Vol.43, No. 2 JOURNALOFVIROLOGY, Aug. 1982, p. 756-760
0022-538X/82/080756-05$02.00/0
Synthesis of the
Viral
Glycoprotein
of Rous-Associated Virus-2
PAULAJ. ENRIETTO,t* ANTHONY F.PURCHIO,AND RAYMOND L. ERIKSON
Departmentof Pathology, School of Medicine, University of Colorado HealthSciencesCenter, Denver, Colorado 80262
Received21 December1981/Accepted 14April 1982
The synthesisof the viral glycoproteinof Rous-associated virus-2 was studied
in vitroinacell-freesystemprogrammedwith viral RNA andsupplementedwith
dog pancreas membranes. Theprotein synthesized was related structurally and
immunologically to those found in Rous-associated virus-2-infected chicken
embryo fibroblasts. This work confirms and extends earlier work on the nature
and synthesis ofviralglycoproteins.
The envelope (env) gene of avian leukemia
sarcoma viruses encodes theenvelope proteins
ofthevirion, gp85 and gp37, whicharelinkedby
disulfide bonds (7, 10, 24). These proteinshave
been shown tobe derived from a glycosylated
polyprotein precursor (molecular weight,
92,000),Pr92env(3, 10, 12, 14, 15), the
predomi-nant species found in the cell. Translation of
viralmRNAofvarious sizesallowed the
identifi-cationof themRNAforthe envgeneproductas
a molecule of21Sfor leukosis viruses and 28S
forsarcomaviruses(16, 17,22, 24).The
primary
product encoded by these mRNAs isaprotein of
65,000 (65K) to 70,000 daltons (70K), which is
specifically immunoprecipitated by antisera
raised against the envelopeglycoproteingp85.
The purpose of the experiments described
here was to study the
synthesis
andprocessing
of the viral
glycoprotein
in a cell-free systemprogrammed by viral RNA and supplemented
withdog pancreasmembranes inamanner
anal-ogous tothatdescribed
by
Katzetal.(11)
fortheglycoprotein
of vesicular stomatitis virus. Indoing so we wanted to
identify
theprotein
product of the 21S viral RNA from
Rous-associ-atedvirus-2(RAV-2)andRAV-2-infected
chick-en embryo fibroblasts(CEF) and relate it
struc-turallyandimmunologically tothe
predominant
form of the viral
glycoprotein
found in theinfectedcell, Pr92env. Inaddition,wewanted to
determinethe natureofthe
protein
synthesized
in a cell-free system supplemented with dog
pancreas membranes and determine its
relation-shiptoPr92en .
To this endpolyadenylated subgenomic
frag-mentsof RNAfromRAV-2and
polyadenylated
RNA from RAV-2-infected CEFwere
prepared
asdescribedby Purchioetal.(19)and sizedon5
t Present address: ImperialCancer Research Fund, Lon-don, England WC2A3PX.
to 20% neutral sucrose densitygradients. RNA
21S to 23S in size was collected in each case,
precipitated,and resedimented on a second 5 to
20% neutral sucrose gradient; it was then
pooled, ethanol precipitated, and prepared for
translation.
Translation reactions were carried out with
rabbit reticulocyte lysates (18) and were
pro-grammed with 21S RAV-2 RNA, prepared as
above, using either [35S]methionine or
[35S]cys-teine as a label as described by Purchio et al.
(19). As can be seenin Fig. 1 the predominant
protein synthesized by 21S RAV-2 RNA has a
molecular weight of approximately 64K, as
de-scribed by others (16, 17, 22). A small amount of
protein with a molecular weight of76K is also
synthesized and probably represents Pr76
pro-grammed bygag-gene mRNAcontaminatingthe
21S fractions obtained from the sucrosedensity
gradients.
In addition to the 76K-dalton and64K-dalton
proteins,
severalproteins
oflowermolecular
weight
were alsosynthesized
andprobablyrepresentproducts ofpremature
termi-nation of protein synthesis.
