Copyright©1977 AmericanSociety forMicrobiology Printed in U.S.A.
Binding Characteristics of
Rauscher Leukemia Virus
Envelope
Glycoprotein gp7l
to
Murine Lymphoid
Cells
A. K. FOWLER,l* D. R.TWARDZIK,1 C. D. REED,1 0. S. WEISLOW,2 AND A. HELLMAN'
National CancerInstitute'andLitton Bionetics,Inc.,2Frederick Cancer Research Center, Frederick,
Maryland21701
Receivedforpublication 15 July 1977
The major envelope glycoprotein (gp71) purified from Rauscher leukemia
virus (R-MuLV) binds efficiently to murine lymphoid cells but not to either murinenonlymphoid cellsorlymphoidcells from other species. Binding of 125I-labeledR-MuLVgp7lwascompetitively inhibitedbyunlabeled glycoprotein, as wellasby whole R-MuLV, butnotbymurine xenotropic viruses, R-MuLVp30, and several unrelated proteins. Polyacrylamide gel electrophoresis profiles of
iodinatedgp7lafter bindingtolymphoid cellswere similar to prebound profiles. Antibodyto R-MuLV gp7l prevented binding, whereas normal serum had no effect. Adsorption of the glycoprotein to murine lymphoid cells occurs rapidly and is time and temperature dependent. The procedure described is sensitive fordetecting thebindingactivity of approximately104cells.Binding was propor-tional up to 2.5 X 105cellsperml andplateaued above 107 cells perml. In the
presence of excess R-MuLV gp7l, BALB/c thymocytes bound approximately
2.4 X 10 moleculespercell.
Oncornavirus infection of mice results in the
depressionofboth humoral and cellular immune reactions in vivo (for review,see5,22).
Further-more, evidence that in vitro cellular immunity
is impairedin virus-infected mice has been
re-ported (10, 12). These observations have more
recently been extended by the demonstration that freeze-thawed extracts of Rauscher leuke-mia virus (R-MuLV) suppress in vitro cell-me-diated immune reactions of normalmouse
lym-phocytes (A. K. Fowleretal.,in press).Similarly, UV-inactivated feline leukemia virus also
sup-pressesin vitroblastogenic responsesofnormal
cat lymphocytes (L. L. Hebebrand et al., in press). Sincemost, ifnotall,mammals contain
genetic information for related type C viruses-xenotropic viruses-thatare inducible in vivo in the host byhormonal (6, 7, 11) and
immunological (14) stimulation, we have
sug-gested that virion components endogenous to the host function as regulators in normal
im-munological processes (13). Indeed, xenotropic
viruses are actively expressed in maternal (8)
and fetal (15, 17) tissues during pregnancy, a
periodofintricate hormonal andimmunological interaction.
Theprimaryeventinvirus-cellinteraction is the adsorption ofthe virion,
presumably
via aviral envelope component, to specific cellular
receptors. Recent progress in
identifying
andisolating oncornaviral proteins now permits a
more detailed elucidation of virus-cell
interac-tion. Accordingly, we have undertaken studies
todeterminetheeffect ofpurified virion proteins on in vitro murine lymphocyte transformation andtoexamine theirbinding characteristics, the
receptorsinvolved, and the mechanisms of viral
protein-cell membrane interaction that lead to modification of cell behavior.During these stud-ies, wehave observed that the major envelope
glycoprotein(gp7l)ofR-MuLV, like AKRgp7l
(18),induces transformation ofnormal
lympho-cytes, whereas alower-molecular-weightvirion
protein(s) appears to depress T-cell function (Fowleretal.,inpress). Inthis communication,
weextendourprevious findingsby characteriz-ingthebindingof R-MuLVgp7ltolymphocytes
and othercells derived from various organsof several strains of mice.
MATERIALS AND MERTHODS
Cellpreparation. Thymuses, spleens,or
epididy-mides were excised and minced in 20 ml of RPMI 1640 medium and gently sieved through a 60-mesh
stainless-steel filter. Thymic cell preparations were
thenfurther washed three timesbyrepeated centrif-ugation (250 x g)andresuspensionwith fresh medium.
