Copyright © 2000, American Society for Microbiology. All Rights Reserved.
Polarization of Allogeneic T-Cell Responses by
Influenza Virus-Infected Dendritic Cells
SANGKON OHANDMARYNA C. EICHELBERGER* Center for Immunization Research, Department of International Health,
Johns Hopkins University, Baltimore, Maryland 21205 Received 17 March 2000/Accepted 25 May 2000
The developing immune response in the lymph nodes of mice infected with influenza virus has both Th1- and Th2-type characteristics. Modulation of the interactions between antigen-presenting cells and T cells is one mechanism that may alter the quality of the immune response. We have previously shown that the ability of dendritic cells (DC) to stimulate the proliferation of alloreactive T cells is changed by influenza virus due to viral neuraminidase (NA) activity. Here we show that DC infected with influenza virus A/PR/8/34 (PR8) stimulate T cells to produce different types of cytokines in a dose-dependent manner. Optimal amounts of the Th1-type cytokines interleukin-2 (IL-2) and gamma interferon (IFN-␥) were produced from T cells stimulated by DC infected with low doses of PR8, while the Th2-type cytokines IL-4 and IL-10 were produced only in response to DC infected with high doses of PR8. IL-2 and IFN-␥levels corresponded with T-cell proliferation and were dependent on the activity of viral NA on the DC surface. In contrast, IL-4 secretion required the treatment of T cells with NA. Since viral particles were released only from DC that are infected with high doses of PR8, our results suggest that viral NA on newly formed virus particles desialylates T-cell surface molecules to facilitate a Th2-type response. These results suggest that the activity of NA may contribute to the mixed Th-type response observed during influenza virus infection.
The immune response to influenza virus has typical Th1-type characteristics, with production of interleukin-2 (IL-2), gamma interferon (IFN-␥), and cytotoxic T lymphocytes. However, cells that have Th2-type characteristics are also evident: in situ hybridization and enzyme-linked immunosorbent spotting analysis have demonstrated the presence of IL-4-, IL-5-, and IL-10-secreting cells in infected mice (2, 3, 26). The types of cytokines present during the initiation of an immune response are reflected by the isotypes of antibodies produced (30). The heterogeneity of influenza virus-specific antibody isotypes present in infected mice (20) may result from such mixed Th populations. It is interesting that isotype predominance de-pends on the replicative capacity of influenza virus and the site of immune induction (20). This suggests that the quantity of virus present in particular lymph nodes or the way in which the virus is presented at a particular site may direct the types of cytokines secreted by Th cells.
We therefore proposed that the quantity and quality of the T-cell response are altered by the infection of antigen-present-ing cells with influenza virus. We have demonstrated that alloreactive T-cell proliferation stimulated by dendritic cells (DC), the primary antigen-presenting cell type, is altered by influenza virus in a dose-dependent manner (24). Enhanced proliferation is a result of desialylation of DC surface mole-cules by viral neuraminidase (NA) (23), one of the major surface glycoproteins that are required for the release of newly formed virions from the host cell (1, 18). Like NA from other sources, viral NA cleaves the terminal sialic acid from glyco-conjugates on the cell surface. This substrate, sialic acid, plays an important role in regulating the interactions between cells. For example, adhesion between cells is increased (22), result-ing in an enhanced capacity of DC to activate T cells (6) when
the heavily sialylated glycoprotein CD43 is blocked with mono-clonal antibodies. Similarly, when macrophages are treated with bacterial NA, allospecific cytotoxic-T-lymphocyte re-sponses are enhanced (10). Interestingly, the eukaryotic lyso-somal NA gene is located in the major histocompatibility com-plex of genes (21), suggesting that it may play a role in immunity. Indeed, it is upregulated on activated T cells (15) and has been implicated in IL-4 production. T cells from mice that lack expression of lysosomal NA do not secrete IL-4 unless treated ex vivo with soluble NA (4). It is therefore reasonable to predict that viral NA may contribute to the quality of the T-cell response during infection.
We therefore examined the cytokines produced by T cells that had been stimulated by DC infected with different doses of influenza virus A/PR/8/34 (PR8). The types of cytokines pro-duced were dependent on the dose of PR8 and on NA activity. IL-2 and IFN-␥were optimally produced when T cells were stimulated by NA-treated DC or DC infected with low doses of PR8, while IL-4 and IL-10 were observed only in response to DC infected with high doses of PR8. We show that the type of response is determined by the cell group that is the target of NA activity: Th1-type cytokines are secreted when DC are desialylated, while Th2-type cytokines are secreted when T cells are desialylated.
MATERIALS AND METHODS
Virus preparation and titration.PR8 virus was cultured in 10-day-old embry-onated chicken eggs. The infected allantoic fluid was harvested, and aliquots were stored at⫺80°C. An NA-deficient NWS-Mvi virus was a kind gift from Gillian Air (University of Oklahoma Health Sciences Center). A stock was cultured in MDCK cells in the presence of both trypsin (2.5g/ml; Quality Biologicals, Gaithersburg, Md.) andVibrio choleraeNA (1 mU/ml; Boehringer Mannheim, Mannheim, Germany) (17). The virus was inactivated by UV irra-diation (short wave, i.e., 254 nm). The NA activities of live and UV-inactivated viruses were similar. Virus titers were determined by infection of MDCK cells as previously described (15). UV-inactivated PR8 did not contain any infectious virus.
