gene expression of Pseudomonas aeruginosa virulence factors
.
White Rose Research Online URL for this paper:
http://eprints.whiterose.ac.uk/151491/
Version: Published Version
Article:
Ahmed, Syed A K S, Rudden, Michelle orcid.org/0000-0001-9617-087X, Smyth, Thomas J
et al. (3 more authors) (2019) Natural quorum sensing inhibitors effectively downregulate
gene expression of Pseudomonas aeruginosa virulence factors. APPLIED
MICROBIOLOGY AND BIOTECHNOLOGY. pp. 3521-3535. ISSN 0175-7598
https://doi.org/10.1007/s00253-019-09618-0
eprints@whiterose.ac.uk https://eprints.whiterose.ac.uk/ Reuse
This article is distributed under the terms of the Creative Commons Attribution (CC BY) licence. This licence allows you to distribute, remix, tweak, and build upon the work, even commercially, as long as you credit the authors for the original work. More information and the full terms of the licence here:
https://creativecommons.org/licenses/
Takedown
If you consider content in White Rose Research Online to be in breach of UK law, please notify us by
GENOMICS, TRANSCRIPTOMICS, PROTEOMICS
Natural quorum sensing inhibitors effectively downregulate gene
expression of
Pseudomonas aeruginosa
virulence factors
Syed A. K. S. Ahmed1&Michelle Rudden2&Thomas J. Smyth3&James S. G. Dooley1 &Roger Marchant1& Ibrahim M. Banat1
Received: 12 October 2018 / Revised: 2 January 2019 / Accepted: 4 January 2019 / Published online: 9 March 2019
#The Author(s) 2019
Abstract
At present, anti-virulence drugs are being considered as potential therapeutic alternatives and/or adjuvants to currently failing antibi-otics. These drugs do not kill bacteria but inhibit virulence factors essential for establishing infection and pathogenesis through targeting non-essential metabolic pathways reducing the selective pressure to develop resistance. We investigated the effect of naturally isolated plant compounds on the repression of the quorum sensing (QS) system which is linked to virulence/pathogenicity in
Pseudomonas aeruginosa. Our results show thattrans-cinnamaldehyde (CA) and salicylic acid (SA) significantly inhibit expression of QS regulatory and virulence genes inP. aeruginosaPAO1 at sub-inhibitory levels without any bactericidal effect. CA effectively downregulated both thelasandrhlQS systems withlasIandlasRlevels inhibited by 13- and 7-fold respectively compared to 3- and 2-fold reductions with SA treatment, during the stationary growth phase. The QS inhibitors (QSI) also reduced the production of extracellular virulence factors with CA reducing protease, elastase and pyocyanin by 65%, 22% and 32%, respectively. The QSIs significantly reduced biofilm formation and concomitantly with repressed rhamnolipid gene expression, only trace amount of extra-cellular rhamnolipids were detected. The QSIs did not completely inhibit virulence factor expression and production but their administration significantly lowered the virulence phenotypes at both the transcriptional and extracellular levels. This study shows the significant inhibitory effect of natural plant-derived compounds on the repression of QS systems inP. aeruginosa.
Keywords Trans-cinnamaldehyde . Salicylic acid . Quorum sensing . Quorum sensing inhibitor .Pseudomonas aeruginosa
Introduction
A review commissioned by the UK government in 2014 (https://amr-review.org/) predicted that there will be more deaths in the world due to antimicrobial resistance (AMR) than cancer by the year 2050. Antibiotic usage creates an evolutionary stress response in the bacterial population that over time leads to the emergence of resistant strains. Extensive use of antibiotics coupled with the diminished pipe-line of new antibiotics has seen a rapid evolution of resistance
that has culminated in the development of multi-drug-resistant pathogens that are extremely difficult to treat.
Pseudomonas aeruginosa, a Gram-negative opportunistic pathogen, is prevalent in immunocompromised patients suffer-ing from cystic fibrosis (CF) and human immunodeficiency virus (HIV). These bacteria are notorious biofilm producers. The biofilm provides a stratified environment with the core being more anoxic with low bacterial growth and metabolic rates. These metabolically inactive biofilm cells are resistant to β-lactam antibiotics (Anwar and Costerton 1990; Werner et al. 2004) ciprofloxacin, tetracycline and tobramycin (Brown et al.1988). The biofilm layer also acts as a diffusion barrier reducing the rate of antibiotic penetration, preventing sufficient accumulation of antibiotics and allowing time for expression of resistance genes (Jefferson et al. 2005). In CF,
P. aeruginosa forms biofilms and readily adapts to the lung environment eventually leading to prolonged inflammation and chronic lung infections that are very difficult to treat using conventional antibiotic methods. In addition, the presence of inducible (MexXY) and constitutive (MexAB-OprM) efflux pumps and the poor permeability of the outer membrane also
* James S. G. Dooley jsg.dooley@ulster.ac.uk
1 School of Biomedical Sciences, Ulster University, Coleraine BT52
1SA, UK 2
Department of Biology, University of York, Wentworth, York YO10 5DD, UK
contribute to the reduced susceptibility ofP. aeruginosato a broad range of antibiotics (Aghazadeh et al. 2014, López-Causapé et al.2017). Effective treatment of P. aeruginosais therefore becoming increasingly challenging with the bacteri-um showing resistance to even the third and the fourth genera-tions of carbapenems and cephalosporins (Luna et al.2013; Patel et al.2014). Therefore, it has become critical to find alter-native therapies to successfully clearP. aeruginosainfections.