More than 90%of thethymocyteswereviablebythe trypan dye exclusion test. Splenic cell suspensions
werewashedoncewith fresh medium and thenlayered
on a Ficoll-Hypaque gradient. The
lymphocyte-en-richedlayerwascollected and washedthreeadditional timeswithfresh medium. Theviabilityof thesplenic
lymphocyteswasconsistently above90%. Forsperm
cellpurification,theepididymalcellpreparationswere
729
on November 10, 2019 by guest
http://jvi.asm.org/
730 FOWLER ET AL.
initially low-speed centrifuged (200xgfor3 min)to
remove particulate material. The cells remaining in thesupernatant werethen concentrated by
centrifu-gation (1,000xgfor20min) and furtherpurified by
discontinuous sucrose gradient (20:50:80%)
centrifu-gation (approximately 80,000xgfor60min)at40C. Thespermcells collectedatthe50:80%sucrose inter-facewerewashedanadditional threetimes with fresh
medium. The finalspermpreparationcontained fewer
than 5% contaminating cells. No estimate ofsperm viabilitywasmade. Peripheral lymphocyteswere pre-pared from heparinized blood by Ficoll-Hypaque sep-aration andwereprocessed identicallytothe splenic
lymphocyte preparations. Greater than 90% of the
cellswere viable. Blood used for thepreparation of
peripheral lymphocyteswascollected from rodentsby
cardiacpunctureand fromprimatesbyvenipuncture. Erythrocytes used in these studieswerealso washed threetimeswith fresh medium.
Glycoprotein purification and
characteriza-tion. BandedR-MuLV (1012 virus particlesperml),
produced in JLS-V9 cells and having an infectious titer of2x108focus-forming unitsperml,was
freeze-thawed twiceandcentrifugedat105,000xgfor90mi at40C. Thesupernatantwaslyophilized and dialyzed
againsta solution containing 0.01 M
NN-bis-(2-hy-droxyethyl)-2-aminoethanesulfonic acid (BES) (pH
6.5),0.001MEDTA, and 1.0M NaCland appliedto
aSephadex G-100 column (1.5 by 90 cm)equilibrated
withthesamebuffer. Peak fractionscontaining
enve-lopeglycoproteinwere identified bysodium dodecyl
sulfate-polyacrylamide gel electrophoresis stained with either Coomassie blue orSchiffreagentand, if
contanatedwithbovineserumalbumin, further
pu-rifiedbyphosphocellulose chromatography as previ-ously described (25). The purified glycoprotein was
labeled with'25I(1x10Wto5x1iO cpm/ngofprotein),
using the chloramine-T method(9). Afteriodination,
95 to98% ofthe acid(10% trichloroacetic acid)-precip-itableglycoproteinwasprecipitable with specific
an-tiserumprepared against purified R-MuLV gp7l and demonstrated less than a 2%immune precipitation with anti-bovineserumalbumin. Sodiumdodecyl
sul-fate-polyacrylamide gel electrophoresis of the iodi-nated glycoprotein demonstrated a single band
mi-gratinginthe71,000-dalton region (see Fig. 3A) and hereafter will be referredtoasgp7l.
Binding assay. Cells were incubated at 370C in
thepresenceof['MI]gp7lin1.0 ml ofmedium
contain-ing1%bovineserumalbumin.Cellconcentrationwas
maintainedat 10i cellsperml unlessotherwise indi-cated.All bindingassayswereperformedin polysty-renetubes (12 by75 mm) that had beenprewashed withmedium containing bovineserumalbumin.
Dur-ing incubation, cells were gently mixed on a roller
drum(10 rpm). Afterincubation, the cellswere
cen-trifugedat400xgfor 10minat40C.Thepelletwas
resuspended in2.5ml ofcold mediumcontaining bo-vineserumalbumin andrecentrifuged. This procedure wasrepeatedtwoadditionaltimes.Theradioactivity associated with the final cell pellet was measured
directly in a Nuclear-Chicago gamma counter. To
estimatenonspecific binding of the radiolabeled gp7l
toreaction vessels, control tubes,towhich no cells were added, were processed identically. This value,
which was consistently less than 300 cpm per tube, wassubtracted from theradioactivity associated with
correspondingtestculturestoderiveacorrected
spe-cificbindingvalue.