* Corresponding author. Mailing address: Department of Interna-tional Health, Johns Hopkins University, Room 5026, 615 N. Wolfe St., Baltimore, MD 21205-1901. Phone: (410) 614-3407. Fax: (410) 955-7159. E-mail: meichelb@jhsph.edu.
7738
on November 9, 2019 by guest
http://jvi.asm.org/
Mice.Five- to six-week-old female C57BL/6 (B6) and BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and housed at Johns Hopkins University. They were used at 6 to 10 weeks of age.
DC.Bone marrow from B6 mice was prepared as previously described (23) and cultured at 5⫻105to 10⫻105cells/ml in complete medium containing 500 U
of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Pharmingen, San Diego, Calif.)/ml. On days 2 and 4, 75% of the medium was removed from each well and replaced with fresh medium containing 500 U of GM-CSF/ml. On day 6 of culture, DC aggregates were purified by 1⫻gsedimentation over 50% fetal calf serum (FCS) (12). These aggregates were resuspended in complete medium containing GM-CSF, and after overnight culture, the nonadherent cells were pelleted. The cells were identified as DC by microscopic examination (large cells with dendritic extensions), fluorescence-activated cell sorter analysis (stained with antibodies to CD11c, B7-1, B7-2, and major histocompatibility complex class II cell surface molecules, and their excellent capacity to stimulate allogeneic T-cell responses. Each preparation contained more than 90% CD11c-positive cells as determined by flow cytometry.
T cells.T cells from BALB/c mouse spleens were prepared by depletion of B cells and macrophages. Red blood cells in splenocyte suspensions were lysed. The lymphocytes were then washed and resuspended in serum-free RPMI me-dium at 107cells/ml. Rat anti-B220 (RA3-6B2) and anti-Mac1 (M1/70)
antibod-ies (Pharmingen) were added at 4g/ml, and the cells were incubated on ice for 30 min before washing with medium. Anti-rat immunoglobulin-coated magnetic beads (Dynal, Oslo, Norway) were added and used to remove B220- and Mac1-positive cells by following the manufacturer’s instructions. The remaining cells were counted for use in experiments. Each preparation contained more than 95% CD3⫹T cells, as determined by flow cytometry.
Virus infection and NA treatment.To infect DC, different quantities of virus were added to tubes containing 106cells in 2 ml of phosphate-buffered saline
(PBS) to obtain multiplicities of infection (MOI) that ranged from 1.25 to 50 infectious virus particles/cell. After 1 h of incubation at 37°C, 10 ml of RPMI 1640 (Life Technologies, Rockville, Md.) containing 10% FCS (Biofluids, Rock-ville, Md.), 2 mM glutamine, and penicillin and streptomycin (both from Quality Biologicals) (complete medium) was added, and the cells were incubated for 3 h at 37°C. Uninfected DC were treated in the same way, except that virus was not added.
Viral NA (N8) was a kind gift from Graeme Laver (John Curtin School of Medical Research). To treat cells with NA, DC or T cells (106/ml) were
incu-bated with 5 mU of purified NA for 2 h at 37°C in complete medium. Control cells were maintained under identical conditions in the absence of NA.
Measurement of cytokines in mixed DC–T-cell supernatants.After virus in-fection or NA treatment,H-2bDC were irradiated (3,000 rads), washed, and
diluted to 5⫻104cells/ml in complete medium. T cells (H-2d) were resuspended
at 3⫻106cells/ml in complete medium. Equal volumes (100l) of DC and T
cells were added to quadruplicate wells in a 96-well round-bottomed tissue culture plate (Costar, Cambridge, Mass.). In some experiments the NA inhibitor zanamivir (kindly provided by Glaxo Wellcome Laboratories) was added at a final concentration of 1 mM. Polyclonal goat antihemagglutinin (anti-HA) or anti-NA (National Institutes of Health, Bethesda, Md.) was added at 1g/ml. The ability of these antibodies to inhibit hemagglutination and NA activity was confirmed in our laboratory. The hemagglutination inhibition titer of 1g of the anti-HA preparation/ml was 4,056, while 1g of the anti-NA preparation com-pletely inhibited the NA activity of 25⫻106infectious units of PR8. Whereas
anti-HA inhibits the attachment of virus and therefore the infection of cells, anti-NA allows infection but inhibits the detachment of newly formed viral particles from the host cell surface (13). Like anti-NA, zanamivir inhibits the enzyme activity of NA and therefore allows the infection of cells but not the release of newly formed influenza virus particles from the cell surface (31). Supernatants were removed from cultures on a daily basis, and the cytokines were quantitated by enzyme-linked immunosorbent assay (ELISA), using anti-body pairs purchased from Pharmingen.
The cytokine ELISA used Immunolon I plates (Dynatech, Chantilly, Va.) coated overnight at 4°C with a 2-g/ml concentration of monoclonal anticytokine that had been diluted in 0.1 M Na2HPO4(pH 9.0). After the plates were washed
with 0.05% Tween 20 (Sigma, St. Louis, Mo.) in PBS three times, they were blocked by the addition of 200l of 10% FCS to each well. The plates were washed, 100l of culture supernatant was added per well, and they were then incubated overnight at 4°C. Detecting antibody (50l of 0.5-g/ml biotinylated anticytokine) was diluted in PBS containing 0.05% Tween 20 and 10% FCS and added to washed plates. The plates were incubated at room temperature for 1 h, washed, and then incubated with 100 l of 0.5-g/ml phosphatase-labeled streptavidin (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) for 30 min at room temperature. An alkaline phosphatase substrate,p-nitrophenyl phos-phate (Sigma), was added after the plates were washed eight times. The absor-bance was measured after 1 h at 405 nm on a kinetic microplate reader (Mo-lecular Devices, Palo Alto, Calif.).
