity, but whenthevirusdose is varied attachment rate is the same irrespective of mul-tiplicity (Fig. 2B). The cellconcentrationappears to be the major parameter in determining the efficiency of attachment, with higher rates of attachment at higher cell concentrations. The "percentage law" (1) formulated for virus-anti-bodyreactions thusappears to apply also to virus-cell receptor interaction, and consequently the receptors are probably in great excess at the multiplicitiesused.Attachment of
"4C-labeled
virus. Since the cell concentration determined the rate ofadenovirus attachmentandtheratewasuninfluencedby virus multiplicity from0.15 to 3, the kinetics of attach-ment of 4C- and 32P-labeled virus could be per-formed in separate experiments. 14C- and 32p_10 20 30 40 50 60 10 20 30 40 50 60
Time in min.
FIG.2. Effect ofmultiplicityupon attachmentof
2P-labeled adenovirus type 2. (A) Cellconcentration was
variedfrom2X 108toI X 107cells/ml,and thesame,
virusdose wasused,giving the indicated
multiplicities-(M). (B) Virus concentration was varied, giving the
indicated multiplicities (M), and the cell dose was
constantat5X 107cells/ml.
J. VIROL.
on November 11, 2019 by guest
http://jvi.asm.org/
[image:3.462.38.231.447.626.2] [image:3.462.242.432.462.587.2]labeled viruswasmixed together withKBcellsat a celldensity of5 x 107 andamultiplicity of 1, and the mixtures were
incubated
at 37 C. At varioustime intervals, sampleswere removed and the supernatant fluid was assayed for radioac-tivity.Figure
3 shows that 32p_ and "4C-labeled virus attachatthesame rate,althoughthe steady state may be reached somewhat earlier with the 14Clabel, with 89 and 80% of the 32p and "4G-label, respectively, attachedat 60min.Elutionof32P-andl4C-labeledvirus. Inspite of thefact that
32p_
and'4C-labeled virus attachedtothecellsatthesamerate, itwas
judged
necessary to study the elution of thetwoisotopes
from the cells subsequenttoattachment. Theprocedure is outlined in Materials and Methods, andcellsatadensity
of 5 X107/ml
and32p_
and 14C-labeledvirusat a
multiplicity
of1 wereused. After attach-ment for 15 min, the elution was followed atvarioustimes for60min.Figure3showsthatless than 1% of the 32P label is eluted from the cell within the
period studied,
but the 14C label elutesto a
slightly
higher
rate, and6% of the labelwasrecovered in thesupernatantfluidat60min. The
fraction
eluted varied between 5 and 8% indif-ferent
experiments.
All of the 14Cactivity
eluting
from the cellswasacid-insoluble.
Characterization of eluted 14C label. It was
tentatively assumed,
based on the resultsde-scribed above, that the
slight differential
elution between 14C and 32P label maybe
ascribed topartial
disintegration
of thevirus
at, or in closevicinity of,
the cell surface. To establish theapproximate size
of the elutedfraction,
11C-'b I' .0
0 0 L.
1.04
0.1
D
-.0
*03 q
It
0212
0 I.. *o1
10 20 30 40 50 60
Time in min.
FIG. 3. Attachment and elution of 32P- and 14C-labeled adenovirus type2. Symbols: @, 32Plabel; 0,
14C label; solidline, fraction unadsorbed; dashedline,
fractioneluted.
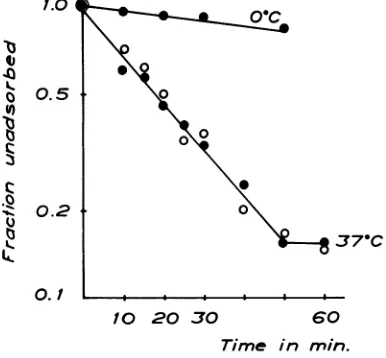
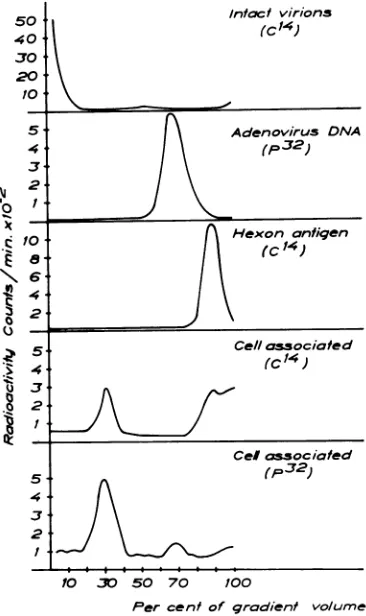
labeled hexon antigen, intact 'IC-labeled virus, and eluted 'IC label were examined in sucrose gradients asdescribed in Materialsand Methods and the radioactivity was continously recorded. Figure4shows that the'IClabel eluted from the infected cellsresides instructures sedimenting at a slower rate than that of intact virus and at the same rate aspurified hexon antigen. Hexon anti-genicity could also be demonstratedat the peak of the14Cactivity.