P. aeruginosaproduces a variety of virulence factors, in a coordinated system, that are reported to enable host colonisation and adaptation (Valderrey et al.2010; Gellatly and Hancock 2013; Sousa and Pereira2014). These virulence factors include the production of biofilm, pyocyanin, elastase and rhamnolipid and are under the control of a cell density-dependent signalling regulation known as quorum sensing (QS) (Stover et al.2000; Lee and Zhang2014; Sousa and Pereira2014) The canonical QS system inP. aeruginosaincludes thelasand therhlsystems both consisting of LuxI type synthases (LasI and RhlI) which produce specific acyl homoserine lactone (AHL) molecules, N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-L-homoserine lactone (C4-HSL). At sufficiently high bacterial concentrations, these AHL molecules then bind to the LuxR type receptors (LasR and RhlR) to form transcrip-tional activation complexes which regulate the transcription of various genes involved with virulence of P. aeruginosa
(Papenfort and Bassler2016).
Interfering with this QS system through application of QS inhibitors (QSI) is a novel therapeutic target that has shown to effectively reduce virulence in opportunistic pathogens. The dis-ruption of QS communication can be achieved through the en-zymatic degradation of AHL molecules by lactonases, acylases and oxidoreductases or by using small structural molecules that inhibit the QS signal molecule from binding to its cognate reg-ulatory protein (Morohoshi et al.2009; Kalia2013; Kisch et al. 2014; Gupta et al.2015). A synthetic derivative of a furanone, compound C30 (C30F), has been shown to supress bacterial QS in mice lung models through interference with AHL production (Wu et al.2004) and through attenuation of QS-regulated pro-duction of virulence factors (Hentzer et al.2003). In the recent past, a range of plant compounds have shown to be effective as anti-QS and anti-biofilm agents (Musthafa et al. 2010; Jayelakshmi et al.2016; Ouyang et al.2016; Luo et al.2017). Kim et al. (2015), using an in silico approach, predicted that natural gingerol could bind to the QS regulator LasR protein. They then demonstrated, using standard assays, a decrease in production of several virulence factors and biofilm formation following exposure to gingerol, consistent with interference of the binding of the cognate signal molecule, 3-oxo-C12-HSL, to LasR. Moreover, access to crystal structure of LasI (Gould et al. 2004) and LasR (Bottomley et al.2007) along with the avail-ability of computer-aided programs like structure-based virtual screening (SB-VS) and molecular docking have been useful in identifying more compounds with potential anti-QS abilities.
A SB-VS experiment unlocked six drugs with LasR struc-tural similarity including salicylic acid (SA), nifuroxazide and chlorzoxazone (Yang et al. 2009). These compounds were able to significantly inhibit QS gene expression and pheno-types inP. aeruginosa. In another study, molecular docking results showed that a plant compound, trans-cinnamaldehyde (CA), was able to interact with the LasI substrate binding sites by forming hydrophobic andπ-πbonds with phenylalanine-27 and 105, tryptophan-33 and a hydrogen bond with arginine-30 in the LasI synthase (Chang et al. 2014). Since QS is related to several virulence mechanisms in
P. aeruginosa, therefore the ability of compounds like SA and CA to interfere with the QS system can open the possi-bility of utilising these as effective anti-QS agents for control-ling the pathogenic phenotypes ofP. aeruginosa.
The QS system allows bacteria to adapt to changing envi-ronmental conditions at the population level, with the adapta-tion mediated at the transcripadapta-tional level via regulated expres-sion of the QS genes in response to metabolic and environmen-tal stimuli (Wagner et al. 2003; Scott and Hwa 2011). Understanding the transcriptional expression of the QS genes is therefore essential for understanding the physiology of the cell under QS inhibitory conditions. The current information on the ability of CA and SA to reduce QS activity inP. aeruginosa
is very limited and has been mostly acquired through crude estimations of virulence proteins or by using high throughput microarray analysis for identifying changes to gene expression (Prithiviraj et al. 2005; Yang et al.2009). Therefore, in this study, we have used a very robust and MIQE (minimum infor-mation for publication of quantitative real-time PCR experi-ments) compliant reverse transcription quantitative PCR (RT-qPCR) assay, a gold standard for low-medium throughput quantitative expression analysis, to study the changes in transcriptomic profiles whenP. aeruginosais subjected to CA and SA treatments at sub-inhibitory concentrations. To correlate the effects of the gene expression on the phenotypic profiles following QSI treatment, the QS-regulated virulence factors rhamnolipid, elastase, protease and pyocyanin were estimated.
Materials and methods
Bacterial strains and media
The fully sequenced and widely reported laboratory strain
P. aeruginosaPAO1 (ATCC 15692) was used in the study. Overnight cultures were prepared from−80 °C frozen culture
positive control for QS was included using 10 μM C30F (Skindersoe et al.2008). All experiments were carried out in biological triplicates. The experimental compounds were pur-chased from Sigma-Aldrich, UK, unless otherwise stated.