RESULTS
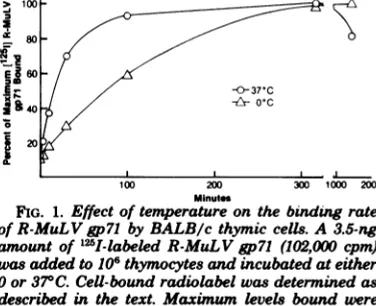
The binding of
[1"I]gp71
to murine splenic andthymiccellswasinitiallylinearand temper-ature dependent (Fig. 1). At 370C, bindingoc-curred rapidly, and within 15 min 50% of the maximum bound level was attained. The rate ofbinding subsequentlydecreased, and after100 min therewasminimalincrease intotalbinding level. After prolonged incubation (24 h) total
cell-associated label decreased approximately 20%,presumably duetodegradationofthe gly-coprotein. Incomparison, at00C (wet ice) the rateofbindingwasmuchslower, with 50% sat-urationoccurringatapproximately75min. For
maximum binding at 00C, nearly 6 h was
re-quired, but the total level boundwassimilarto that observed at370C, 3,693 versus 3,607 cpm per
101
thymic cells,respectively.To further investigate binding specificity, thymiccellswerepreincubatedfor 1 hat370C withvaryinglevels ofunlabeled R-MuLVgp7l
andp30,as wellaswithseveral unrelated
pro-teins (bovine senun albumin V, ovalbumin, myoglobin, and cytochrome c), before
adding
['25I]gp71.
Afteranadditional 30min of incuba-tion, only theunlabeled gp7lcompetitively
in-hibited binding (Fig. 2). The
preincubation
of cells with as little as 3 ng of unlabeled gp71 reducedbindingby 10%,andincreasingthis level to 6 and 13 ng reduced binding 20 and50%,
respectively. Total competition, however,re-quired approximately a50-fold excess of
unla-beled to labeled
glycoprotein
(200 ng per 106>100 10 0030 00 20
, looS~~~~~in t
860- K <
WE E m 37°C
!~~~~~~~~~~~~~~~OC
100 200 300 1000 2000
Minutes
FIG. 1. Effect oftemperature onthe binding rate
ofR-MuL V gp71 byBALB/c thymic cells.A3.5-ng
amountof125I-labeled R-MuLVgp7l (102,000 cpm)
wasaddedto106thymocytes and incubated at either
0 or370C. Cell-boundradiolabel wasdetermined as described in the text. Maximum levels boundwere 3,693 and3,607 cpmat0and
370C,
respectively. Eachpoint represents the mean value of quadruplicate
cultures.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.491.261.449.468.621.2]100
'
O , 80
_ rL
, o60
E>
0
40
c2 !
ILCM 20
10-1 100 101 102 103 104
Unlabeled RLVgp7lAdded(ng)
FIG. 2. Competitive inhibition by unlabeled R-MuLVgp7l of '25I-labeled R-R-MuLVgp7l binding by BALB/c thymic cells. Thymocytes (106/ml) were
in-cubatedfor60minat37C withvarying amountsof unlabeledgp71 before3ngof
I5IIgp71
(30,200 cpm) wasadded.Afteranadditional 30minof incubation,cell-bound radiolabelwasdetermined asdescribed inthetext.Eachpointrepresentsthemeanvalueof
duplicatecultures. Thepreincubation of thymocytes with 6ngofunlabeled gp71 reduced binding20%.
cells). Similarly (data not shown), antibody to R-MuLV gp7lprevented binding,whereas
nor-mal serum and antimurine immunoglobulin G and immunoglobulin Mserahadnoeffect.
Ad-ditionalevidenceofspecificitywasalsoobtained
by thepretreatmentof thymic cells with
infec-tiousR-MuLVaswellastwomurinexenotropic
viruses, NZB-C135 (21) andM-55 (1). At
equiv-alentconcentration, 1010 virus particlesperml,
R-MuLV reduced binding 80%, whereas no
ef-fect was observed with the xenotropic viruses.
The observationthatunlabeledgp7l
compet-itivelyinhibitedbinding indicates thatthe iodi-nated and unlabeled R-MuLV gp7l molecules
are biologicallyverysimilar and thatthe
mea-sured binding is not an artifactresulting from
thenonspecific binding of by-productsof
iodin-izationorfromtheuptake ofsmalllevels offree
"I.