Measurement of cytokines produced by DC.Uninfected or PR8-infected DC were washed and distributed into 96-well plates containing 200l of complete medium with 500 U of GM-CSF/ml and then cultured at 37°C. Supernatants were harvested at 12, 24, 48, 72, and 96 h postinfection, and cytokines were measured by ELISA as described above. Cytokine-specific antibody pairs were purchased from Pharmingen. Controls included uninfected DC stimulated with
V. choleraelipopolysaccharide (LPS) (Sigma) added at 50 ng/ml and uninfected DC stimulated with 0.01 mg of anti-CD40/ml or a control antibody of the same isotype (Pharmingen).
Analysis of data.The significance of the difference between values was com-pared using the nonparametric Wilcoxon rank test. Unless otherwise specified, all data are expressed as means⫾standard deviations (SD).
RESULTS
IL-2 and IFN-␥are optimally produced in response to allo-geneic DC infected with low doses of PR8, while IL-4 and IL-10 are optimally produced in response to DC infected with high doses of PR8.We tested the consequences of influenza virus infection on the ability of DC to stimulate an allogeneic T-cell response in a mixed culture system. DC were cultured from the bone marrow ofH-2bB6 mice, infected, washed, and irradiated
(23). Infected DC analyzed by immunostaining with polyclonal anti-NA and anti-HA showed approximately the same propor-tion of cells infected by influenza virus at MOI of 2.5 and 25 (60 to 70%). However, the level at which HA and NA were expressed was greater when DC were infected with a high dose of influenza virus (24). They were then incubated with T cells from the spleens ofH-2dBALB/c mice. Each assay used serial
dilutions of DC to stimulate 3⫻105T cells/well. Since optimal proliferation was observed with 5⫻103DC/well, the quantity of cytokines secreted with this number of cells is presented. Cytokines were measured at different times in the supernatants of mixed lymphocyte cultures. Maximum amounts of Th1-type cytokines IL-2 and IFN-␥were present on day 3 postculture, whereas Th2-type cytokines IL-4 and IL-10 reached maximum on day 4 postculture (results not shown).
The supernatant of T-cell cultures that were stimulated by allogeneic DC contained a significant amount of IL-2 and IFN-␥. Both IL-2 and IFN-␥levels increased when DC were infected with influenza virus at low MOI but decreased with increasing doses of PR8 (Fig. 1A and B). The amounts of these cytokines were consistent with the magnitude of T-cell prolif-eration as measured by incorporation of [3H]thymidine (24). In contrast, secretion of the Th2-type cytokines IL-4 and IL-10 increased when greater numbers of viral particles were used to infect the DC (Fig. 1C and D). This was particularly clear for IL-4, which was at negligible levels in the supernatants of T cells stimulated with DC that were either left uninfected or infected with low doses of PR8.
Secretion of IL-2 and IFN-␥is dependent on viral NA when cultures are stimulated by DC infected with low doses of PR8. Cytokines were measured in the supernatants ofH-2bT cells
that were stimulated by DC infected at either a low (MOI⫽
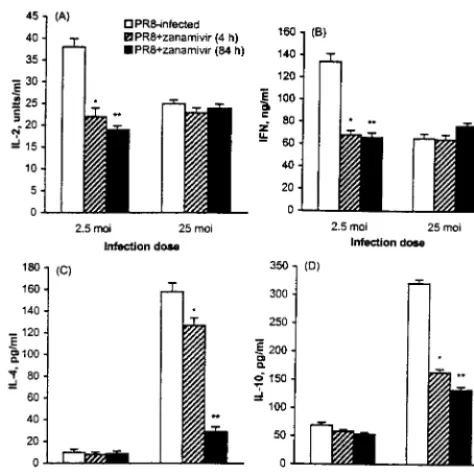
2.5) or high (MOI ⫽ 50) dose of PR8 in the presence of polyclonal anti-HA or anti-NA. Levels of IL-2 and IFN-␥ se-creted by cultures that contained anti-HA and anti-NA during the 4-h infection period, as well as during further culture with T cells, are shown in Fig. 2A and B. At low MOI, the produc-tion of IL-2 and IFN-␥was reduced in the presence of anti-NA but not of anti-HA. When antisera were added after DC were infected (present during mixed culture only), there was no change in cytokine production and proliferation (results not shown). The NA dependence of the IL-2 and IFN-␥response was also evident when T cells were stimulated by DC infected with low doses of PR8 in the presence of the NA inhibitor zanamivir (Fig. 3A and B). Inhibition was evident even when zanamivir was added during the first 4 h of infection, i.e., before mixing with T cells.
Unlike IL-2 secretion in response to DC infected at low MOI, there was no change in the production of IL-2 when T cells were stimulated with DC infected with high doses of virus in the presence of either anti-HA or anti-NA throughout
on November 9, 2019 by guest
http://jvi.asm.org/
fection and culture (Fig. 2A) or in the presence of zanamivir (Fig. 3A). In contrast, IFN-␥production was reduced in the presence of anti-NA when T cells were stimulated by DC infected at high MOI (Fig. 2B). This small reduction was con-sistently observed in repeated experiments and suggests that NA contributes to the IFN-␥response stimulated by DC in-fected with high doses of PR8. However, when zanamivir was added during the culture period, this reduction was not ob-served (Fig. 3B).