To determine whether a message21S in size
isolated from RAV-2-infected CEF could also
program the synthesis ofa protein of this size,
polyadenylated RNA wasisolatedfrom
RAV-2-infectedCEFby oligodeoxythymidylic
acid-cel-lulose column chromatography and sized on
sucrosedensitygradientsasdescribed
previous-ly (18). Using 21S RNA to program the rabbit
reticulocyte cell-free protein-synthesizing
sys-tem, it can be seen(Fig. 1) that aproteinof64K
daltons was synthesized along with a variety of
other proteins which probably represent
pro-teins encoded bycellular mRNAs21S insize.
Tocharacterize the primary product of these
translationreactions and to relate thisproduct to
the viral glycoprotein two experiments were
carried out. Inthefirstexperiment, theproduct
756
on November 10, 2019 by guest
http://jvi.asm.org/
viralRNAb 2Is
I- + Ip I-cell 215-s
_i
-64k
T7 1 2 3 4 5 6 7
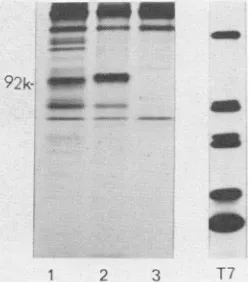
FIG. 1. Cell-free products programmed by21S
vi-ral RNA and 21S RAV-2-infected CEF RNA. Viral
21S RNA and polyadenylated 21S RNA from
RAV-2-infected CEFwere isolated as described previously (19) andusedtoprogram amessage-dependent rabbit reticulocyte lysate cell-free protein synthesizing sys-tem(18).Proteins synthesizedwereeither immunopre-cipitated asdescribedpreviouslyoranalyzed directly by sodium dodecyl sulfate-PAGE, fluorography, and autoradiography. Lanes 1 through4, proteins synthe-sized in thepresenceof21SRNAisolated from RAV-2. Lane 1, no exogenous RNA; lane 2, products programmedby 21SRNAfromRAV-2;lanes3and4,
immunoprecipitation of products programmed by 21S RAV-2RNAwithimmuneserum B(lane 3)ornormal rabbitserum(lane 4). The label used in these transla-tions was [35S]cysteine (25 ,uCi, Amersham Corp.).
Lanes 5 through 7, product synthesized in lysates programmed with 21S RNA from RAV-2-infected CEF; lane 5, total cell-free product; lanes 6 and 7, products immunoprecipitated with immune serum B
(lane 6)ornormal rabbitserum(lane7). The label used in these experiments was [35S]methionine (25 p.Ci,
AmershamCorp.).
ed by immunoprecipitationof labeled disrupted
virions (datanotshown) and by
immunoprecipi-tation of[35S]methionine-labeled
RAV-2-infect-ed CEF cell extracts. As can be seen in Fig. 2,
both serarecognized the polyprotein precursor
Pr92en , aswellas,in thecaseofimmune serum
A, Pr76, the precursor to theinternal structural
proteins. Little of the mature glycoproteins gp85
or gp37 was immunoprecipitated from these
cells. Pr92, immunoprecipitated by these two
sera, appeared tomigrate slightly differently by
polyacrylamide gel electrophoresis (PAGE), a
difference which appears to reflect the
specific-ity of theantisera fortwodifferent forms of Pr92
which vary in glycosylation and degree of
sulf-hydryl bonding (data notshown).
To show an immunological relationship
be-tween the proteins synthesized in vitro by 21S
viralorcellular RNA and theviralglycoprotein,
we dissolved cell-free product in RIPA lysis
buffer (9) and immunoprecipitated the product,
asdescribed previously (4), with immune serum
B ornormal serum. As can be seen inFig. 1, the
64K-dalton protein was specifically precipitated
from cell-free lysates programmed with viral or
cellular 21S RNA, using antiserum against the
viral glycoprotein, thus relating these proteins
immunologically.
