0022-538X/83/061061-05$02.00/0
Copyright© 1983,AmericanSocietyfor Microbiology
Stoichiometry of
Large T
Antigen and pp53 in Complexes
Isolated from Simian Virus 40-Transformed Rat Cells
MARTINI. FREED, IRA LUBIN, AND DANIEL T. SIMMONS*
Schoolof Lifeand Health Sciences, Universityof Delaware, Newark, Delaware 19711
Received 30 December1982/Accepted 17March1983
Simian virus40-transformed cellssynthesize high-molecular-weight protein com-plexes (22to30S) that consist of the virus-coded large T antigen (81,500 daltons) and the cellular antigen pp53. These complexes were partially purified from lysatesof transformedratcells bysucrosevelocity sedimentation. The stoichiom-etry of the two proteins in the complex was studied by direct enzyme-linked immunosorbent assays, using alkaline phosphatase-conjugated T and anti-pp53 monoclonal antibodies.The resultsfromtheseexperiments indicate that the Tantigen-to-pp53 ratio in the complex is 0.87 ±0.27. Nostatistically significant differenceswerefound in this ratio for faster- andslower-sedimenting complexes. These results fromenzyme-linkedimmunosorbentassaysandprevious molecular weightestimates of the complex suggest that this complexis composed, on the
average, of four molecules ofTantigen and fourorfive molecules of pp53.
Cells transformed with simian virus40(SV40) containaproteincomplexmade upofthe virus-coded T antigen and a cellular phosphoprotein named pp53 (12,16). Thiscomplex sedimentsat
22 to 30S (7, 16) andappears to be resistant to
heat, partialreduction (9, 12), highsalt concen-trations (9, 15), and incubation with chelating
agentsandnonionic detergents (9). The cellular
pp53 protein has a molecular weight of50,000
(50K)to56K(dependinguponthe speciesfrom
which it is isolated) and wasoriginally detected inlysates ofSV40-transformedcellsby immuno-precipitation with hamster anti-T serum(3, 11-13,17, 24). Cells infected with SV40 (9, 17, 23) andsomelinesof normal, uninfected cells (5, 13, 22, 24) also accumulatepp53, although thelevels of the proteinaregenerallylower than in
trans-formed cells. Substantialamountsoftheprotein are made in cellstransformed with other papo-vaviruses (12, 22), adenovirus (21), retroviruses (4, 20), orchemicals (4). Interestingly, the ade-novirus tumorantigen (Elb product) (21) binds to
pp53
incells transformedbythese viruses. In SV40-transformed and -infectedcells, nearlyall ofthe pp53 is complexed with T antigen (7-9,16), and in some transformed cell lines that
produce largeamountsofpp53, nearlyallofthe T antigen is present in this complex (7; this report).
Thefunction ofthecomplex in the cell is not known,andlittleis known about its structure. In previous work, ithas not beenpossibletoobtain areliable estimate ofthe ratio ofT antigen to
pp53 molecules in this aggregate (7, 9,15,16). In
the present study, we examined the
stoichiom-etry of the two proteins in the complex by di-rect enzyme-linked immunosorbent assays (ELISAs). We chose to use ELISAs because this method isverysensitive and givesaccurate estimates ofantigen (and antibody) concentra-tions(25). Furthermore, it circumvents the diffi-culties inherent inestimatingthe levels ofthese
two proteins in the cell from labeling
experi-ments(1).
It was firstnecessary to find aline of
SV40-transformed cells in which the majority of theT
antigenandpp53arecomplexedto oneanother.