Production of IL-4 and IL-10 in response to DC infected with high doses of PR8 is dependent on viral NA activity. There was little production of either IL-4 or IL-10 when DC were infected at low MOI, and incubation with either anti-HA or anti-NA (Fig. 2C and D) or NA inhibitor (Fig. 3C and D) did not alter this pattern. At high MOI, there was no IL-4 produced when anti-HA was added at the time of DC infection (results not shown), confirming that IL-4 secretion is in re-sponse to infected cells only. Addition of anti-HA to T-cell cultures stimulated by high-dose-infected DC did not alter the production of IL-4 significantly (Fig. 2C). At high MOI, IL-4 production was dramatically decreased by the addition of anti-NA during the mixed-culture period (Fig. 2C). To determine at what point of viral replication the NA facilitates IL-4 produc-tion, NA inhibitor was added for 4 h only (prior to the addition of T cells) or throughout the duration of the culture period. When inhibitor was added for the first 4 h of infection, IL-4 production was reduced. However, this decrease was small compared to the inhibition observed when inhibitor was added to the mixed lymphocyte culture (Fig. 3C).
The production of IL-10 was also NA dependent, as
dem-onstrated by its decrease in the presence of anti-NA (Fig. 2D) as well as of zanamivir (Fig. 3D). However, there was still a significant amount of IL-10 produced in the presence of anti-NA, suggesting that its production may not be dependent solely on NA. In contrast to the IL-4 response, when zanamivir was added to DC infected with high doses of PR8 during the first 4 h only, the amount of IL-10 was decreased almost to the same degree as when this inhibitor was present during the entire culture period (Fig. 3D). Unlike the IL-4 response, which required the presence of infected DC, a small amount of IL-10 (70 pg/ml) was secreted by T cells responding to DC that had been incubated with a high dose of PR8 in the presence of neutralizing anti-HA.
Production of IL-4 requires viral replication.To confirm the NA dependence of these responses, T cells were cultured with allogeneic DC that had been infected with NWS-Mvi, a repli-cation-competent mutant virus that lacks NA, or UV-inacti-vated PR8 that has active NA but cannot replicate in cells. Cytokines were measured in the supernatants of alloreactive T cells 3 or 4 days after stimulation with these DC. The increased IL-2 response to DC infected at low MOI was dependent on the presence of functional NA (there was no increased re-sponse to NWS-Mvi) and did not require infection (there was still increased IL-2 production when T cells were stimulated by UV-inactivated virus) (Fig. 4A). The IFN-␥response was sim-ilar to the IL-2 response (Fig. 4B).
[image:3.612.55.292.70.320.2]In contrast, IL-4 was not produced when DC were treated with UV-inactivated virus (Fig. 4C), showing that the replica-tion of influenza virus in DC is required for this response. This
FIG. 1. Cytokine levels in cultures of allogeneic T cells and DC infected with different doses of PR8. DC were prepared by in vitro culture of bone marrow cells fromH-2bmice. After 4 h of infection at various MOI representing increas-ing numbers of PR8 viral particles per cell, the DC were irradiated (3,000 rads), washed, and mixed with 3⫻105T cells from the spleens ofH-2dmice. The cultures were incubated at 37°C in round-bottomed 96-well plates, and the supernatants were removed on a daily basis for cytokine analysis by ELISA. The data (average and SD of quadruplicate cultures) are shown for IL-2 and IFN-␥ in supernatants on day 3 and IL-4 and IL-10 in supernatants on day 4. Similar results were obtained in three repeated assays.
FIG. 2. IL-2, IFN-␥, IL-4, and IL-10 production in mixed cultures of PR8-infected DC with allogeneic T cells in the presence of antibodies to neutralize HA (anti-H1) or NA (anti-N1).H-2bDC were cultured, infected, and prepared for culture with H-2dT cells as described for Fig. 1. IL-2 and IFN-␥were measured in cultures that contained 1g of anti-H1 or anti-N1/ml during the first 4 h of DC infection as well as during the 3-day mixed-culture period. IL-4 and IL-10 were measured in cultures that contained 1g of H1 or anti-N1/ml during a 4-day culture period only (antibodies were not added during the infection of DC). The data are the averages and SD of quadruplicate cultures. Statistically significant differences between cytokine levels in the presence or absence of antibodies for which thePvalue is⬍0.05 when compared by Wilcoxon rank test are shown (ⴱ).
on November 9, 2019 by guest
http://jvi.asm.org/
[image:3.612.314.549.386.626.2]response was also NA dependent, since there was no induction of IL-4 when T cells were stimulated with NWS-Mvi at high doses (Fig. 4C).
Unlike the IL-4 response, IL-10 was measured in the super-natants of cells stimulated with DC treated with UV-inacti-vated virus (Fig. 4D). However, the amount was small (120 pg/ml) in comparison to that measured in the supernatants of cultures stimulated with infected cells (330 pg/ml), suggesting that the IL-10 response is enhanced by viral replication. These assays also confirmed the dependence of the IL-10 response on viral NA, since this cytokine was not induced by NWS-Mvi (Fig. 4D).