Eclipse ofadenovirus. Theeclipse of the virion was assayed by
following
the fate of32P-labeled
virus after an attachment period of 10 min, as
described
in Materials and Methods. Acell
density of5 X 107cells/ml andamultiplicity of1 wasused. Thecountsinthedifferentfractions ina
typical experiment
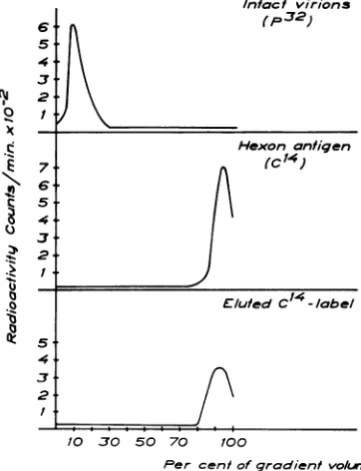
are shown in Table 2, andfrom these datathe percentageofDNA
suscepti-bleto
deoxyribonuclease
atdifferent
timescanbecalculated,
provided
thatthe32P-label
is confined tothe DNA of thevirus. The results of GreenandPifia (9) supply
thebasis forthis
assumption. AsshowninTable2and
Fig.
5,therateof release of adeoxyribonuclease-susceptible
structureisrapid,
and, within 20 min
after
thearbitrarily
assignedtime-zero,
maximaluncoating
of the DNA hastaken place. In
different
experiments, between 65Intact virions
6-*Ap32)
C.4
Hexon antigen
10 E/lutedC 1 -l/abel
c
5.
10 30 50 70 100
Per centofgradientvo/urne
FIG.4. Characterizationof the eluted14Clabel from
adenovirus-infected KB-cells. Linear sucrosegradients
from20to40%sucrosecentrifuged for 1 hr at100,000' X g.Fromtoptobottom,intact32P-labeled adenovirus type 2,purified "4C-labeled hexon antigen, and the
eluted14Clabelfrominfected KB-cells.
on November 11, 2019 by guest
http://jvi.asm.org/
[image:4.462.236.417.366.602.2] [image:4.462.31.222.438.627.2]TABLE 2. Fate ofadenovirus type 2 labeled with3SP
Cell-associated counts In medium
PercentDNA
(B)
~~~~~~~~~~~~DNA
available susceptibleTime (min) (cid-insob) e foreclipse, to
deoxyri-(Aci-noul
sensitiveto (C) (D) (E) A+C+ E bonuclease,Acidinsouble deoxyri- Acid-soluble Acid-insoluble Acid-soluble B/F X100 bonuclease
5 1,854 576 244 323 0 2,098 27
10 1,810 852 172 322 0 1,982 43
15 1,997 1,042 150 372 0 2,147 48
20 1,900 1,360 193 391 0 2,093 65
30 1,723 1,433 319 389 0 2,111 68
45 1,657 1,387 390 317 0 2,149 65
and 85 %of virusDNA wassusceptibleto deoxy-ribonuclease. A minor fraction of the
32P-label,
not exceeding 10% of the DNA in 4 to 6 hr, is subsequently converted to acid-soluble material insidethecell.
Multiplicity of infection andrelease of a deoxy-ribonuclease-susceptible structure. The influence
of
multiplicity
upondeoxyribonucleasesensitivity
wastested bothby addingastandardvirusdoseto cells ofvaryingdensities andby varyingthevirus
dose at the same cell
density
level.32P-labeled
virus was attached for 10 min at 37 C, and the percentage of 32P label
susceptible
todeoxyribo-nuclease was determined after an additional
period
of 30 min at 37C,
as described above.Figure 6 shows that less
deoxyribonuclease-sensitive material isreleased athighcelldensities (A) and that the percentage ofthis material de-creases at
high multiplicities
of virus(B).