To confirm thisrelationship, it wasnecessary
to show structural similarity between the
pro-tein synthesized in vitro, the 64K-dalton
pro-tein, and the predominant form of the
glycopro-tein found in vivo, Pr92env. This was
accomplishedby two-dimensional chymotryptic
-S
ofcell-free translation was related to the viral
glycoprotein
immunologically,
and inthe secondexperiment, theproductwasrelatedstructurally
bypeptidemapping. Bothexperiments reliedon
the availability of antisera capable of
recogniz-ing the viral glycoproteinand, in this case, the
polyprotein
precursor Pr92env. Two types ofantisera were used in these experiments.
Im-mune serum A was prepared by
injecting
8- to10-week-old New Zealand white rabbits with
500,ug of RAV-2viruswhich had beenprepared
asfollows. The virus was harvested every 12 h
from RAV-2-infected CEF, clarified at 10,000
rpm, pelleted onto apadof70%sucrose in 0.01
MTris(pH7.2),andspun on a sucrosegradient
(20 to70%)in 0.01MTris(pH7.2)at26,000rpm
for 15 h. Injections were given a total of four
times at 10-day intervals. Immune serum B, a
generous gift of D. P. Bolognesi, was prepared
againstthepurifiedviral glycoprotein gp85 (21).
The specificities ofthese sera were
demonstrat-92k- O
-1 2 3 T1
FIG. 2. Immunoprecipitation of RAV-2-infected CEFwithimmuneseraAand B.RAV-2-infected CEF
were labeled with 150 ,uCi of [35S]methionine and
immunoprecipitated as previously described (4).
Im-munoprecipitatedproteinswereanalyzedon10%
sodi-um dodecyl sulfate-polyacrylamide gels (13), which
were soaked for30min in a 1 M solution ofsalicylic acid (5),dried,andexposedat-70°C,usingKodak X-omat X-ray film. Lane 1, immune serum A; lane 2, immuneserumB; lane 3, normalserum.
so
..
4.0iomil.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.491.43.243.77.231.2] [image:2.491.289.415.435.577.2]758 NOTES
peptide fingerprint analysis of 64K-dalton and
Pr92env proteins immunoprecipitated from
[35S]methionine-labeled RAV-2-infected CEF,
using immune serum A. Peptide mapping was
carried out onthe [35S]methionine-labeled
pro-2
3 A
teins essentially as described by Erikson etal.
(8). As canbeseeninFig. 3 (A and B) all of the
major92K-daltonpeptides could be found in the
64K-dalton protein, with five peptides in
com-mon. Itisapparentthat the64K-dalton
protein
is02 B
4
*w
2 43
i?
2 C
3
5
2
FIG. 3. Chymotryptic peptidemapsof92K-dalton,64K-dalton,and mixedproteins. (A)RAV-2-infected CEF
were labeledfor2 h with150 jiCi of[35S]methionine, immunoprecipitated as described previously (21) with
immune serum A, and analyzed by PAGE. The 92K-dalton protein was localized by autoradiography and
prepared for analysis by two-dimensional chymotryptic peptide mappingasdescribedpreviously (8). Proteins to
be analyzedwere digestedatanenzyme/protein ratio of 1:24, using chymotrypsin (Worthington Biochemical Corp.) and were separated in the first dimension by ascending chromatography in 20 butanol-N-propanol-isoamyl alcohol-pyridine-water (1:1:1:3:3). Separationinthe second dimensionwasby high-voltage
electropho-resisatpH 3.5.(B)[35S]methionine-labeledcell-free product from translation reactionsprogrammed with viral
21SRNA wasanalyzed by sodium dodecyl sulfate-PAGE. The 64K-dalton proteinwaslocalizedby autoradiog-raphy and analyzedasdescribed above. (C) Equalnumbersofcounts of 64K-dalton and 92K-daltonproteins were prepared and digested as described above. These were mixed and subjected to chromatography and
electrophoresis. Ineachcasesharedpeptidesarenumbered.
..s^:^:
s8..:'&.H<-J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.491.55.457.145.570.2]VOL. 43, 1982
subject to moreextensive cleavage than the
92K-dalton protein, possibly because of the
differ-ence in glycosylation; the 64K-dalton protein
was unglycosylated under these circumstances.