Tofurtherexamine this possibility,theso-diumdodecylsulfate-polyacrylamide gel electro-phoresis profile of
['"I]gp71
bound to spleniccells after a 60-min incubation period at370C
wascomparedwith nonreacted labeled
glycopro-tein (Fig. 3). Although several minorpeaks of radioactivitywerenoted in thecellularextract,
themajorpeakofactivitycorresponded tothe
standard labeled R-MuLVgp7l, indicatingthat
no significant change in molecular weight
oc-curred duringthebindingassayprocedures.The
minorpeaks undoubtedlyrepresentaggregation
and degradation products of the glycoprotein
andare consistent with theinterpretationthat
the observed reduction in cell-bound label after prolonged incubation is largelyattributable to
glycoprotein degradation.
The effect of celldensityonbindingwas
stud-ied by adding 3 ng of
['II]gp71
to BALB/c thymic cells. As showninFig. 4,the assaywassensitive for detecting the receptor activity of 104 cells, and binding was proportional up to a cell density of 105 cells per ml. At higher cell concentrations, the relative binding level grad-ually decreased andeventually plateaued above concentrations of107/ml. Aconsistent observa-tion made throughout these experiments was that only a portion of the input radiolabeled
glycoprotein, rangingfrom 15 to40%,depending ontheprobepreparation,wasboundby murine lymphoid cells despite a large excess of cells (107). Although receptor site masking or inter-ference may beinvolved, it is reasonable that a
significant portion of the iodinated gp7l main-taining imnmunological reactivity is not
biologi-callyactive basedonitsbinding properties. Data favoring thisinterpretationhave been obtained by multiple reincubation of reactant superna-tantsremoved from thymus cells (107/ml) pre-viously pulsed and incubated with
['5I]gp71
withfreshthymus cells. Fresh cells, during thesecond and third incubations of the reactant supernatants, boundonly 6.7and 3.1%,
respec-tively, of the residualimmunologically reactive
[1251]gp71.
In comparison, 29.8% of the imnmu-nologicallyreactiveglycoproteinwasbound dur-ing the first incubationperiod. The total[1"I]-gp7lbound during four reincubationcycles
rep-resented only41.7% of theimmunologically re-activeglycoproteininput.The observed variable
bindingefficiencyprobablyreflectsprocedurally
related degradation of the glycoprotein; how-ever,anaturalheterogeneityof the gp7l mole-culemustalso be considered.
Toestimate the number of receptor sites per
cell, BALB/cthymiccellswereincubated with an excess of
"2I-labeled
glycoprotein. As may benoted inFig.5, the total iodinatedgp71bound per 106 cellsincreased uptoapproximately
2.7 ng (38.5 x 10-15 mol) as the level ofsupple-mentedglycoproteinwaselevatedto107
ng/ml.
Extrapolating from this value, the number of R-MuLVgp71
molecules bound per cell isap-proximately2.5x 104.
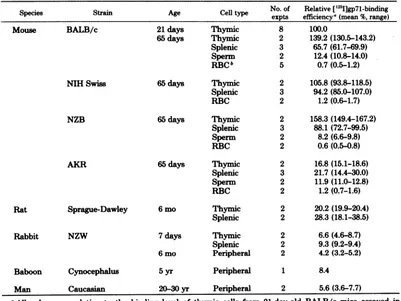
Cellspecificity ofR-MuLVgp7l bindingwas examinedtwoways and is summarized in Table 1.The firstcomparedthebindinglevelof murine
lymphoid cells to nonlymphoid cells
(erythro-cytes and sperm), and the second determined the relativebindinglevel of
lymphoid
cellsfrom several species. In general, murine lymphoidcells exhibited thehighest
potential
forbindingR-MuLV
gp71,
and, in most strainsstudied,
thymus-derivedcells boundmoreviralenvelope glycoproteinthanspleniccells.Anotable excep-tiontotheobserved highrelative bindinglevel oflymphoidcellsforR-MuLVgp7l
wasconsist-ently noted in the AKR mouse, in which the binding level of both
thymic
andsplenic
cells.. I- I-
I-VOL. 24,1977
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.491.57.217.52.172.2]732 FOWLER ET AL.