Minimum inhibitory concentration determination
The minimum inhibitory concentration (MIC) of the test inhibi-tors against P. aeruginosaPAO1 was determined using the resazurin microtiter plate assay (Elshikh et al.2016) which used the redox indicator resazurin that changed colour from blue to pink in the presence of viable cells. The MIC was determined as the concentration at which there was no colour change following 4 h incubation of the overnight cells with 0.015% resazurin.
RNA isolation and purity assessment
The cell pellets were collected from different growth phase cul-tures by spinning them at 13,000×gfor 2–3 min at room temper-ature and the RNA extracted using JetGene RNA Purification Kit (Thermo Fisher Scientific). The cells were lysed with occasional vortexing in a buffer solution with 1× TE buffer, 15 mg/ml lyso-zyme and 20 mg/ml proteinase K (Promega). The samples were then transferred to a 2-ml Lysing Matrix A tube (MP Biomedicals) withβ-mercaptoethanol containing RLT buffer (provided in the kit) for enhanced lysis. The contents in the lysing matrix tubes were then homogenised using the FastPrep™FP 200 cell disrupter at speed 5.5 for 30 s. A double DNA-digestion treatment was done to ensure that the RNA was free of any genomic DNA (gDNA) contamination. The RNA isolated was quantified using the Nanodrop spectrophotometer with A260/ A280ratio of 1.8–2.1 being considered as pure. The integrity of the samples was checked by agarose gel electrophoresis for pres-ence of two sharp distinct bands representing 23S and 16S rRNA. The integrity was further verified by analysing the sam-ples in an Agilent 2100 Bioanalyzer where RNA Integrity Number (RIN) values greater than 8 were observed for all sam-ples. The RIN is based on a numbering system from 1 to 10 with 1 being the most degraded and 10 being the most intact. The RNA samples were aliquoted and stored at−80 °C.
Reverse transcription quantitative polymerase chain
reaction
First-strand cDNA was synthesised using Superscript™Reverse Transcriptase II (Invitrogen). Each reaction mix contained DNase-treated RNA (500 ng), 20–250 ng random primers (Promega), 10 mM dNTPs and RNase free water to make to the reaction volume 15.6μl. The reactions were heated at 65 °C for 5 min before adding 5× strand buffer, 0.1 M DTT and RNase inhibitor (RNAse out™Invitrogen) in final concen-trations of 1×, 10μM and 40 units, respectively. The reactions were incubated at 25 °C for 2 min before adding Superscript™II
Reverse Transcriptase (200 units final concentration) (Invitrogen). The RT reactions were carried out at a series of temperature starting with 25 °C for 10 min, 42 °C for 50 min and 70 °C for 15 min. The first-strand cDNA synthesis was performed for all the biological triplicates from each time point. A negative reaction without reverse transcriptase was included in every run. All cDNA samples were stored at−20 °C prior to use.
The cDNA synthesised was then used as a template for real-time PCR amplification using the ROCHE LightCycler LC480 system with a SYBR-Green probe. Since PCR efficiency is highly dependent on primer specificity, therefore a qPCR cali-bration curve was generated from each primer set using PAO1 gDNA. Only those primers which gave a calibration curve with a slope value between−3.1 and−3.6 that translated into
am-plification efficiencies of 90–110% were used for PCR quanti-fication. The binding specificity of these primers were also validated post-amplification by generating a melt curve for each primer set with the presence of a single sharp peak eliminating the chances of any non-specific binding.
The qPCR 10μl reaction mix each contained 2× SYBR Green master mix (1×), forward and reverse primers (1μM), cDNA template and molecular grade water. Negative controls in form of –RT (no reverse transcriptase) and no template control NTC (no DNA template added) were included to rule out any contamination during the preparation process. A pos-itive control in the form of gDNA was also included. The cut-off values for residual gDNA amplification and NTC were set at greater than 35 and 40 cycles, respectively. The cycling parameters were as follows: initial denaturation at 95 °C for 5 min, 40–50 cycles of denaturation at 95 °C for 10 s, anneal-ing at 59 °C for 10 s, extension at 72 °C for 10 s.
Reference gene validation
A total of six candidate genes (gyrB, proC, cysG, rpoD, rpoBand
Relative gene expression data analysis
System (LC480 software, version 2)-generated analysis was performed on the real-time PCR data. The threshold values (Cq) from each of the qPCR run were extracted from the LC480 system using the second derivative maximum method (Rasmussen2001). Data analysis was performed by taking the arithmetic mean of the Cq values of the technical replicates and transferring it into log values to generate the relative quan-tities (RQ). The RQ values of the target genes were then di-vided by geometric mean of reference gene RQs (rpoDand
proC) to give normalised relative quantity value (NRQ). The NRQ value was then divided by the experimental calibration which in the experiment was relative expression at early log (6-h) and was set to 1. The output was the calibration normal-ised ratio (CNRQ) which was used in extrapolating informa-tion on the expression profile of the target genes.