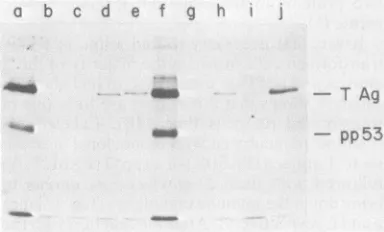
Figure1 shows thatthis is thecaseforalineof
transformed rat cells (line 14B). Labeled
ex-tracts wereincubated with monoclonal
antibod-iesto Tantigen(PAblOl)or topp53 (PAb122)(8)
followed with fixed Staphylococcus aureus to
bring down the immune complexes (Fig.1,lanes aandf, respectively). After the reaction withthe first antibody, the cleared supernatants were incubated with eithernonimmune medium as a control (Fig. 1, lanes b andg), PAblOl (Fig. 1, lanescandh),PAb122(Fig. 1,lanes d andi),or hamsteranti-tumorserum(Fig.1,laneseandj). Theprecipitated proteins were subjectedto so-diumdodecyl sulfate-acrylamide gel electropho-resis(23),and thelabeledproteinsweredetected
in thegel by fluorography at -80°C. The X-ray
film was scannedwith a densitomerto obtain a quantitative estimate of the intensity of each band (data not shown). PAblOl (anti-T) and PAb122 (anti-pp53) precipitated both Tantigen
and pp53 from the cell extracts (Fig. 1, lanes a
and f). Greater than 95% of the pp53 in the
extract wasinitially precipitatedwithPAblO1;a
1061
on November 10, 2019 by guest
http://jvi.asm.org/
very small fraction remained it camedown withPAb122(compa a and d). This indicated that ne
pp53 was complexed to T
antil
about85% of the labeled Tantige
was initially precipitated with P
mainder (15%) being brought d( tumorserum(compare Fig. 1,lano indicated thatalarge proportionc in this cell line was complexec additional point is that about !
antigenin the extractwasrecogni;
(compare Fig. 1,lanesaande),sh
monoclonal antibody binds to r
notall)of the Tantigen molecules
with the results ofGurney et al. To carry out the ELISAs, mc bodies PAblOl and PAb122 were
hybridoma medium by protein
(Pharmacia Fine Chemicals) cl
accordingtothe method describe
(6) exceptthat3MNaSCNwas u
antibodies.PAblOl andPAb122z
types immunoglobulinG2a and G
ly (Hybridoma Profiles, August
CancerInstitute).The purified ml
bodieswereconjugatedtobovine
phatase (Sigma Chemical Co.; t:
cordingto the method of Voller(
same amountof each antibody (1
a b cd ef g h
_ _
FIG. 1. ImmunoprecipitationofI and pp53 from SV40-transformed
transformed rat(line 14B) cells wer(
with [35S]methionineataconcentrati
The cells werelysed, andimmunopi tionswerecarriedoutinantibodyexc
describedby Gurneyetal. (8). The reacted witheitherPAblOl(lane a)o0 The supernatants (lanes b-e for PA PAb122)wereused in asecond imrr with nonimmune medium (lanes b (lanes c andh), PAb122 (lanes d ar anti-tumor serum (lanes e and j). proteinsweredenatured with sodiun andappliedtoasodiumdodecylsulfa (23). The labeledproteinsin thegeli
fluorography.
n solution and pledto2.5mgofenzyme(900 U/mg)sothatthe
reFig. 1,lanes two conjugated antibodies had similar specific
-arly all of the activities. Extracts of SV40-transformed rat
gen.
Similarly,
cells weresubjected
to sedimentationthrough
nin theextract gradients of5to20% sucrosetopartiallypurify
Ab122, the re- thecomplexes. Gradientfractions were
collect-own with anti- ed, and serial twofold dilutions (starting with
esf andj).This 1:4)weremade inprechilled disposable,96-well Afthe Tantigen (flat-bottomed) polystyrene microtiter plates
I to pp53. An (Linbro)with thecoating buffer of Volleretal.