Effect
of metabolic inhibitors upon theappear-anceof deoxyribonuclease sensitivity. The
partici-pation
of cellular metabolism in theeclipse
wasevaluated
by studying
theefficiency
ofuncoating
in thepresenceof
actinomycin
Dandpuromycin,
which inhibit
DNA-dependent
RNAsynthesis
and
protein synthesis, respectively.
The dose was5 mg of
actinomycin
perml and 50,ug
ofpuro-mycin
per ml. These concentrations were showntoinhibitRNA
andprotein synthesis,
respectively,
to 1% ofactivities incontrolKB cells within 30 min after addition of the
inhibitors,
when in-organic 32pwasincorporated
into RNA or 14C-threonineintoproteins
of cells inspinner
cultures. KB and HeLacellswerethereforepretreated
for varyingperiodsat37Cinspinner mediumin the presence and absence of the inhibitors at these concentrations.Viruswassubsequently
addedby
resuspendingthecells in attachmentmediumwith andwithout inhibitors butwith32P-labeledvirus. After15minofattachment,thecellswerewashed
and
again
incubated at 37 C in the presence orabsence ofinhibitorsfor30minat 37
C,
and the percentage ofdeoxyribonuclease-sensitive
ma-qJ
qj
U)
U)
qj Q
0..
70 T
. 60t
50+
40+
30+
20
t
10
t
10 20 30 40 50
[image:5.462.234.442.101.456.2]Time in
min.FIG. 5. Kinetics of conversion of intact adenovirus
intoadeoxyribonuclease sensitive structure after
virus-cellinteraction.
terial was determined. Figure 7 shows that ir-respective of the time for pretreatment actino-mycin does not
affect
the transfer of virus into deoxyribonuclease-sensitive material. The pres-ence of puromycin appeared to slightly increase thecapacityofthecells to eclipse virus, especially ifit waspresent for periods of 2 to 4 hr prior to virus attachment. No difference between HeLa and KB cells could be discerned with regard to the effect ofpuromycin.Characterization of the cell-associated32p_ and
"4C-labeled
virus.The attached virus thusappears to be rapidly eclipsed into a deoxyribonuclease-sensitiveform, and thisprocessdoes notappearto require RNA or protein synthesis. Since a core structure ofadenoviruses has been observed by electronmicroscopy (6, 14), it wasjudgedon November 11, 2019 by guest
http://jvi.asm.org/
L.
'
b 60
|, 50
o 4
.--0 t 40~ <. 30 q:
A
1o
B
0.1 1 10 0.1 1 10
[image:6.462.32.222.57.228.2]Multip/icily
FIG. 6. Effect ofmultiplicityuponthe conversion of
intact adenovirus into a deoxyribonuclease-sensitive
structure. (A) The cell dose was varied to give the
indicatedmultiplicities. (B) The virus dose was varied
togivethe indicatedmultiplicities.
Methods, and the fractions were monitored by radioactivity and immunodiffusion. Pure 1C-labeled hexon antigen preparedasdescribed else-where (21),
extracted32P-labeled
DNA (10), and intact'4C-labeled virus were used as standards to measure thesedimentationrateofthe productsin sucrose gradients. The standard preparations wereaddedto sonically treated normal KB cells at the same cell density as in theexperimental
samples. Figure 8 shows that themajor peak of
32P-activity
recovered fromcell-associatedadeno-virus preparations is confined to amaterial sedi-menting midway between viral DNA and virus infectivity. Only about
10%
of the 32Pactivity
sedimentsatthesame rate asviralDNA,but very littleasintact virions.The 32P
activity
ofthe inter-mediate peak issusceptible
todeoxyribonuclease.
When
cell-associated
14Clabelwasanalyzed,
itwas first demonstrated that radioactivity was
qJ1.
.U
E
qj 100qj
80
0
s 60 c 40
q 2
Q.
0
-..°
^I.
e- --0f
I.