A Bovine
Serum Ovalbumin Albumin
Cyctochrome-C
B
10 2
10 20 30 40 50 60 70 80
FractionNumber
FIG. 3. Polyacrylamidegelelectrophoresis of lu2I-labeled R-MuLVgp71run on75to25% linear gradients
(cylindricalgels10cminlength) inthepresenceof0.1%sodiumdodecyl sulfate. Sampleswereprepared by
heating for2minat1XfCinasolutioncontaining50mMTris-hydrochloride(pH 6.7),2%sodiumdodecyl
sulfate,0.5%,8-mercaptoethanol,and0.01%bromophenolblue.After16 hofelectrophoresis (9 mApergel, 75
pulsesper s,0.5 ,uF), gelsweresliced into 1.3-mmfractions.Arrows indicatepositions of marker proteins. (A) ControlR-MuLVgp71; (B) BALBIcspleniccellextractafter a 60-min incubation at37°C with R-MuLV gp71.Before extraction,cellswerewashedasdescribedinthetext.
was approximately 20% of that noted for the
otherstrains. Of the murinenonlymphoid cells
examined, erythrocytes exhibited the lowest
bindinglevel(<2%ofreferencecontrol), whereas
the binding level of sperm cells was slightly
higher (10to15% of referencecontrol).
Except for thebinding level of the
Sprague-Dawleyrat(20%ofreferencecontrol), the
bind-ing of R-MuLV gp7ltolymphoid cellsfromthe
other species tested (rabbit, baboon, human)
wasconsistentlylow(<10%).
DISCUSSION
The results presented demonstrate that the
major envelope glycoprotein purified from
R-MuLV binds efficiently to murine lymphoid
cells. The rate of R-MuLV gp7l binding to
splenic and thymic cells is rapid and initially
linear but, in contrast to that noted for
fibro-blastic cells(4), is temperaturedependent. The
rateofbindingat370Cwasapproximately
five-fold that observed at 0°C (wet ice), although
the maximum extents ofbinding were similar
atboth temperatures. These findings are
con-sistentwith earlier data showing that the rate
ofpoliovirus adsorption to cells in vitro is
de-creasedbyareductionintemperature (16)and
mayreflectachangeinthe randomprobability
ofvirus-cell interaction.
The adsorption of gp71 tolymphoid cells is
highlyspecific. This is indicated by the
inhibi-tion ofbinding by antiserum toR-MuLV gp7l
x
C,
r._ 0. cm -J
-j z
CM
i-8
10
x
a
0L
6
4
21
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.491.95.409.51.423.2]and the competition for available membrane
receptors by either murine ecotropic virus (R-MuLV) orits purified viral envelope
glycopro-tein(R-MuLV gp7l). Murine xenotropic viruses
a
i
a
0
i3
Iol
a2
4 5 6 7
CellNumbe (LOgic)
FIG. 4. Effect of celldensityonthebindingof
R-MuLV gp71 by BALBIc thymocytes. Thymocytes at
varyingconcentration(104to107/ml)wereincubated
for45min at370C in the presence of3 ngof
125I-labeledR-MuLVgp7l (25,000 cpm). Cell-bound
ra-diolabel was determined as described in the text.
Eachpointrepresents themeanvalue of triplicate
cultures.
U',
20 40 60 50 100
112511 gpil1Added(ng)
FIG. 5. Effect ofR-MuLVgp7l concentration on
the level boundbyBALB/cthymiccells. Thymocytes
(106/Ml) were incubatedfor 60 min at370C in the presence of increasing amounts of '251-labeled R-MuL V gp71. Maximum input(11I0 ngofgp71) con
[image:5.491.292.413.66.225.2]tained525,000) cpm.Cell- boundradiolabelwas deter-minedasdescribed in thetext.Eachpointrepresents themeanvalueofduplicatecultures.