90% of the T (25) as the diluent. The plates were incubated zed
by
PAblOl overnight at 4°C and washed three times withLowing
that this buffer A (0.05 M Tris-hydrochloride [pH 7.8],nost (although 0.001 M MgCl2, 1% bovine serum albumin,
s,inagreement 0.01% NaN3) (10). After these washes,200 ,ulof
(8).
alkaline phosphatase-conjugated PAblOl or )noclonal anti- PAb122immunoglobulin G(1.25 ,ug ofantibodye purifiedfrom permlin bufferA)wasaddedtoeach well.After
A-Sepharose
an overnightincubation at 4°C, the plateswerehromatography extensivelywashedwithphosphate-buffered
sa-edby Ey etal. line(0.0027 MKCI, 0.0015 M KH2PO4,0.14M
sedtoelute the NaCl, 0.02 M Na2HPO4 [pH 7.4]) containing
areof
antibody
0.05%Tween20(25), and 200puloffreshlymadei2b, respective-
substrate solution (1 mgofp-nitrophenylphos-1982,
National phate [Sigma] per ml in 9.7% diethanolamine, onoclonal anti- 0.0049 M MgCl2, 0.02% NaN3 [pH 9.8]) was alkalinephos- addedtoeach well.Theplateswereincubated in ypeVII-T)
ac- the dark at room temperature, and theabsor-etal.
(25).
The banceat405 nmwas measured withan MR580 I mg) wascou- Microelisa Autoreader (Dynatech Instruments). Thereactionswereallowedtocontinue untilan absorbance ofgreaterthan 1.0 was obtained in themostactive sample.Figure 2 shows a plot of enzyme activity
versus fraction number for the reactions with anti-Tand
anti-pp53
antibodies. Thetwoprofiles - T A are similar, peaking around fractions 7 or 8.9 Althoughthepeaksappeartobequite broad,the
trueantigen distribution is undoubtedly
quanti-- 53
tatively
different because, under thesecondi-tions,
a twofold drop in antigen concentration doesnotresult inatwofolddropinactivity(seebelow).
The peak fractions correspond to asedimentation coefficient of 27 to 29S. This
value lies within estimates of the sedimentation
ratcellsn (STVA)
coefficientof thecomplexobtainedbyothers(2,r
ateled
for 3 h 7,16)andbyus(datanotshown)asdeterminedion of 20
uCi/mlr
by immunoprecipitation reactions of gradientrecipitationreac- fractions with anti-T and
anti-pp53
antibodies.:essessentiallyas Thenearcoincidence of the anti-T andanti-pp53
lysates
werefirst reactionprofilesinFig. 2indicatesthat the two rPAb122(lane f). antigens are similarly distributed in the gradient.blO1and g-jfor Since the majority of the T antigen and pp53
rlunoprecipitation molecules are complexed to one another in this
and g), PAblOl cellline(Fig. 1),it is reasonableto assumethat
The precipitated the profilesinFig. 2 are, for the most part, those
Thdodecyl sulfate forT antigen and
pp53
in complexed form.Lte-acrylamide gel To determine the molar ratio of T antigento
weredetectedby
pp53
in thecomplex, itwasnecessarytocalcu-late the relative antigen concentration of these
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.488.53.245.398.514.2]0
w 0 w
ct
0
1.2 I.0
0.8 0.6 0.4 0.2
0.0
2 4 6 8 10 12 14 16 18 20 FRACTION
FIG. 2. ELISA of T antigen-pp53 complexes. SV40-transformed rat (line 14B)cells were grownto confluency in roller bottles. The cells were washed
three times with ice-cold phosphate-buffered saline and lysed in 4ml of0.01 M Tris-hydrochloride (pH 9.0)-0.14M NaCI-0.001 M dithiothreitol-0.5% Noni-det P-40. The lysatewasclarified by centrifugation at 4°Cfor10minat16,000xgandconcentratedto0.6 ml by dialysis against dry Sephadex beads at 4°C. The concentratewaslayeredon a5to20% sucrose
gradi-ent in 0.01 M Tris-hydrochloride (pH 7.4)-0.14 M NaCI-0.001Mdithiothreitol and centrifugedat21,200 rpm for 15 h at 4°C in a Beckman SW41 rotor. Fractions (0.6 ml) were collected in the cold and assayed with enzyme-conjugated anti-T (0) and anti-pp53 (0) antibodies as described in the text. The plotted absorbances at 405 nm (A405) were derived fromassaysofa1:8 dilution of each fraction andare
correctedbytheplatereader forextraneousnoise due
todirt orocclusions ontheplastic. Sedimentation is
fromrighttoleft, and the position of HeLa cellrRNAs isnotedatthetop.
twoproteins in various fractions of the gradient.