4 3 2 1 Control
Timeofpretreatment
[image:6.462.239.422.284.592.2]in hours
FIG. 7. Effect ofmetabolic inhibitors on the
con-version ofintact adenovirus into a
deoxyribonuclease-susceptiblematerial. KB cells werepretreated for the
indicatedtimeswithactinomycin(A\)at aconcentration
of5,ig/mlorpuromycin (0) at aconcentrationof50
,tg/ml.HeLacells(0) werealso tested with puromycin atthesame concentration.
tanttoattemptto characterize the
cell-associated
product of adenovirus. The cell-associated "2P labelwasfirstexamined.
32P-labeledvirus was attachedata
multiplicity
of 1 andacell
density
of5 X 107cells for 15 min at 37C, and, afteraneclipse
period
of60min at 37C,thecellswerewashedtwice,
resuspended in0.5 ml, and
sonically
treated for 15 sec. Thismaterial was fractionated by sucrose gradient
centrifugation
as described in Materials and10 3 50 70 100
Percent of gradient volume
FIG.8. Characterization of thze cell-associated 32P
and14Clabels fromadenovirus-infectedKBcells. Linear
sucrose gradients from 5 to
25%c
sucrose centrifuged for3hr at 100,000X g. ThepatternsshIowIz
are, fromtop to bottom: 14C-labeled intact virions; 32P-labeled
adenovirus DNA; 14C-labeled hexon
antigent;
cell-associated14Clabel and cell-associated 32p label.
on November 11, 2019 by guest
http://jvi.asm.org/
[image:6.462.32.225.290.481.2]PHILIPSON
acid-insoluble aftereclipse periods of 30 min to 4 hr. On the other hand, the 'IC label rapidly converted to a structure not
sedimenting
at 45,000 x gfor 45 min. Intact virions are, how-ever,sedimented
atthisspeed.
Therateof appear-ance of thislighter
structure was similar to the rate ofappearance ofadeoxyribonuclease-sensitive
material, previously shown in
Fig.
5. When thecell-associated
'4C-labeledactivity
wasanalyzed
in sucrose gradients, about 45% of the activity wasrecovered inastructure
sedimenting midway
betweenviralDNAandintact
virions,
andatthe same rate as themajor
peak of cell-associated39P
activity,
as shown inFig.
7. Theremaining
14C
activity
wasrecovered inmaterialsedimenting
at the same rate as hexon
antigen.
A similar pattern wasobservedaslateas4 hrafterinfection;
i.e., most of the
cell-associated
32P-activity
was recovered in the structure with anintermediate
sedimentation
rate and 40 to50%01
of the 14Cactivity
wasalso recovered in thisfraction.DISCUSSION
The
following
model of adenovirus attachmentand
eclipse
has beenadopted
from the presentedresults.
Adenovirus
particles
arerapidly
attached to cells(80to90% in60min),
andpartof
theprotein coatappearsto be releasedat,orinclosevicinity
of,
thecell
surface,
exposing
anucleoprotein
structureinside the
cell,
thenucleic acid of which is sensitive todeoxyribonuclease (65
to 85%efficiency).
Thefurther breakdown of thisstruc-ture into DNA and
protein
cannot bedetected
biochemically
at4 hrafter infection. Thepresenceof14Clabel inthesizerangeof the hexon
antigen
inthecellmediumand thewashed
pellets,
aswell as the intermediate sedimentation ratebetween
virus and free DNA, for the
major
part ofthecell-associated
32plabel andhalf of the14Clabel,
support this theory.
Possibly
such a structure couldcorrespond
totheadenoviruscore,observed
in the
electron
microscope (6, 14),
butadditional
evidence
concerning
thenature andfinal fate of thisstructureisrequired.
Alternatively,
the inter-mediate structure may be released DNA with attached hexonantigen,
since thisprotein
has ahighDNA
affinity
(unpublisheddata).
Considering
the datafurnishing
the basis forthismodel,it was first shown thatadenovirustype 2infectivity
rapidly
attachedtoKBcells and that purified radioactive virusattachedatthesamerateasinfectivity,in
spite
ofthe fact that theefficiency
of the plaqueassay hasbeenestimated tobe3% ofthe number of
particles (11).