Production of virulence factors
An overnight PAO1 culture was used to inoculate PPGAS medium and incubated for 24 h under continuous shaking at 37 °C. The supernatant was collected, and filter sterilised for use in the following assays:
ProteaseThe amount of LasA protease produced by PAO1 following incubation with and without the inhibitors were estimated by adding 0.1 ml culture supernatant to a reaction mixture containing 0.8% azocasein in 500μl of 50 mM K2HPO4(pH 7) and incubating at 25 °C for 3 h. The reaction was terminated by adding 0.5 ml of 1.5 M HCl and then keeping it on ice for 30 min. The precipitated protein was removed by centrifugation (10,000×g for 10 min). NaOH (1 N) was added to the supernatant in equal ratios and the concentration of acid soluble azopeptides measured spectro-photometrically at 440 nm.
ElastaseThe LasB elastase production was measured by adding 1 ml of the culture supernatant to a 2-ml reaction buffer (100 mM Tris-HCl, 1 mM CaCl2) containing the substrate elastin-Congo red and incubating for 3 h at 37 °C with shaking at 180 rpm. The reaction was terminated by adding 2 ml of 0.7 M sodium phos-phate buffer (pH 6) and placing it on ice for 15 min. The absor-bance of the supernatant was measured at 495 nm.
Pyocyanin The pyocyanin concentration was estimated by adding 7.5 ml filtered supernatant to 4.5 ml of chloroform and vortexed until the colour changed to greenish blue. The samples were centrifuged (10,000×gfor 10 min) and 3 ml of the resulting blue coloured liquid was transferred to a new tube containing 1.5 ml of 0.2 M HCl and shaken until the blue colour turned to pink. The pink layer was transferred to a cuvette and the absorbance measured at 520 nm. The
concentration was calculated inμg/ml by multiplying the ab-sorbance by factor 17.072 (Essar et al.1990).
Rhamnolipid extraction and purification
The extraction of rhamnolipid was performed following the method of Smyth et al. (2010). The culture supernatant (50 ml) from PAO1 grown in PPGAS medium for 24 h was acidified to pH 2 and extracted with ethyl acetate three times. The organic solvent containing rhamnolipid was dried with anhydrous MgSO4to remove residual water. Rhamnolipid was isolated from the ethyl acetate solvent in the form of yellow gummy residue after removing the organic solvent in a rotary evaporator. The rhamnolipid crude extract was then purified using solid phase extraction by running the samples through Strata SI-1 Silica (55 μM, 70A) Giga tubes (Phenomenex). After conditioning and removing the impurities from the col-umn with chloroform, rhamnolipids were eluted using chloro-form and methanol in ratios of 5:0.3, 5:0.5 and 1:1.
Rhamnolipid separation and analysis
by high-performance liquid chromatography mass
spectrometry/mass spectrometry
Analysis of the extracted rhamnolipid mixture was performed using a LCQ™quadrupole ion trap with a negative electrospray ionisation (ESI) interface connected to a Thermo HPLC Spectra system. A reverse phase C18 column with 5μm particles was used to separate the rhamnolipids. The parameters included desolvation gas at 65 units and source temperature 250 °C, 20μl injection volume and 0.5 μl/min flow rate. Two mobile phases were used: HPLC grade water (A) and acetonitrile (B). The rhamnolipid congeners were resolved in a linear gradient mobile phase starting with 70%A:30%B to 30%A:70%B over 50 min and then back to 70%A:30%B for 55 min with a final hold of 5 min. Tandem mass spectrometry was carried out using ESI in a negative mode using collision-induced dissociation (CID) at 35% peak within the MS range of 50–800m/z.
Statistical analysis
All statistical analysis was performed using the GraphPad prism v5.
Results
Growth phase-dependent expression of QS genes
systems were expressed in a cell density-dependent manner with expression levels increasing upon entering the stationary phases of growth (Fig.1b). Maximum expression levels for all genes were detected in the mid-late stationary phase corresponding with highest cell density. In bothlas and rhl systems, the autoinducer synthase genes (lasIandrhlI) were expressed earlier and at much higher relative concentrations in comparison to their cognate regulatory protein genes (lasRandrhlR). At high concentrations, LasR and RhlR bind to their cognate N-acyl homoserine autoinducer molecules; the bound complex is then a transcriptional regulator of several genes inP. aeruginosa.
The QS system regulates productions of most of the
P. aeruginosavirulence factors including the low molecular weight glycolipids rhamnolipids that are under the direct
regulation of the RhlR-RhlI system. The rhamnolipid biosyn-thetic genes display a differential expression profile where
rhlAandrhlBare expressed earlier relative torhlC, which is only maximally expressed after significantrhlABexpression (Fig.2a–c). The products ofrhlABare responsible for the first step in rhamnolipid biosynthesis, which produce mono-rhamnolipids. Mono-rhamnolipids are in turn the substrate for the rhlCgene product to produce di-rhamnolipids. The differential sequential expression pattern observed for the rhamnosyltransferases from this data is suggestive of a coor-dinated regulation based on the substrate availability.