These valueswereestimated by plotting
absorb-ance (enzyme activity) versus antigen dilution
for the fractions (Fractions 6 through 11)
con-taining most of the complex (Fig. 3). These
curves describe the reactions of T antigen and pp53 with their corresponding monoclonal
anti-bodies and, for most of these fractions, are
sigmoidal in shape. To estimate relative antigen
concentrations, the point on the curve that is
mostsensitivetochanges in antigen
concentra-tion was determined. This point is called the
50% point and can be used as an estimate of
antigen concentration. It is definedasthat point
which is halfway between the reaction plateau
values athigh and low antigen concentrations.
The low plateau values were obtained directly
from the figure. The high plateau values were
estimated by extrapolation of thecurvesshown
in Fig. 3. These extrapolations were somewhat
difficultfor fraction 9 andarelikelytobe slightly
different from the true values. However, the
anti-T and anti-pp53 reaction curves for this
fraction are nearly superimposable, and
there-fore theirplateau valuesareprobablyvery
simi-lar. Table 1 lists the relative antigen.
concentra-tions of both T antigen and pp53 at the
calculated 50% point for each of fractions 6
through 11 of the complex peak. The ratio of
these two values is also shown in Table 1 and
represents the deduced T antigen-to-pp53 ratio
in each of these six fractions. The average
antigen ratio (and hence molar ratio since the
antibodies are monoclonal) is 0.87 ± 0.27.
Ex-tending these calculations for the fractions
be-yond 6 and 11 had little effect on the average
molar ratio. Sincenoconsistent differenceswere
observed between the calculated molar ratios in
differentfractions, itappearsthat sedimentation
did not separate complexes with different
rela-tiveamounts of Tantigen and pp53.
Indetermining theaccuracyof the calculated
Tantigen-to-pp53 ratio,weshould consider that
someof the anti-Tactivity (upto15%) shownin
Fig. 2 may be due to a reaction with small
amounts of uncomplexed T antigen (Fig. 1)
present in fractions 6 through 11. This point
alone might indicate that the calculated ratio is
toohigh. However, notall(about 90%) ofthe T
antigen molecules are recognized by PAblOl
(see above), and consequently the T antigen
concentration measuredby ELISAissomewhat
lower than it should be. This would have an
approximately equal effectin theopposite
direc-tion, and thereforewethink thatavalue of 0.87
± 0.27 isreasonablyaccuratefor this ratio. The
simplest interpretation of these data is that T
antigen andppS3are, ontheaverage,present in
approximately a one-to-one molar ratio in the
complex.Thisinterpretationdoesassume,
how-ever,that the two proteinsof thecomplex are, on the average, equally available for antibody
binding. This assumption is not unreasonable
because PAblOl and PAb122 precipitate the
complex with about equal efficiency
(unpub-lished data).
It is possible to obtain an independent
esti-mate of the composition of the complex from
published data. Bradleyetal.(2) determinedthe
molecular weights of monomers, dimers, and
tetramersofTantigen by gel exclusion
chroma-tography. The values they obtained
[image:3.488.45.239.59.189.2]corre-sponded, within ±10%,to the values estimated
TABLE 1. Calculation of Tantigen/pp53ratios
Relativeantigenconcn
Fraction
no. T antigen pp53 Tantigen/
pp53a
6 1/28 1/24 0.85
7 1/27 1/30 1.11
8 1/22 1/20.5 0.93
9 1/16 1/12 0.75
10 1/46 1/19.5 0.42
11 1/23.5 1/27.5 1.17
aAverage =0.87+ 0.27.
28S 18S 4S
4
4
4
46,
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.488.252.445.569.666.2]0 O
0
S
1.20
FRACTION 9 | FRACTION 10 FRACTION II0 0.