Amixingexperiment (Fig. 3C)wasthencarried
out in which equal numbers of methionine
counts of 92K and 64K-daltons, digested by
chymotrypsin, were analyzed. All numbered
peptides appeared to comigrate. This analysis
then relates the 64K-dalton protein to Pr92eM '
structurally as well asimmunologically.
Having characterizedthe primary product of
RAV-2 viral and cellular 21S RNA and having
shown its relationship to the primary product
found in the infected cell, the glycosylated
Pr92en', it was of interest to study theprocessing
of primary product to the precursor pM92en'.
Analogousstudiescarriedoutby Katz et al. (11)
provided an in vitro model system in whichthis
could be accomplished. In this system, dog
pancreas microsomal membranes, added to the
cell-free protein-synthesizing system, provide
the enzymemachinery required forthe
process-ing (i.e., glycosylation) ofglycoproteins, ashas
been described inavariety ofsystems(1,2, 11).
Rough endoplasmic reticulum vesicles were
isolated from dog pancreas, essentially as
de-scribed byKatzetal. (11). Thesewereaddedto
thereticulocyte cell-freesystembefore the
addi-tion ofRAV-2 viral RNA 21S in size and were
present during translation. Proteins synthesized
in the presence and absence ofpancreas
micro-somalmembranes wereanalyzed and, ascanbe
seen inFig. 4, a64K-daltonproteinaswellas a
92K-daltonprotein was synthesizedin the
pres-ence of membranes. In the absence of
mem-branesonly the 64K-dalton proteinwas
synthe-sized(Fig. 1).
Because proteins synthesized and processed
in the presence of the microsomal membrane
fractionappear tobetransferred intothe lumen
of the membrane vesicles, they are protected
fromdigestion byenzymes suchastrypsin(11).
To determine whether any ofthe proteins
syn-thesized inoursystemwere resistant totrypsin
treatment aftertranslation, partofthe reaction
mixture was treated with trypsin as described
previously (11). After incubation, the products
of the translation reactions carried out in the
presence orabsence of membranes andwith or
without trypsin treatment were analyzed. Only
the 92K-dalton protein and a protein
endoge-nous tothecell-free systemwereprotectedfrom
trypsin digestion, suggestingthat the 92K-dalton
protein had been sequestered within the lumen
of the microsomal membrane vesicles(Fig. 4A).
Todetermine whether the 92K-dalton protein
synthesized in the reticulocyte cell-free system
programmed with 21S RAV-2 RNA was
glyco-sylated, its bindingto columns of lens culinaris
coupled to Sepharose was tested. Binding
oc-curred, but elution of the protein with
CX-D-methyl-mannopyranoside could not be
accom-plished. Thus, it was not possible to say
conclusively that the 92K-dalton protein
synthe-sized in our system wasglycosylated. We were
able to demonstrate, however, that the protein
synthesized inthissystemwasimmunologically
related to the 92K-dalton protein precipitable
from RAV-2-infected cells.
Immunoprecipita-tion ofcell-free product from translation
reac-tions carried out in the presence of membranes
wasdonewith immune serum B and with normal
serum. As canbeseeninFig. 4B,92K- and
64K-daltonproteinsareimmunoprecipitablewith
im-mune serum. Otherproteins of lower molecular
weight which were specifically
immunoprecipi-tated arethought to beeitherpremature
termina-tion productsorproteolytic cleavages of64K-or
92K-daltonproteins. Inaddition,the92K-dalton
protein, synthesizedinvitro and
immunoprecip-itated, could be shown to comigrate with the
92K-daltonproteinimmunoprecipitated with
im-mune serum B from the infected cell (datanot
shown).