The other virulence-associated genes responsible for the production of the exoprotease LasA, and elastase LasB, were also transcriptionally expressed in a cell density-dependent
6 9 12 24
0 10 20 30 40
Log Early
Stationary
Mid-Late Stationary
lasR
***
Time (h)
Relative Expression Level
6 9 12 24
0 20 40 200 400 600
800 Log Early
Stationary
Mid-Late Stationary
lasI
***
Time (h)
Relative Expression Level
6 9 12 24
0 50 100 150
Log Early
Stationary
Mid-Late Stationary
rhlR
***
Time (h)
Relative Expression Level
6 9 12 24
0 8 16 200 250 300 350 400
Log Early
Stationary
Mid-Late Stationary
rhlI
***
Time (h)
Relative Expression Level
0 3 6 9 12 24 48 72
0.01 0.1 1 10
PAO1
Log Early
Stationary
Mid-Late Stationary
Time (h)
OD
600nm
Log
10
a
b
Fig. 1 LasandrhlQS regulatory
genes inP. aeruginosaPAO1 are differentially expressed in a cell density-dependent manner.a Growth ofP. aeruginosain phosphate-limited media (PPGAS).bTranscriptional expression of QS regulatory geneslasR, lasI, rhlRandrhlI. Expression levels were quantified by RT-qPCR, relative mRNA levels for target genes were normalised to the geometric mean of two reference genes (rpoDand
[image:6.595.174.541.250.715.2]manner with maximum expression observed in mid-late sta-tionary phase (Fig.2d–e). Thelas-regulated virulence genes
lasA and lasB were shown to be significantly upregulated during the mid-late stationary phase with expression levels > 300-fold relative to log phase levels (p< 0.001).
Quorum sensing inhibitors effectively downregulate
the QS regulatory system
Selectively interfering with QS systems is a novel strategy targeted at disarming virulent opportunistic pathogens such asP. aeruginosa. In Gram-negative bacteria, QS is typically mediated by acyl-HSLs and rational analogues have been de-signed to specifically target these systems. Several phenolic compounds have been be shown to effectively disrupt QS
systems in Gram-negative bacteria (Hossain et al.2017). In this part of the study, we investigated the anti-QS abilities of naturally isolated plant compounds CA, SA and a synthetic furanone compound, C30F, reported to attenuate virulence in
P. aeruginosa(Fig.3a) (Hentzer et al.2003; Yang et al.2009; Chang et al. 2014). The minimum inhibitory concentration (MIC) of the test compounds CA and SA was determined as 11.35 mM and 18.1 mM, respectively. The use of the quorum sensing inhibitors (QSIs) at the sub-inhibitory concentrations (1/5th MIC) did not affect the growth phenotype of
P. aeruginosa PAO1 (Fig. 3b). CA treatment resulted in a longer lag phase but reached similar optical densities to un-treated PAO1 within 6 h of incubation. Since QS genes were significantly expressed in the stationary phase (Fig.1b), we tested the effect of the QSIs on the expression of QS-rhlA
6 9 12 24
0 100 200 1000 2000 3000 *** Time (h) R e la tiv e E x pr e s s ion Le v e l rhlB
6 9 12 24
0 100 200 300 400 500 600 700 ** Time (h) R el at iv e E xp res si o n L eve l lasA
6 9 12 24
0 100 200 300 400 500 *** Time (h) Rel at iv e E xp res s io n L e ve l lasB
6 9 12 24
0 100 200 300 400 *** Time (h) Re la tiv e E x p res s io n Le ve l rhlC
6 9 12 24
0 50 100 150 ** Time (h) R e la tiv e E x pr e s s ion Le v e l
a
b
c
d
e
Log EarlyStationary Mid-LateStationary Log EarlyStationary Mid-LateStationary
Log EarlyStationary Mid-LateStationary
Log EarlyStationary Mid-LateStationary Log EarlyStationary Mid-LateStationary
Fig. 2 QS-regulated virulence-associated genes are highly expressed in stationary phase in
P. aeruginosaPAO1. Relative transcript levels of virulence-associated rhamnolipid
biosynthetic genesarhlA,brhlB, crhlCand exoproteasedlasAand elastaseelasB. Relative mRNA levels for target genes were normalised to the geometric mean of two reference genes (rpoDand
[image:7.595.175.544.52.519.2]associated regulatory and virulence genes during mid to late stationary phase of growth, when the cell density was at its highest.