Treatment of T cells with viral NA results in the production of IL-4 in mixed cultures.To determine whether treatment of either cell type with purified viral NA results in an altered alloreactive cytokine response, DC and T cells were treated with NA, washed, and then cultured with untreated T cells or DC. There was greater IFN-␥production in cultures that con-tained NA-treated DC than in cultures containing NA-treated T cells (Table 1), whereas treatment of either cell type in-creased the amount of IL-2 in the supernatants of mixed cul-tures. Treatment of either DC or T cells also resulted in increased amounts of IL-10 in supernatants. However, the amount did not approach that obtained with virus-infected DC. In contrast, when T cells but not DC were treated with NA, a substantial amount of IL-4 was measured in mixed cultures (Table 1). The amount secreted was similar to that secreted in response to DC infected with PR8 at high MOI. The quantity of IL-4 secreted by alloreactive NA-treated T
cells was not affected by infection of the stimulating DC with either low or high doses of PR8 (Fig. 5).
The production of IL-10, IL-12, and transforming growth factor1 (TGF-1) in PR8-infected DC cultures is dependent on the virus dose. In addition to cell surface interactions (14, 25), cytokines (9, 16) and chemokines (8) influence the polar-ization of the Th1-Th2 responses. To determine whether PR8-infected DC secrete cytokines in a dose-dependent manner, the supernatants from a large number of DC (106cells in 200
l) were harvested at several time points after infection with PR8 at an MOI of 5 or 50. The maximum production of IL-12 was measured 24 h after infection of DC with PR8 at an MOI of 5 or treatment with LPS or anti-CD40 (Table 2). DC
[image:4.612.55.292.72.309.2]in-FIG. 3. Effect of the viral NA inhibitor zanamivir on cytokines present in mixed cultures of PR8-infected DC and allogeneic T cells.H-2bDC were cul-tured, infected, and prepared for culture withH-2dT cells as described for Fig. 1. Zanamivir was added at 1 mM to DC during the 4-h infection with PR8, as well as during the culture with T cells. Cytokines were measured in the culture supernatants by ELISA. Data (averages and standard errors of four separate assays) are shown for IL-2 and IFN-␥in supernatants of day 3 cultures and for IL-4 and IL-10 in supernatants of day 4 cultures. Statistically significant differ-ences between cytokine levels in the supernatants of cultures with and without inhibitor were determined by Wilcoxon rank test and are shown (ⴱ) for aPvalue of⬍0.05 (n⫽4).
FIG. 4. IL-2, IFN-␥, IL-4, and IL-10 production in mixed cultures containing DC treated with UV-inactivated virus or infected with NWS-Mvi.H-2bDC were cultured, infected, and prepared for culture withH-2dT cells as described for Fig. 1. The results are shown for cultures containing 5⫻103DC and 4⫻105T
[image:4.612.316.547.72.335.2]cells/well. Data are the averages and SD of IL-2 and IFN-␥levels after 3 days of culture and of IL-4 and IL-10 levels after 4 days of culture in quadruplicate wells. Similar results were obtained in three separate experiments.
TABLE 1. Effect of NA treatment on cytokine production in mixed cultures of DC and allogeneic T cells
Cell type treated with NA
Quantity of cytokine in supernatant of mixed culturea IL-2
(U/ml) (ng/ml)IFN-␥ (pg/ml)IL-4 (pg/ml)IL-10
None 18⫾1.2 67⫾5.1 13⫾1.2 53⫾3.9
DC 45⫾2.6 138⫾9.2 23⫾1.3 78⫾4.7
T cells 51⫾3.8 89⫾5.6 213⫾9.2 96⫾4.7
aDC (H-2b) and purified T cells (H-2b) (106each) were treated with 5 mU of
viral NA for 2 h at 37°C. The cells were washed twice with RPMI 1640, and 5⫻ 103DC and 4⫻105T cells were cocultured in round-bottomed 96-well plates.
After 3 days, 100l of culture supernatant was used to measure the amount of IL-2 and IFN-␥by ELISA. IL-4 and IL-10 were quantitated after 4 days. Data presented are the means⫾SD for the quantity of each cytokine, as measured in the supernatants from quadruplicate culture wells.
on November 9, 2019 by guest
http://jvi.asm.org/
fected with PR8 at an MOI of 50 produced less IL-12. Both IL-10 and TGF-1 levels were also dependent on the virus dose but increased with increasing numbers of virus particles per cell. The production of each of these cytokines was NA dependent (24). The presence of active TGF-1 in superna-tants of DC infected with high doses of PR8 is likely the result of the activation of latent molecules present in the culture medium by viral NA (24).
DISCUSSION
During viral infection of mice, a mixed type of Th response is generated (2, 3, 26). As suggested by the antibody isotype profile (20), the cytokine pattern elicited during the influenza virus response is likely to differ depending on viral replication and the location at which it is initiated. The dependence of IL-4 production on the expression of cellular NA (4) suggests that influenza virus NA could polarize the T-cell response toward a Th2 type. We questioned whether infection of DC, the primary antigen-presenting cell type, with influenza virus would polarize an alloreactive T-cell response toward a type that produces IL-4.
Our previous work showed that the ability of DC to stimu-late the proliferation of alloreactive T cells is changed by in-fection with influenza virus. At low doses of PR8, the stimu-latory capacity of DC is enhanced by the activity of NA on the DC surface (23). This effect, however, is dependent on the dose of PR8, so that at higher numbers of virus particles per cell, this increased response is not observed. This change is abrogated in the presence of antibodies that neutralize TGF-1 (24). In this report we show that the pattern of cyto-kines secreted in response to influenza virus-infected DC is also dose dependent. At low MOI, the response favors pro-duction of Th1-type cytokines IL-2 and IFN-␥, while at high MOI, Th2-type cytokines IL-4 and IL-10 are secreted (Fig. 1).