TABLE 1. Relativebinding of'25I-labeledR-MuLVgp71 to different cells
No.of Relative
[fnI]gp71-binding
expts efficiency(mean%,range)Mouse BALB/c 21days Thymic 8 100.0
65days Thymic 2 139.2(130.5-143.2)
Splenic 3 65.7(61.7-69.9)
Sperm 2 12.4(10.8-14.0)
RBCb 5 0.7(0.5-1.2)
NIHSwiss 65days Thymic 2 105.8(93.8-118.5)
Splenic 3 94.2(85.0-107.0)
RBC 2 1.2(0.6-1.7)
NZB 65days Thymic 2 158.3(149.4-167.2)
Splenic 3 88.1 (72.7-99.5)
Sperm 2 8.2(6.6-9.8)
RBC 2 0.6(0.5-0.8)
AKR 65days Thymic 2 16.8(15.1-18.6)
Splenic 3 21.7 (14.4-30.0)
Sperm 2 11.9(11.0-12.8)
RBC 2 1.2(0.7-1.6)
Rat Sprague-Dawley 6mo Thymic 2 20.2(19.9-20.4)
Splenic 2 28.3(18.1-38.5)
Rabbit NZW 7days Thymic 2 6.6(4.6-8.7)
Splenic 2 9.3(9.2-9.4)
6 mo Peripheral 2 4.2(3.2-5.2)
Baboon Cynocephalus 5yr Peripheral 1 8.4
Man Caucasian 20-30yr Peripheral 2 5.6(3.6-7.7)
aAU
values are relative to the binding level ofthymic cells from 21-day-old BALB/c mice assayed inparallel. Cells (106n/ml)wereincubated for 60min at370C in the presence of 3 to4ngofR-MuLV[I251]gp7l.
Cell-boundradiolabelwasdeterminedasdescribed in thetext.The number ofreplicateculturesperexperiment
rangedfromtwo tofour. RBC,Erythrocytes.
24,1977 733
,11.
.0 I I
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.491.70.216.106.228.2] [image:5.491.46.447.316.617.2]734 FOWLER ET AL.
(NZB and M-55), on the other hand, failed to interfere with R-MuLV gp7l adsorption to lymphoidcells, indicating that mouse lymphoid cells, like mouse fibroblastic cells (4), contain a population of membrane receptors for ecotropic virus that have no demonstrable affinity for mouse xenotropic virus. This is further evi-denced by the highbinding affinity for R-MuLV gp7l exhibited by lymphoid cells from NZB mice, a strain known to produce high titers of xenotropic virus (20) and whose thymocytes ex-pressconsiderable endogenous xenotropic viral envelopeglycoproteinontheir cell surface (19). Similarly, thymocytes from BALB/c mice, re-centlyreported tonaturally express endogenous xenotropicviral envelope glycoprotein (3), also bindhighlevels of R-MuLVgp71.
Several differenceswereapparent in the rela-tivebinding capacityofcells from different or-gans and different strains of mice. Lymphoid
cellsderived from thespleen, the primary target organ of R-MuLV-induced erythrocytopoietic
disease, consistently exhibited a much greater capacity for binding R-MuLV gp7l than did either sperm cellsorerythrocytes.Thymic cells, by comparison, bound as much viral envelope glycoprotein as splenic cells, and thymic cells fromsomestrains bound
significantly
morethan spleniccells.Thehigh degreeofthymocyte bind-ing of R-MuLV gp7l is best explained by a cross-reactivity of murineecotropic viruses for the same cell surface receptors. Thus, thymo-cytes, the target cells for certain murineeco-tropic viruses,suchasMoloneyleukemiavirus,
contain receptors in vivo thatprobablybind R-MuLV and other murine ecotropic viruses
equally well. Indeed, murine ecotropic viruses havebeen shown touse thesame receptors on mousefibroblast cellsinvitro(2, 4, 24). Further-more, the observed reduced binding of R-MuLV
gp7lby AKRlymphoid cells,astrainknown to
synthesize endogenous ecotropic virus, is con-sistentwiththisinterpretation, since viral syn-thesis would result in in vivo saturation of the available cellular receptor sites forecotropic
vi-ruses.Inpreliminarystudies,wehave alsonoted
asignificant reductionof R-MuLVgp7l binding tolymphoid cells of BALB/c mice after exoge-nous infection with either R-MuLV or Friend leukemia virus(unpublished data).