The ineffective particlesthus appeartoattach anduncoatto the same extent asthoseinitiating
infection,
and thehighratio ofparticlesto PFU maybedue to in-efficiencyatsubsequentstepsofthemultiplication cycle.This is inagreement with thefindings in the vaccinia virus system (15, 16), butin contrastto the poliovirus system (18). In the latter system, however, the ratio of PFUtoparticles isaslowas 0.003.
Itis of interest that the attachment of adeno-virus particles to cells at low multiplicities appears to follow some of the characteristics established for virus-antibody union. Thus, the attachment rate is primarily dependent on cell concentration, andthe"percentage law" (1) ap-pears to applywhen the samecellconcentration is used but the virus dose is varied (Fig. 2). Fazekas de St. Groth (7) made the same pre-diction for
virus-erythrocyte
complexes.The initial phase in adenovirus eclipse, the exposure ofviralDNA to
deoxyribonuclease,
isa rapid process with regard to the long eclipse phase in the adenovirus system. A preliminary note by Lawrence and Ginsberg (Federation Proc. 24:379, 1965) described similar observa-tions. In contrast to the vaccinia virus system (15,16), cellular protein and nucleic acidsynthesis do notappear to be required in thisprocess; on the contrary, inhibition of protein synthesis in-creases slightly, butsignificantly, theappearanceof the
deoxyribonuclease-susceptible
material(Fig. 7).
The core structure in adenoviruses proposed from electron microscopic studies (6, 14) may possibly correspond to the cell-associated struc-ture containing both 14C and
32p
label (Fig. 8).The
prolonged
eclipse phase of adenovirusmayinthatcasebe dueto theuncoating of this material ifadditional uncoating is necessary for transcrip-tionortranslationfrom the viralgenome.Recent findings in the vaccinia virus system (19, 25) indicate thatacoatedgenome canbe transcribed. No enhancement of
eclipse
could be observedat
higher
multiplicities;
on the contrary,eclipse
was
relatively
less efficientathigher multiplicities
and at
higher
cell concentrations. Withvaccinia
virus (15, 16), DNA uncoating was in contrast morerapid at
higher
multiplicities, which,
how-ever, maybeinfluencedby
theapparentinduction mechanisminvolvedinuncoating inthat system. Theimplicationsofthefurnished resultsforthe mechanismofviruspenetrationandeclipse
merit a comment.Generally,
animal viruses are pro-posedtoenterthe cellbypinocytosis
followedbydisintegration
withinpinocytotic
vesicles(4,
17).The present results are, however, not
entirely
compatible withtheelectron
microscopic
dataonadenovirus
penetration
andeclipse (3).
Thus, the rapid disintegration of 65 to 85% of the virions J. VIROL.on November 11, 2019 by guest
http://jvi.asm.org/
observed in this study is in contrast to the slow penetration and accumulation of intact particles within the cell, observed by electron microscopy. Differences in methods of purification of the virions maypossiblyexplainthediscrepancy.
The pinocytosis concept for virus penetration should, furthermore, be considered in the light of Cohn's studies on the effect of antimetabolites upon pinocytosis of macrophages (2), where actinomycin andpuromycin in concentrations of 0.01 and 0.1,ug/ml,respectively, inhibit pinocyto-sis toaround
107%
of the controlactivity. Inthe present study, actinomycin and puromycin at 5 and 50 ,ug/ml, respectively, failed to inhibit adenovirus disintegration. In addition, studies on picornavirus eclipse have furnished evidencethat the virion is modified or even uncoated in the presenceofcell membrane preparations (13, 22). Furtherstudiesontheuncoatingofpoliovirus by intact cellswashampered bythe elution ofvirus from the cells (18), probably complexed with receptor structures (23), andbytherapidbreak-down of the parental genome into acid-soluble
form(18).
Thus, it is stillpossiblethat animal viruseswith cubicsymmetry willnotbeengulfed bythe
viro-pexis
mechanism, incontrast tothelipid-contain-ing viruses.
ACKNOWLEDGMENTS
Thisinvestigationwassupported bygrantsfrom the
DamonRunyonMemorialFund,theSwedishMedical
ResearchCouncil,andtheSwedishCancerSociety.
LITERATURE CIED
1. ANDREWES, C. H.,AND W. J. ELFORD 1933.
Ob-servations on antiphage sera. Brit. J. Exptl.