The dose-dependent IL-2 and IFN-␥response reflects the proliferative response. Like proliferation, the increase ob-served in response to DC infected with low doses of PR8 is dependent on NA activity. This dependence is demonstrated by a lack of IL-2 and IFN-␥production when allogeneic T cells are stimulated with DC infected with NWS-Mvi, a mutant virus that lacks NA (Fig. 4), and by inhibition of IL-2 and IFN-␥
production in the presence of NA-specific antibodies (Fig. 2) or zanamivir (Fig. 3). To observe the increased Th1-type re-sponse, infection is not required, since UV-inactivated PR8 (Fig. 4), PR8 that is neutralized by the addition of anti-HA (Fig. 2), and purified NA (results not shown) generate similar increases. The possible mechanisms by which NA facilitates this response have been described in a previous publication (23).
Since IL-12 supports the production of IFN-␥by T cells (9), this cytokine, which is secreted by DC infected with low but not high doses of PR8 (Table 2), is likely to support the NA-dependent Th1-type response. Other investigators also did not detect IL-12 in the supernatants of human macrophages that were infected with an apparently high dose of an H3N2 virus (a 1/10 dilution of allantoic fluid containing the virus was used to infect cells). However, under these conditions, the production of IFN-␥was retained and was supported by the production of IFN-␣/ and IL-18 (27). We have not included a complete analysis of cytokines present in our cultures; therefore, it is not possible to accurately predict which may be more important, especially since different pathways promote a Th1-type re-sponse (5) and various outcomes are dependent on the mixture of cytokines present. For example, TGF-inhibits the devel-opment of Th1 cells (28) but in the presence of IL-4 supports IL-12-independent Th1 differentiation (16). Our future exper-iments will therefore address mechanisms that may result in T-cell polarization in this in vitro system and will determine the relationship between NA and each of the relevant cytokines.
[image:5.612.67.282.74.279.2]Since alloreactive T-cell proliferation is largely dependent on IL-2, it is not clear whether increased proliferation follows an increase in IL-2 secretion or whether the increased amount of IL-2 simply reflects the number of T cells in the culture. Clearly the reduced proliferation observed in cultures that were stimulated with DC infected with high doses of PR8 was not due to a lack of IL-2—supplementation with large amounts
FIG. 5. IL-4 produced by alloreactive T cells stimulated by PR8-infected DC. DC were cultured and infected with PR8 at an MOI of either 2.5 or 25, as described for Fig. 1. Part of the DC and T-cell preparation was treated with 5 mU of purified NA for 2 h at 37°C. Infected DC that were either left untreated or treated with NA were mixed with either untreated or NA-treated T cells. The amount of IL-4 was determined after 4 days of culture. Results are shown for wells that contained 5⫻103DC and 4⫻105T cells. The averages and SD of
IL-4 levels in quadruplicate culture wells are shown. Similar results were ob-tained in three separate experiments.
TABLE 2. Cytokines produced in cultures of PR8-infected DCa
Infection dose
(MOI) (pg/ml)IL-10 IL-12
b
(ng/ml) TGF-1
c (pg/ml)
0 538⫾61 2.8⫾0.11 45⫾5
2.5 1,266⫾144 5.9⫾0.26 62⫾13
25 2,647⫾205 2.0⫾0.13 1,077⫾86
aCulture supernatants were removed at serial time points from DC that were either left uninfected or infected at an MOI of 2.5 or 25. The results shown are for IL-10 at 106cells/200l after 48 h of culture, IL-12 at 1.7⫻105cells/200l
after 24 h of culture, and TGF-1 at 5⫻103cells/200l after 24 h of culture.
These are time points that yielded the maximum expression of each cytokine. bAs a positive control, DC were stimulated with LPS and anti-CD40. Optimal production of 9.5 and 6.8 ng of IL-12/ml was measured after 24 h of stimulation with each of these reagents, respectively.
cWe have demonstrated that the production of TGF-1 is NA dependent (24), probably because viral NA can activate the precursor (29). Since the inactive precursor is present in high quantities in serum, most of the TGF-1 that is measured in these cultures is not produced by DC but rather by activation of latent molecules in the serum. The quantity of TGF-1 produced by DC was estimated by subtracting the quantity of TGF-1 present in acidified medium (total in medium) from the quantity of TGF-1 present in DC cultures. Approx-imately 49, 114, and 268 pg of TGF-1/ml were produced by 106uninfected and
PR8-infected DC (MOI of 2.5 and 25) in 200l, respectively.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:5.612.311.552.534.591.2]of this cytokine did not restore proliferation to levels obtained in cultures stimulated with either uninfected DC or DC in-fected at low MOI (results not shown).
In mixed cultures, the amount of IL-10 produced is depen-dent on the multiplicity of influenza virus particles (Fig. 1D) and IL-10 is produced in small amounts even when the virus is not infectious (Fig. 4D). It is noteworthy that IL-10 secretion is inhibited by the inclusion of zanamivir during the first 4 h of infection (Fig. 3D), suggesting that IL-10 production is facili-tated by changes on the desialylated DC surface. Unlike IL-2 and IFN-␥production, which parallels the number of T cells present in mixed cultures, IL-10 secretion continues to in-crease when DC are infected with high doses of PR8 and T-cell proliferation is reduced. This suggests that the changes that occur in mixed cultures stimulated by DC that are infected at high MOI support the increased production of IL-10. TGF-1 supports the production of IL-10 (19). Since latent TGF-1 can be activated by influenza virus NA (29) and increased levels of TGF-1 in culture supernatants are observed when DC are infected at high but not low MOI (Table 2), the NA dependence of IL-10 production in our culture system may be due to the support of activated TGF-1.