The existenceof cross-reactive bindingsites
forR-MuLVenvelope glycoproteinoncells from
at least one other species, the rat, is also
sug-gestedfrom these data. Theextentof R-MuLV
gp7l bound byrat lymphoid cells, though low
compared with mouse lymphoid cells (20 to 30%), was consistently observed. This binding
appeared to be specific by competition
experi-mentsand isconsistent with the earlier
obser-vation that newbornrats arehighlysusceptible
toR-MuLV infection (23).
In thepresence of excess iodinated
glycopro-tein, thymocytes from BALB/c mice bind
ap-proximately2.7ng(38.5 x 1015mol)of R-MuLV per 106 cells. This isequivalentto anestimated binding level ofapproximately 2.4 x
10W
mole-cules perlymphoidcell.Bycomparison, murinefibroblasts-cellsofdifferent derivation and
ex-hibiting morphological characteristics widely
different fromlymphoidcells, includingalarger cellsurface area-bind5.3 X
10'
molecules percell(4), orapproximately20-fold that observed for thymocytes. Such estimates, however, are based on the assumption that all cells within thepopulation examined contain the same fam-ily orfamilies of receptors atequivalent numbers and thattheybind the sameamountof glyco-protein. These assumptions may be more valid with certain murine cell populations thanwith
others;certainly,the datapresentedhere do not rule out thepossibilitythat thebinding efficien-cies of specific lymphoid cell subpopulations vary, that the numbers of receptors per cell
differ,orthatmultiple receptorforms exist on
individualcells.Indeed,thedifference noted be-tween the relative binding level of thymocytes andsplenic cells from NZB and BALB/c mice suggests that one ormore of thesepossibilities islikelyin vivo. It shouldalso be notedthat,in addition to lymphoid cells serving as vehicles for virus adsorption and replication, immuno-cytes may also bind virus via antigen-specific receptors as part of the normal events leading toantiviralimmunity.Althoughantigen-binding cells are present in only low numbers before immunization, we cannot completely rule out their involvement in the binding studies per-formedhere;however,preliminary blocking ex-periments with anti-mouse immunoglobulins in-dicate that their participation is minor. Addi-tional studiesareneededto
clarify
these points andtodetermine the extent of cell type specific-ity and maturation level in the regulation of virus-cell receptor activity in vivo. Such infor-mation mayprovide insights into the mechanismof viralprotein-cell membrane interaction that
leads to the modification of cell behavior in immunity andoncogenesis.
ACKNOWLEDGMENT
Thisworkwassupported by Public Health Service contract
NO1-CO-254-23from theNational Cancer Institute.
LITERATURE CITED
1.Allen, P.T.,J. A.Mullins, G. A. Saviolakis,J. E. Strickland,A. K.Fowler, andA.Hellman. 1977. Direct isolation ofxenotropic retraviruses from the uterusofthe normaladult NIH Swissmouse.Virology
79:239-243.
2. Besmer, P., and D. Baltimore. 1977. Mechanism of J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
R-MuLV gp71 BINDING BY LYMPHOID CELLS
restriction of ecotropic andzenotropicmurineleukemia virusesand formation of pseudotypes between the two viruses. J. Virol. 21:965-973.
3. Cloyd, M. W., D. P. Bolognesi, andD. D. Bigner.
1977.Immunofluorescent analysis of expression of the RNA tumor virus major glycoprotein, gp7l, on the surfaces of normal murinecells. Cancer Res. 37:931-938. 4. DeLarco, J.,and G. J. Todaro. 1976. Membrane recep-tors for murineleukemia viruses: characterization using the purified viral envelope glycoprotein, gp7l. Cell 8:365-371.
5. Dent, P. B. 1972. Immunodepression by oncogenic
vi-ruses.Prog. Med. Virol. 14:1-35.
6. Fowler, A. K., N. M. Kouttab, P. D. Kind, J. E. Strick-land, and A. Hellman. 1975. Oncornaviral protein modulation in mouse uterine tissueby estrogen. Proc. Soc.Exp.Biol. Med. 148:14-18.
7. Fowler, A. K., C. D. Reed, G. J. Todaro, and A. Hellman. 1972. Activation of C-type RNA virus markers in mouse uterine tissue. Proc. Natl. Acad.Sci. U.S.A.69:2254-2257.
8. Fowler,A.K.,J. E.Strickland,N. M.Kouttab,and A. Hellman. 1977. RNA tumor virus expression in mouseuterinetissueduringpregnancy. Biol.Reprod. 16:344-348.