Pathol. 14:367-383.
2. COHN,Z.A.1966. Theregulationofpinocytosisin
mousemacrophages. I.Metabolicrequirements
as defined by the use of inhibitors. J. Exptl.
Med. 124:557-571.
3. DALES, S. 1962. An electronmicroscope study of
the early association between twomammalian
viruses and their hosts. J. Cell Biol.
13:303-322.
4. DALES, S. 1965. Penetration of animalvirusesinto
cells. Progr. Med. Virol. 7:1-43.
5. EAGLE, H. 1959. Aminoacid metabolism in
mam-malian cell cultures. Science130:432-437.
6. EPSTEIN, M. A.,S. J. HOLT,AND A. K. POWELL.
1960. The fine structure and composition of
type S adenovirus; an integrated electron
mi-croscopical and cytochemical study. Brit. J.
Exptl.Pathol. 41:567-576.
7. FAZEKAS DE ST.GROTH,S. 1962.The
neutraliza-tion ofviruses. Advan. VirusRes. 9:1-125.
8. GAREN,A.,ANDL. M. KOZLOFF. 1959.The
initia-tion of bacteriophage infection, p 203-236.
In F. M. Burnet and W. M. Stanley [ed.],The
viruses, vol. 2,AcademicPress, Inc., New York.
9. GREEN, M., AND M. PINA. 1963. Biochemical
studiesonadenovirusmultiplication.IV.
Isola-tion, purification and chemical analysis of adenovirus. Virology 20:199-207.
10. GREEN, M., AND M. PINA. 1964. Biochemical
studiesonadenovirus multiplication. VI.
Prop-erties of highly purified tumorigenic human
adenoviruses and their DNA's. Proc. Natl. Acad. Sci. U.S. 51:1251-1259.
11. GREEN, M., M. PINA, AND R. C. KIMES. 1967. Biochemical studies on adenovirus
multiplica-tion. XII. Plaquing efficiencies of purified
humanadenoviruses.Virology 31:562-565.
12. HERSHEY, A. D., AND M. CHASE. 1952. Independ-entfunctionsof viralproteinand nucleic acid in
growth ofbacteriophage. J. Gen. Physiol. 36:
39-56.
13. HOLLAND, J. J. 1962. Early stagesof enterovirus
infection. Cold Spring Harbor Symp. Quant.
Biol.27:101-111.
14. HORNE, R. W. 1963. Architectural symmetry in
viruses and their components. Perspectives
Virol. 3:43-57.
15. JoKLIK, W. K. 1964. Theintracellular uncoating ofpoxvirus DNA. I. The fate ofradioactively
labeled rabbit poxvirus. J. Mol. Biol. 8:263-276.
16. JOKLIK, W. K. 1964. The intracellular uncoating ofpoxvirus DNA. II. The molecular basis of theuncoatingprocess. J.Mol. Biol.8:277-288.
17. JOKLIK, W. K. 1965. The molecular basis of the
viraleclipsephase. Progr.Med.Virol. 7:44-96.
18. JOKLIK, W. K., AND J. E. DARNELL. 1961.
Adsorb-tion and early fate ofpurified poliovirus.
Virol-ogy13:439-A47.
19. KATES, J. R., AND B. R. MCAUSLAN. 1967.
Mes-senger RNA synthesis by a "coated" viral
genome. Proc. Natl. Acad. Sci. U.S.
57:314-320.
20. PETTERSSON, U., L. PHILIPSON,AND S. HOGLUND.
1967. Structural proteins of adenoviruses. I.
Purificationand characterizationofadenovirus
type 2hexonantigen. Virology,inpress.
21. PHILIPSON, L. 1961. Adenovirus assay by the
fluorescent cell-counting procedure. Virology
15:263-268.
22. PHILLPSON, L., AND M. LIND. 1964. Enterovirus eclipsein acell free system. Virology 23:322-332.
23. PHILIPSON, L., AND S. BENGTSSON. 1962.
Inter-action of enteroviruses with receptors from
erythrocytes and host cells. Virology
18:457-469.
24. WOODSON, B. 1967. Vaccinia mRNA synthesis
under conditions which prevent uncoating.
Biochem.Biophys. Res. Commun. 27:169-175.