From thework described here it is clearthat,
A
21s RNA+ MEM
92k-
-P B
92k
-we
Uo
64k- _
2
2 3
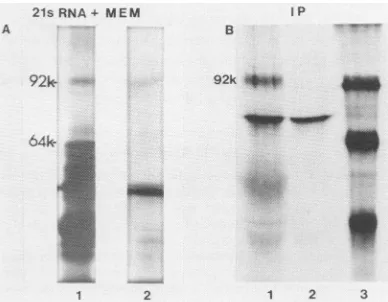
FIG. 4. Translation and immunoprecipitation of
21S viral mRNA in the presence of dog pancreas
microsomal membranes.(A)Viral mRNA 21S in size was used to program a message-dependent rabbit
reticulocyte lysate supplemented with dog pancreas
microsomal membranes andprepared asdescribedby Katz etal. (11). The label used in these experiments
was[35S]methionine(25 ,uCi, Amersham Corp.). Lane
1, total cell-free product synthesized in the presence of
dog microsomal membranes. Lane 2, total cell-free
productsynthesized in the presence of dog microsom-al membranes and treated after translation with
pan-creatic RNase A (1 ,ul of a 300-,ug/ml solution) and
trypsin(5,ulof a3-mg/ml solution) for 20 min at 23°C. (B) Cell-free product synthesized in the presence of
dog microsomal membranes, without trypsin treat-mentand immunoprecipitated with immune serum B
(lane 1) or normal serum(lane 2). Lane 3,
molecular-weightmarkers.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.491.254.448.351.502.2]760 NOTES
invitro, the primaryproduct of RAV-2 viral and
cellular mRNAs is a protein of64K molecular
weight whichcould be related immunologically
and structurally tothe polyproteinprecursor to
the mature viral glycoproteins Pr92enV. This
work confirms andextends the results of others
(16, 17, 22). Inaddition,we wereableto
demon-stratethesynthesis ofaprotein of92,000daltons
in rabbit reticulocyte lysates programmed with
21S RNA from RAV-2 supplemented with dog
pancreas microsomal membranes. This protein
could be relatedimmunologically tothe
precur-sor Pr92eMY, precipitable from RAV-2-infected
CEF. These results imply that the 64K-dalton
primary product is processed by microsomal
membranes during its synthesis to give rise to
the glycosylatedprecursor, Pr92enV, which isthe
predominant form of the viral glycoprotein
found in the infected cells (3, 12). In vivo, the
64K-dalton proteincan only beseenunder
con-ditions which inhibit glycosylation, such as
growthintunicamycin(6,23),lendingsupport to
the notion that processing of the 64K-dalton
proteininvitromimicsthe process
occurring
invivo. Moreevidenceto support thishypothesis
comes from the recent work of Purchio et al.
(20), in which they demonstratedthat the
64K-daltonprotein (and by extension the 92K-dalton
protein) was synthesized exclusively on
mem-brane-boundpolyribosomes. Further
analysis
ofthis in vivo
processing
shouldclarify
thepath-way by which this glycoprotein is
synthesized
and inserted into the cell membrane.
LITERATURECITED
1. Blobel, G.,and B.Dobberstein.1975.Transfer ofproteins across membranes. I. Presence ofproteolytically proc-essed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myelo-ma.J.Cell. Biol. 67:835-851.
2. Blobel, G., andB.Dobberstein. 1975.Transfer ofproteins acrossmembranes. II.Reconstruction of functionalrough microsomes from heterologous components. J. Cell. Biol. 67:852-862.
3.Buchhagen, D. L., and H. Hanafusa. 1978. Intracellular precursorstothemajor glycoproteinof avian oncoviruses inchicken embryofibroblasts. J. Virol. 25:845-851. 4. Brugge, J.S.,and R. L.Erikson. 1977.Identification ofa
transformationspecific antigen induced byanavian sarco-mavirus. Nature (London) 269:346-348.
5. Chamberlin,W.1979.Fluorographicdetection of radioac-tivity in polyacrylamide gels with thewatersolublefluor,
sodiumsalicylate. Anal. Biochem. 98:132-135. 6. Diggelmann,H.1979. Biosynthesisofanunglycosylated
envelopeglycoproteinofRous sarcoma virusinthe pres-enceoftunicamycin.J.Virol.30:799-804.