9. Greenwood,F.C.,W.M.Hunter, andJ.S.Glover.
1963. The preparation of13"I-labelled human growth
hormone of high specific activity. Biochem. J.
89:114-123.
10.Hayry,P.,D.Rago,andV.Defendi.1970.Inhibition ofphytohemagglutinin- and alloantigen-induced
lym-phocyte stimulation by Rauscher leukemia virus. J. Natl.Cancer Inst. 44:1311-1319.
11.Hellman, A.,and A. K. Fowler. 1971. Hormone-acti-vated expression of theC-type RNA tumor virus ge-nome.Nature(London)NewBiol.233:142-144. 12. Hellman,A.,A. K.Fowler, H. G.Steinman,and P.
M.Buzzerd.1972.Studies of theblastogenicresponse ofmurinelymphocyte.III.Specificviral transformation. Proc.Soc.Exp.Biol. Med. 141:106-109.
13. Hellman,A.,A.K.Fowler,J. E.Strickland,andN. M. Kouttab. 1976.Apossible physiological function for type C RNA viruses. Bibl. Haematol. (Basel)
43:161-165.
14. Hirsch,M.S.,P. H.Black, G. S.Tracy,S.Leibowitz,
andR.S. Schwartz.1970.Leukemia virus activation
in chronic allogenic disease. Proc. Natl. Acad. Sci. U.S.A. 67:1914-1917.
15.Huebner, R. J., P. S. Sarma, G. J. Kelloff, R. V.
Gilden,H.Meier,D. D.Myers,and R. LPeters.
1971. Immunological tolerance to RNA tumor virus genome expressions: significance to tolerance and pre-natal expressions in embryogenesis and tumorigenesis. Ann.N. Y.Acad. Sci. 181:246-271.
16.Joklik,W.K., and J. E. Darnell. 1961. The adsorption
andearlyfate ofpurifiedpoliovirus in Hela cells. Virol-ogy 13:439-447.
17.Kalter, S. S., R. L Heberling, R. J.Helmke, M. Pan-igel, G. C. Smith, D. C. Kraemer, A.HeIlman,A. K.Fowler,and J. E.Strickland.1975. A comparative study on the presence of C-type viral particles in pla-centas fromprimatesand otheranimals,p. 391401. In Y. Ito and R M. Dutcher(ed.),Comparativeleukemia
research.University of Tokyo Press, Tokyo.
18.Lee, J.C., andJ.N. Whle. 1977. Characterization of the blastogenic and cytotoxic responses of normal mice to ecotropicC-type viral gp7l. J. Immunol. 118:928-934. 19. Lerner,R.A., C. B. Wilson,B.C.DelVillano, P. J. McConahey, and F. J. Dixon. 1976. Endogenous
on-cornaviral geneexpression in adult and fetal mice: quan-titative,histologic,andphysiologic studiesof the major viral glycoprotein, gp7O. J. Exp. Med. 143:151-166. 20. Levy,J. A. 1973. Xenotropic viruses:murineleukemia
viruses associated with NIH Swiss, NZB, and other mousestrains.Science 182:1151-1153.
21. Levy,J.A.,P.Kazan,0. Varnier, andH.Kleiman.
1975.MurinexenotropictypeCvirusesI.Distribution andfurther characterization of the virus in NZB mice. J.Virol.16:844-853.
22. Notklas,A.L.,S. E.Mergenhagen,andR.J. Howard.
1970.Effectof virusinfections onthe function ofthe immunesystem. Annu. Rev.Microbiol. 24:525538.
23. Rauscher,F.1962.Avirus-induced disease of mice char-acterizedbyerythrocytopoiesisandlymphoid leukemia.
J.Nati.Cancer Inst.29:515-543.
24. Sarma,P.S., M.Cheong,J. W. Hartley, and R. J. Huebner. 1974. A viral interference test for mouse leukemiaviruses.Virology 33:180-184.
25.Strand, M., andJ. T.August.1976.Structuralproteins
of ribonucleic acidtumorviruses:purificationof enve-lope,core, and internal components.J. Biol. Chem.
251:559-564. VOL. 24,1977
on November 10, 2019 by guest
http://jvi.asm.org/