CA at sub-inhibitory levels (2.27 mM) significantly (p< 0.001) reduced the expression of the QS transcriptional reg-ulatory genes lasR and rhlR(Fig. 3c). CA caused a 7-fold
lasR PAO 1 W T +C A +S
+CA + A +C30 F 0 20 40 60 R e la ti v e E x pr e s s ion Le v e l lasI PAO 1W T PAO 1CA PA O1+ SA PAO1 +C A+SA PA O1+C 30F 0 200 400 600 800 * * * Re la ti v e E x p re ssi o n L e ve l rhlR 0 50 100 150 *** *** ** rhlI PAO1 WT PAO1 +C A PAO 1+SA PAO 1+CA +SA PA O1+C 30F 0 100 200 300 400 Re lat ive E x p ress io nL e v e l * O O O O O Br O Br Trans-cinnamaldehyde [CA] Salicylic acid [SA] Furanone [C30F] PAO 1 PAO 1 PAO 1 PAO 1 S A PA O1 W
T +C A +S +C A+ A
+C30 F
PA O1
PAO 1
PA
O1 PAO 1 S A
a
b
c
0 3 6 9 12 24 48 72
0.01 0.1 1 10 PAO1 CA SA CA+SA C30F Log Early Stationary Mid-Late Stationary Time (h) OD 6 0 0n m Log 10 *
0 3 6 9 12 24 48 72
Fig. 3 Quorum sensing inhibitors (QSIs) significantly reduce
expression oflasandrhlQS systems inP. aeruginosa.aMolecular structure of the natural QSIs used in this study, salicylic acid (SA), trans-cinnamaldehyde (CA) and positive control furanone C30 (C30F).bGrowth ofP. aeruginosawith QSIs at sub-MIC concentra-tions (SA 3.62 mM, CA 2.27 mM and C30F 10μM). cRelative
expression of QS regulatory geneslasR, lasI,rhlRandrhlIwith com-binations of QSI treatments. Relative mRNA levels for target genes were normalised to the geometric mean of two reference genes (rpoD
[image:8.595.75.512.53.589.2]reduction (p< 0.001) inlasRgene expression while the differ-ence between the untreated and treated cells was even higher in the LasR-controlledrhlRexpression with a reduction of 19-fold being observed. CA also effected a significant (p< 0.001) reduc-tion in the AHL synthase gene expressions during the stareduc-tionary phase of growth. The downregulation in therhlIsynthase gene following treatment was 6-fold while inlasIsynthase, it was 13-fold during the late stationary phase.
The second inhibitor tested was the plant hormonal com-pound SA at sub-inhibitory concentration of 3.62 mM. This also caused inhibition in QS gene expressions but unlike CA, the overall reductions were lower (Fig.3c). The compound seemed to have a greater inhibitory effect on thelasQS circuit unlike CA which effectively repressed bothlas andrhl QS synthase and regulatory genes. The downregulation in QS tran-scriptional regulatory geneslasRandrhlRdue to SA treatment was 2-fold and 4-fold, respectively. The transcript levels of the
lasIsynthase gene were three times lower following SA treat-ment, while there was no significant reduction in expression of therhlIsynthase gene in the stationary phase. The behaviour of thelasRandrhlRregulatory genes determines the expression of virulence-related genes associated with the QS mechanisms in
P. aeruginosa; therefore, these results suggest that SA would not produce a very high downregulation in QS-regulated viru-lence gene expressions in comparison to CA.
Although CA and SA when used alone did show reduction in most QS gene transcripts but when used in combination (PAO1 + CA + SA) the results were inconclusive (Fig.3c). The combination treatment influenced thelasI synthase ex-pression where it reduced the transcript level by 5-fold. A similar reduction (3-fold) was also observed in the transcrip-tional regulatorrhlRexpression. However, the combination treatment did not exert any significant effect on the expression levels of thelasRandrhlIgenes. These results suggest that the inhibitory effects of CA and SA on the QS gene transcriptions were compromised when used in combination.
The positive control C30F produced an expected inhibitory effect on therhlcircuit ofP. aeruginosaduring the mid-late stationary phase when used at a concentration of 10μM (Fig.3c). TherhlRtranscript level was reduced 5-fold while the synthase gene rhlIwas repressed by 2-fold. The com-pound did not produce any significant inhibition on the tran-scription levels of thelasRIgenes.
Trans-cinnamaldehyde significantly reduces
expression of QS-regulated virulence factors
After investigating the effect of the experimental inhibitors on the QS master genes—lasRIandrhlRI,the study focused on investi-gating the inhibitory effects on thelasandrhlQS-regulated set of virulence genes. The target genes selected werelas-controlled
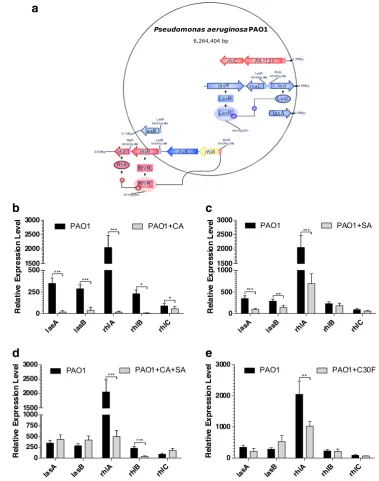
lasAprotease andlasBelastase and rhl-regulated genesrhlA, rhlBandrhlCassociated with rhamnolipid production (Fig.4a).
The target gene expression was normalised using validated refer-ence genes,rpoDandproC,across all culture conditions.
The significant inhibition in lasRI expressions in
P. aeruginosaPAO1 when subjected to CA as seen before af-fected the mRNA transcript levels of thelasAandlasBgenes (Fig. 4b). The relative expression data showed a 19-fold (p< 0.001) reduction inlasAgene expression whilelasBshowed a 7-fold (p< 0.001) reduction when compared to the untreated cells during the mid-late stationary growth phase. The compound was also effective in highly repressing the expression of the rhamnolipid synthesis rhlABCgenes during stationary phase (Fig.4b). The reduction in transcript level ofrhlAwas observed as greater than 100-fold (p< 0.001). A significantly high down-regulation was also observed inrhlB(p< 0.05) expression while a 2-fold reduction (p< 0.05) was observed inrhlCexpression.