80.8
-L
0 0
0.4-1/10 1/100 1/1000 1/10 1/100 1/1000 1/10 1/100 1/1000
ANTIGEN
CONCENTRATION
FIG. 3. Antigen dilution curves of ELISAs of T antigen-pp53 complexes. Cell lysateswere subjected to sedimentation in sucrose gradientsasdescribed in the legend to Fig. 1. Fractions were collected and tested by ELISAs with monoclonal anti-T(O)andanti-pp53(0)antibodies asdescribedin the text. Corrected absorbances at405 nm(A405)wereplotted versus antigen concentration for fractions 6 through 11 of the gradient.
directly from sedimentation coefficients. With
their values for the sedimentation coefficients of
theTantigen-pp53complex and of theTantigen
tetramer, the relationship described by Martin
and Ames (14) gives a calculated molecular weight of 560K ± 10% for the complex. This calculation makes the assumption that the mo-lecular weight values of the complex as deter-minedby gel exclusion and by sedimentationare within 10% of one another. Second, it seems likely that the complex contains a tetramerofT
antigen, becauseMeyersetal. (18) have
report-ed that the tetrameric form of the T antigen analog, D2T, preferentially binds, in vitro, to
SV40 DNA at the origin of replication, and
Reich and Levine (19) recently have shown that
the T antigen-pp53 complex displays the same
SV40 DNA-binding specificity as uncomplexed
(withoutpp53) Tantigen. Consequently, a com-plex with an approximate molecular weight of
560K andtetramericTantigencomponentmight
beexpectedtocontain fourorfive molecules of pp53(basedonmolecularweights of81.5KforT antigenand 53Kforpp53).
Thedeductionsmade above about the compo-sition of the complex and our own estimates derivedfrom ELISAresults areentirely
consis-tent with one another. That four molecules of
pp53are associatedwith theprotein complex is supported by observations of McCormicketal.
(15) which indicate that the pp53 isolated from F9embryonalcarcinoma cells is inatetrameric form and binds to purified T antigen in vitro. However, a complex consisting of four mole-cules ofT antigen and five molecules of pp53
cannotbeexcluded. Althoughnotenough
infor-mationis availabletopredicttheactual arrange-ment of the individual protein subunits, addi-tional information on the structure of the
complex mightprovide insights into its function
in thecell and itspossible role in cellular trans-formation with SV40 and otherpapovaviruses.
We thank E. Gurney for the hybridoma cells and David Usherforhelpfulsuggestions.
Thiswork was supported by Public Health Service grant CA25942 from the National Cancer Institute.
LITERATURECITED
1. Benchimol, S., D. Pim,and L. Crawford. 1982. Radio-immunoassay of the cellular protein p53 in mouseand humancelllines. EMBO J. 1:1055-1062.
2. Bradley,M.K., J.D.Griffin,andD. M.Livingston. 1982. Relationship ofoligomerizationtoenzymaticand DNA-bindingpropertiesofSV40largeTantigen.Cell 28:125-134.
3. Chang, C.,D. T.Simmons,M. A.Martin,and P.T. Mora. 1979. Identification and partial characterization ofnew
antigens from simian virus 40-transformedmousecells.J. Virol. 31:463-471.
4.DeLeo,A.B.,G.Jay,E.Apella,G. C.Dubois,L. W.Law, andL.J.Old.1979.Detection ofatransformation-related antigen inchemicallyinducedsarcomasand other
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.488.52.449.58.316.2]formedcellsof the mouse. Proc. Natl. Acad.Sci. U.S.A.
76:2420-2424.
5. Dippold, W. G., G. Jay, A. B. DeLeo,G. Khoury, and L. J. Old. 1981. p53 transformation-relatedprotein: detection by monoclonalantibody in mouse and human cells. Proc. NatI. Acad. Sci. U.S.A. 78:1695-1699.
6. Ey, P. L., S. J. Prowse, and C. R. Jenkin. 1978.Isolation of pure IgG1, IgG2, and IgG,b immunoglobulins from mouse serumusing proteinA-Sepharose. Immunochemis-try 15:429-436.