The production of IL-4 is clearly NA dependent. Following infection at high doses, IL-4 secretion by alloreactive T cells is almost completely inhibited by zanamivir when it is added throughout the culture of DC and T cells (Fig. 3). Under these conditions, DC are infected since NA is not required for in-fection, but virus is not released from the host cell surface (7, 17). When zanamivir was added during the first 4 h of infection only, IL-4 was secreted by the responding allogeneic T cells (Fig. 3C), supporting the idea that removal of sialic acid from the surface of T cells and not of DC facilitates IL-4 production in response to influenza virus-infected DC. It is likely that the desialylation of T cells is required for the production of IL-4 (4). When either DC or T cells are treated with an exogenous source of purified viral NA, the greatest levels of IL-4 are produced in the mixed lymphocyte cultures that contain desia-lylated T cells (Table 1).
Since IL-4 is produced by infection with PR8 at high MOI only (Fig. 1C) and this is dependent on the activity of viral NA (Fig. 2C, 3C, and 4C), we propose that under these conditions, viral NA present in the supernatant cleaves terminal sialic acids from glycoconjugates on the T-cell surface. This idea is supported by the quantitation of sialic acid released from cells under these conditions. The amount of sialic acid in superna-tants of infected DC was dose dependent (24), and T cells treated with an amount of NA that was even smaller than that measured in supernatants of DC infected with high doses of PR8 released sialic acid into the medium. When T cells that had been stimulated with DC infected at high doses were washed and then treated with NA, the concentration of sialic acid measured in the supernatant was 0.312⫾ 0.015 g/ml, compared with 0.598⫾0.019g/ml released by NA treatment from T cells that had been stimulated with uninfected DC. This suggests that some sialic acid was cleaved from T-cell surface glycoconjugates during culture with DC infected at high doses. Electron microscopy, culture of the virus, and quantitation of NA activity show that virus particles that have active NA are released from DC infected with a high but not a low number of infectious particles per cell (24), providing a rationale for these observations of dose dependence. The reason why virus parti-cles are not formed by DC that are infected with a lower dose of PR8 is not clear. DC represent a cell type that, because it is essential for the activation of the adaptive immune response, may have mechanisms different from those of other cells to protect itself from the consequences of viral replication.
Per-haps the inhibition of virus assembly by such a protective mechanism is overcome in the presence of an excessive num-ber of virus particles or by defective interfering particles, which are likely to be present at higher MOI. Under these conditions the production of virions may be permitted, and consequently IL-4 production would be facilitated.
We predict that when NA is tethered to the surface of the antigen-presenting cell, a Th1-type response will predominate, but when NA cleaves both DC and T-cell surface substrates, a Th2-type response can be induced. Our in vitro studies there-fore suggest that the types of cytokines produced during influ-enza virus infection may be determined in part by the location of NA activity.
Other DC surface molecules or cytokines produced by DC that can modulate the immune response probably also contrib-ute to the polarization of the response, since Th1-Th2 devel-opment is the result of the strength of both T-cell receptor and cytokine signals (11). These supporting factors may be NA independent (for example, influenza virus-infected DC have increased expression of ICAM-1 [23]) or NA dependent (for example, the activation of TGF-[24, 29]). Our future studies will therefore determine the mechanisms by which NA facili-tates polarization of the T-cell response and will address the role of NA in polarizing T-cell responses in vivo.
ACKNOWLEDGMENTS
This work was supported by grant AI40489 from NIH.
We thank Glaxo Wellcome for providing zanamivir, Gillian Air for the NA-deficient influenza virus, and Graeme Laver for the purified NA.
REFERENCES
1.Air, G. M., and W. G. Laver.1989. The neuraminidase of influenza virus. Proteins6:341–356.
2.Baumgarth, N., L. Brown, D. Jackson, and A. Kelso.1994. Novel features of the respiratory tract T-cell response to influenza virus infection: lung T cells increase expression of gamma interferon mRNA in vivo and maintain high levels of mRNA expression for interleukin-5 (IL-5) and IL-10. J. Virol.
68:7575–7581.
3.Carding, S. R., W. Allan, A. McMickle, and P. Doherty.1993. Activation of cytokine gene in T cells during primary and secondary murine influenza pneumonia. J. Exp. Med.177:475–482.
4.Chen, X. P., E. Y. Enioutina, and R. A. Daynes.1997. The control of IL-4 gene expression in activated murine T lymphocytes: a novel role for neu-1 sialidase. J. Immunol.158:3070–3080.
5.Cousens, L. P., R. Peterson, S. Hsu, A. Dorner, J. D. Altman, R. Ahmed, and C. A. Biron.1999. Two roads diverged: interferon alpha/beta- and interleu-kin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J. Exp. Med.189:1315–1328.
6.Fanales-Belasio, E., G. Zambruno, A. Cavani, and G. Girolomoni.1997. Antibodies against sialophorin (CD43) enhance the capacity of dendritic cells to cluster and activate T lymphocytes. J. Immunol.159:2203–2211. 7.Griffin, J. A., S. Basak, and R. W. Compans.1993. Effects of hexose
starva-tion and the role of sialic acid in influenza virus release. Virology125:324– 334.