The ability of SA to repress the las QS geneslasIandlasR
(Fig.3c) consequently influenced the virulence gene expres-sions oflasAandlasB. The transcript levels oflasAandlasB
were reduced by 4-fold (p< 0.001) and 2-fold (p< 0.01) re-spectively when treated with 3.62 mM SA (Fig.4c). It can be hypothesised that the inability of SA to produce an inhibitory effect onrhlIsynthase gene expression (Fig.3c) meant there were enough signal molecules to drive the expression of the
rhlABC genes. Although a 3-fold (p< 0.001) reduction was observed inrhlAgene expression, partly due to the reduced expression of therhlRregulatory gene, the overall inhibitory effect seemed small as insignificant reductions in rhlBand
rhlCgene expressions were observed with SA at the concen-tration tested in this experiment (Fig.4c).
The downregulation in thelasIsynthase gene when treated with both 2.27 mM CA and 3.62 mM SA did not correlate in a mRNA reduction of the las-regulated virulence geneslasA
and lasB(Fig.4d). However, the ability of the combination treatment to repress the QS regulatory rhlRgene caused a significant downregulation (p< 0.001) in the rhlAB genes. The combination treatment exerted a 4-fold decrease in the
rhlAgene and 6-fold decrease in the rhlBgene expressions compared to the untreated samples. Interestingly, a minor up-regulation of 2-fold was observed in therhlCgene.
The inability of C30F to reduce las-regulated QS regulato-ry and synthase gene expressions was only validated in the target gene expression analysis with no reduction being ob-served in las-controlled virulencelasAandlasBexpressions (Fig.4e). But C30F’s ability to reducerhlRIhad a consequen-tial effect on the expression ofrhlABgenes with reduction of 2-fold and 3-fold inrhlAandrhlBgene expressions respec-tively at late-mid stationary phases.
QSIs reduce production of extracellular virulence
factors at sub-MIC concentrations
outlined by O’Toole (2011) with slight modifications. The bio-film growth was visualised as a ring of biomass stained with crystal violet at the air-liquid interface. The biofilm formation was evaluated both in the presence and absence of inhibitory compounds, by measuring the absorbance of crystal-violet-stained-adherent-cells solubilised in ethyl acetate at 570 nm from stationary phase cultures (24-h) (Fig.5a). There were significant (p< 0.001) reductions in treated samples compared to the un-treated PAO1. In comparison to CA, SA was more effective in reducing the formation of biofilm in the microtiter wells with an absorbance reduction of 54% compared to the untreated cells. CA was also effective, to a lesser degree, with reductions of 26%. The combined use of CA and SA was the most effective of the
treatment methods with a reduction of 62%. The positive control C30F in comparison was the least effective (24%) in reducing biofilm formation.
LasA proteaseThere were significant reductions in LasA pro-tease activity in the QSI-treated cells as estimated from absor-bance reading resulting from azo dyes released into the medi-um due to proteolytic cleavage of the substrate azocasein. In the presence of SA, the OD440dropped from 0.3 to 0.1 to account for a 31% reduction (p< 0.05) in absorbance reading while CA treatment gave an even higher reduction of 65% (p< 0.01). The combination QSIs (CA + SA) treatment pro-duced the highest reduction in protease production with a
LasR LasI Pseudomonas aeruginosa PAO1
6,264,404 bp
PA 1131 rhlC
lasA
lasB
lasR rsaL lasI
RsaL binding site LasR binding site rhlB rhlR rhlI rhlA LasR binding site AlgR binding site LasR binding site LasR RhlI RhlR RhlR 1.2Mbp 1.6Mbp 2.0Mbp 3.8 Mbp 4.1 Mbp RhlR binding site
a
b
las regulon
rhl regulon
lasA las B
rhlA rhlB rhlC 0 250 500 1500 2000 2500 3000 PAO1 PAO1+CA *** *** *** * * Re la ti v e E xp res si o n L e ve l
lasA lasB rhlA rhlB rhlC 0 500 1000 1500 2000 2500 3000 PAO1 PAO1+SA *** ** *** Re la ti v e Ex pr es si o nL e v e l
lasA lasB rhlA rhlB rhlC 0 250 500 750 1000 1500 2000 2500 3000 PAO1 PAO1+CA+SA *** *** Re la ti v e E xp res si o n L e ve l
lasA lasB rhlA rhlB rhlC 0 1000 2000 3000 PAO1 PAO1+C30F ** R el a ti v e E x pr e ss io n Le v el
c
d
e
Fig. 4 Trans-cinnamaldehyde
(CA) significantly reduces gene expression of virulence-associated genes inP. aeruginosa
PAO1.aThe schematic representation of the genetic location of las and rhl QS systems inP. aeruginosa. Effect of QSIs at the following concentrations ofb 2.27 mM CA,c3.62 mM SA,d 2.27 mM CA + 3.62 mM SA and e10μM C30F on the
transcriptional expression of virulence-associated genes lasA, lasB, rhlA, rhlB and rhlC in
[image:10.595.167.547.53.535.2]reduction of 80% (p< 0.001) absorbance being observed when compared to the untreated PAO1 (Fig.5b).