7. Greenspan, D.S.,andR. B.Carroll. 1981. Complexof
simianvirus 49 largetumorantigenand48,000-dalton host tumorantigen. Proc. Natl. Acad. Sci. U.S.A. 78:105-109. 8. Gurney, E. G., R. 0. Harrison, and J. Fenno. 1980. Monoclonal antibodiesagainst simian virus 40 T antigens: evidencefor distinct subclasses of large T antigen and for similarities among nonviral Tantigens.J. Virol. 34:752-763.
9. Harlow, E., D. C. Pim, and L. V. Crawford. 1981.
Complex of simian virus 40 large-T antigen and host
53,000-molecular-weight proteininmonkeycells. J. Virol. 37:564-573.
10. Kilton, L. J., M. Bradley, C. Mehta, andD.M.Livingston. 1981.Rapid and sensitive quantitative immunoassay for the large simian virus 40 Tantigen.J.Virol. 38:612-620. 11.Kress, M., E. May, R. Cassingena, and P. May. 1979. Simian virus 40-transformed cells express newspeciesof proteinsprecipitable byanti-simian virus 40 tumor serum. J.Virol. 31:472-483.
12. Lane, D. P., and L. V. Crawford. 1979.T-antigenis bound to a host protein in SV40 transformed cells. Nature
(London)278:261-263.
13. Linzer, D. I. H., W. Maltzman, andA.J. Levine. 1979. TheSV40 A geneproductisrequired for the production of a54,000 MW cellulartumorantigen. Virology 98:308-318. 14. Martin, R. G., and B. N. Ames. 1961. A method for
determiningthesedimentation behavior of enzymes:
ap-plication toprotein mixtures. J. Biol. Chem. 236:1372-1379.
15. McCormick, F., R. Clark, E. Harlow, and R. Tjian. 1981.
SV40 Tantigen bindsspecifically to a cellular 53K protein in vitro. Nature(London)292:63-65.
16. McCormick, F., and E. Harlow. 1980. Association of a murine53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J. Virol. 34:213-224. 17. Melero, J. A., D. T.Stitt, W. F. Mangel, and R. B. Carroll. 1979.Identification of newpolypeptidespecies (48-55K) immunoprecipitable by antiserum to purified large T anti-gen and present in SV40-infected and -trans-formed cells.Virology93:466-480.
18. Myers, R., R.Williams,and R. Tjian. 1981. Oligomeric structureof SV40 T antigen in free form and as bound to SV40 DNA. J. Mol.Biol. 148:347-353.
19. Reich, N. C., and A. J. Levine.1982. Specific interactions of the SV40 T antigen-cellular p53 protein complex with SV40 DNA.Virology 117:286-290.
20. Rotter, V.,0. W. Witte, R. Coffman, and D. Baltimore. 1980. Abelson murine leukemia virus-induced tumors elicit antibodies against a host cell protein,p50.Virology
36:547-555.
21. Sarnow, P., Y. S. Ho, J. Williams, andA.J. Levine. 1982. Adenovirus Elb-58kd tumorantigen and SV40 large tu-morantigenarephysically associated with the same 54kd cellularproteinintransformed cells. Cell 28:387-394. 22. Simmons, D. T. 1980. Characterization ofTau antigens
isolated from uninfected and simian virus 40-infected monkey cells and papovirus-transformed cells. J. Virol. 36:519-525.
23. Simmons, D. T., M. A.Martin,P.T. Mora, and C.Chang. 1980. Relationship among Tau antigens isolated from various lines ofsimian virus 40-transformed cells. J. Virol. 34:650-657.
24. Smith,A.E., R. Smith, and E. Paucha. 1979. Character-ization of different tumorantigenspresent in cells trans-formedby simian virus 40. Cell 18:335-346.
25. Voller,A., D.Bidwell, and A.Bartlett. 1976.Microplate enzymeimmunoassaysfor theimmunodiagnosis of virus
infections,p. 506-512. In N. Roseand H. Friedman(ed.), Manual of clinical immunology. American Society for
Microbiology, Washington, D.C.
VOL. 1983
on November 10, 2019 by guest
http://jvi.asm.org/