7. Duesberg, P. H., G. S. Martin, andP. K. Vogt. 1970.
Glycoprotein components of avian and murineRNA tu-mor viruses.Virology 41:631-646.
8. Erikson, E., J. S. Brugge, and R. L. Erikson. 1977. Phosphorylated and nonphosphorylated forms of avian sarcomaviruspolypeptide p19. Virology 80:177-185. 9. Gilead, Z., U.-H. Jeng, W. S. M. Wold, K. Sugawara,
H. M.Rho, M. L. Harter,andM.Green. 1976. Immuno-logical identification of two adenovirus 2-induced early proteinspossibly involved in cell transformation. Nature (London) 264:263-266.
10. Halpern, M. S., D. P.Bolognesi, and C. J. Lewandowski. 1974.Isolationof major viral glycoprotein andaputative precursorfrom cells transformed by avian sarcoma virus-es.Proc. Natl. Acad.Sci. U.S.A. 71:2342-2346. 11. Katz, F. N., J. E. Rothman, V. R. Lingappa, G. Bobel, and
H. F. Lodish.1977. Membraneassemblyin vitro: synthe-sis, glycosylation, andassymmetricinsertionofa trans-membrane protein. Proc. Nati. Acad. Sci. U.S.A. 74:3278-3282.
12. Klemenz, R., and H.Diggelmann. 1978. Thegeneration of the twoenvelope glycoproteins of Rous sarcoma virus froma commonprecursorpolypeptide. Virology 85:63-74.
13. Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685.
14. Moelling, K., and M.Hayami. 1977. Analysis of precur-sors totheenvelopeglycoproteins of avian RNA tumor viruses in chicken and quail cells. J. Virol. 22:598-607. 15. Mosser, A. G., R. C.Montelaro, and R. R. Rueckert. 1977.
Proteins of Rous-associated virus type 61: polypeptide stoichiometry and evidence that gp35 is not a cleavage product of gp85. J. Virol. 23:10-19.
16. Pawson, T., R. Harvey, and A. E. Smith. 1977. The size of Rous sarcoma virus RNA active in cell free translation. Nature(London) 268:416-419.
17. Pawson, T.,P.Mellon, P. H. Duesberg, and G. S. Martin. 1980. env gene of Rous sarcoma virus: identification of the geneproduct by cell-free translation. J. Virol. 33:993-1003.
18. Pelham, H. R. B., and R. J. Jackson. 1976. An efficient mRNA dependenttranslation system from reticulocyte lysate.Eur. J. Biochem. 67:247-256.
19. Purchio, A. F., E. Erikson, and R. L. Erikson. 1977. Translation of35sandsubgenomic regions of avian sarco-mavirus RNA. Proc. Natl. Acad. Sci. U.S.A. 74:4661-4665.
20. Purchio A. F., S. Jovanovich, and R. L. Erikson. 1980. Sites ofsynthesis of viral proteins in aviansarcoma virus-infected chick cells. J. Virol.35:629-636.
21. Rohrschneider, L.,H.Bauer, and D. P.Bolognesi.1975. Group-specific antigenic determinants of the larger enve-lope glycoprotein of avian oncornaviruses. Virology 67:234-241.
22. Stacey, D. W., V. G. Allfrey, and H. Hanafusa. 1977. Microinjection analysisofenvelope-glycoprotein messen-ger activitiesofavian leukosis viralRNAs. Proc. NatI. Acad. Sci. U.S.A. 74:1614-1618.
23. Stohere, R., and E. Hunter. 1979. Inhibition of Rous sarcomavirusreplication by2-deoxyglucose and tunica-mycin:identification ofanunglycosylatedenvgene prod-uct.J.Virol. 32:412-419.
24. VanZaane, D., A. L. J. Gielkens, W. G. Hesselink,and H. P.J. Bloemers.1977.Identification ofRauschermurine leukemiavirus-specificmRNAsfor thesynthesisof gag-and-env-gene products. Proc. Natl. Acad. Sci. U.S.A. 74:1855-1859.
J. VIROL.