8.Gu, L., S. Teng, R. M. Horner, C. Tam, M. Loda, and B. J. Rollins.2000. Control of Th2 polarization by the chemokine monocyte chemoattractant protein-1. Nature404:407–411.
9.Heufler, C., F. Koch, U. Stanzi, G. Topar, M. Wysocka, G. Trinchieri, A. Enk, R. Steinman, N. Romani, and G. Schuler.1996. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interfer-on-␥production by T helper 1 cells. Eur. J. Immunol.26:659–668. 10. Hirayama, Y., K. Inaba, M. Inaba, T. Kato, M. Kitaura, T. Hosokawa, S.
Ikehara, and S. Muramatsu.1988. Neuraminidase-treated macrophages stimulate allogenic CD8⫹T cells in the presence of exogenous interleukin 2. J. Exp. Med.168:1443–1456.
11. Iezzi, G., E. Scotet, D. Scheidegger, and A. Lanzavecchia.1999. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur. J. Immunol.29:4092–4101.
12. Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Mura-matsu, and R. M. Steinman.1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/ macrophage colony-stimulating factor. J. Exp. Med.176:1693–1702. 13. Jahiel, R. I., and E. D. Kilbourne.1966. Reduction in plaque size and
on November 9, 2019 by guest
http://jvi.asm.org/
reduction in plaque number as differing indices of influenza virus-antibody reactions. J. Bacteriol.92:1521–1534.
14. Kato, T., and H. Nariuchi.2000. Polarization of naı¨ve CD4⫹T cells toward the Th1 subset by CTLA-4 costimulation. J. Immunol.164:3554–3562. 15. Landolfi, N. F., J. Leone, J. E. Womack, and R. G. Cook.1985. Activation of
T lymphocytes results in an increase in H-2-encoded neuraminidase. Immu-nogenetics22:159–167.
16. Lingnau, K., P. Hoehn, S. Kerdine, S. Koelsch, C. Neudoerfl, N. Palm, E. Ruede, and E. Schmitt.1998. IL-4 in combination with TGF-favors an alternative pathway of Th1 development independent of IL-12. J. Immunol.
161:4709–4718.
17. Liu, C., and G. M. Air.1993. Selection and characterization of a neuramin-idase-minus mutant of influenza virus and its rescue by cloned neuramini-dase genes. Virology194:403–407.
18. Liu, C., M. C. Eichelberger, R. W. Compans, and G. M. Air.1995. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J. Virol.69:1099–1106.
19. Maeda, H., H. Kuwahara, Y. Ichimura, M. Ohtsuki, S. Kurakata, and A. Shiraishi.1995. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J. Immunol.155:4926–4932.
20. Marshall, D., R. Sealy, M. Sangster, and C. Coleclough.1999. Th cells primed during influenza virus infection provide help for qualitatively distinct antibody responses to subsequent immunization. J. Immunol. 163:4673– 4682.
21. Milner, C. M., S. V. Smith, M. B. Carrillo, G. L. Taylor, M. Hollingshead, and R. D. Campbell.1997. Identification of a sialidase encoded in the human major histocompatibility complex. J. Biol. Chem.272:4549–4558. 22. Nong, Y. H., E. Remold-O’Donnell, T. W. LeBien, and H. G. Remold.1989.
A monoclonal antibody to sialophorin (CD43) induces homotypic adhesion
and activation of human monocytes. J. Exp. Med.170:259–267.
23. Oh, S., and M. C. Eichelberger.1999. Influenza virus neuraminidase alters allogeneic T cell proliferation. Virology264:427–435.
24. Oh, S., M. McCaffery, and M. C. Eichelberger.2000. Dose-dependent changes in influenza virus-infected dendritic cells result in increased alloge-neic T cell proliferation at low, but not high, doses of virus. J. Virol.74:
5460–5469.
25. Ohshima, Y., L. P. Yang, T. Uchiyama, Y. Tanka, P. Baum, M. Sergerie, P. Hermann, and G. Delespesse.1998. OX40 costimulation enhances interleu-kin-4 expression at priming and promotes the differentiation of naı¨ve human CD4⫹T cells into high IL-4-producing effectors. Blood92:3338–3345. 26. Sarawar, S. R., and P. C. Doherty.1994. Concurrent production of
interleu-kin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J. Virol.68:3112–3119.
27. Sareneva, T., S. Matikainen, M. Kurimoto, and I. Julkunen.1998. Influenza A virus-induced alpha/beta and IL-18 synergistically enhance IFN-gamma gene expression in human T cells. J. Immunol.160:6032–6038. 28. Schmitt, E., P. Hoehn, C. Huels, S. Goedert, N. Palm, E. Rude, and T.
Germann.1994. T helper type 1 development of naı¨ve CD4⫹T cells requires the coordinate action of IL-12 and IFN-␥and is inhibited by TGF-. Eur. J. Immunol.24:793–798.
29. Schultz-Cherry, S., and V. S. Hinshaw.1996. Influenza virus neuraminidase activates latent transforming growth factor. J. Virol.70:8624–8629. 30. Stavnezer, J.1996. Antibody class switching. Adv. Immunol.61:79–89. 31. von Itzstein, M., W. Y. Wu, G. B. Kok, M. S. Pegg, J. C. Dyason, B. Jin, T.
Van Phan, M. L. Smythe, H. F. White, S. W. Oliver, et al.1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature363:418–423.