LasB elastaseThe elastase production was estimated through absorbance measurement of Congo red following cleavage of elastin-Congo red substrate by the enzyme elastase produced byP. aeruginosa. In presence of inhibitors CA and SA, the OD495decreased from 0.08 to 0.06 and 0.05 respectively giv-ing subsequent reduction percentages of 22% (p< 0.01) and 28% (p< 0.05) (Fig. 5c). The combination treatment was again the most effective in reducing absorbance with reduc-tion of 46%. However, like the protease assay, C30F was the least effective, confirming earlier results of its reduced inhib-itory effects onlasAandlasBgene expressions.
PyocyaninPyocyanin production is regulated by therhlQS via PQS; hence, the measurement of pyocyanin inhibition is also a good indicator of the effectiveness of the tested compounds as QS inhibitors inP. aeruginosa. The pyocyanin concentration decreased from 3.1μg/ml to 2.1μg/ml and 0.922μg/ml in the presence of CA and SA, respectively. When used together, the inhibitors decreased the yield by 64% (1.1μg/ml) (Fig.5d).
Rhamnolipid estimationThe administration of the QS inhibi-tors caused a reduction in the yield of rhamnolipid with CA and SA both producing a drop in crude weight from 1.72 g/l to 0.7 g/ l (approximately) (Fig.6a). Rhamnolipids are produced as con-geners containing one to two rhamnose sugar moieties giving the compounds their distinctive properties (Chen et al.2010; Chen et al.2013). The structural composition of rhamnolipid
produced in the presence of the QSIs was studied by analysing the purified crude sample using high-performance liquid chro-matography mass spectrometry/mass spectrometry (HPLC-MS/ MS) method. The inhibitor treatment when used alone did not affect the composition of the rhamnolipid with the congener profile resembling the untreated PAO1 sample (Fig. 6c). But in the combination treatment (PAO1 + CA + SA), only two rhamnolipid congeners were detected by the HPLC-MS/MS method (Fig.6d) compared to six in the untreated sample. This combination treatment also effected the maximum reduc-tion in the relative amount of rhamnolipid obtained from a 50-ml culture supernatant. The two predominant congeners identified in all the samples were Rha-Rha-C10-C10(m/z649) and Rha-Rha-C10-C12 (m/z 677). The mono-rhamnolipid detected in greatest abundance was Rha-C10-C10. When the MS data for the two common di-rhamnolipid congeners were compared to untreated sample, the combination treatment and CA treatment showed marked differences (Fig. 6b). SA treatment did not show any noticeable difference in relative quantification of the congeners although it resulted in a decrease in crude weight.
Discussion
Trans-cinnamaldehyde is more effective than salicylic
acid in reducing expression of QS genes
This study is consistent with previous research showing that natural QSIs significantly modulate transcriptional expression of QS regulatory and virulence-associated genes during
Fig. 5 QSIs quantitatively reduce production of extracellular virulence factors inP. aeruginosa
[image:11.595.231.542.54.315.2]stationary phase inP. aeruginosaPAO1. CA effectively inhibited the expression of bothlasandrhlQS systems. Both the regula-tory proteins (LasR and RhlR) and the AHL synthases (LasI and RhlI) were significantly repressed with CA. Downregulation of both these QS systems correlated with repression of their virulence-associated genes. The exact mechanism of action is unknown for these compounds. Several natural and synthetic antagonists have been described for LasR; however, the relative instability of LasR::antagonist complexes has limited biochemi-cal characterisation in vitro. Recently, O’Reilly et al. (2018) used potent agonists rather than known antagonists to stabilise LasR in vitro. They were able to develop a focused library of agonists based on previous tri-phenyl ligands, resulting in several new LasR::agonist complexes available in/from the PDB. From these structures, O’Reilly et al. (2018) determined an important func-tional role for a flexible loop in the ligand binding domain
(LBD), previously unknown, which upon ligand binding pro-motes specific conformational changes that seals the ligand bind-ing pocket from solvent and directs the DNA bindbind-ing domain (DBD) to form a transcriptional activation complex. This sug-gests a plausible mechanism by which agonists stabilise and antagonists destabilise LasR. These structures provide essential information for the fundamental understanding of how LuxR type receptors bind to their cognate autoinducers.
We hypothesise that CA and SA act as QS antagonists. Previously, molecular docking studies have suggested CA to interact with the LasI protein (Chang et al.2014). LasI synthase produces 3-oxo-C12-HSL which is the ligand for LasR. RhlI is 47% homologous with LasI (Chang et al.2014; Gökalsın et al.
2017) and a similar mechanism of action is interpreted in AHL production. In general, LuxI synthases catalyses the transfer of an acyl group bound to acyl carrier protein (ACP) from fatty acid PA O1 PA O1+ CA PA O1+ SA PA O1+ SA +C A 0.0 0.5 1.0 1.5 2.0 ***
Rhamnolipid yeild g/l
PAO 1+CA PAO 1+S A PAO 1+C A+S A 0 50 100
Rha-Rha-C10-C10 Rha-Rha-C10-C12
59% 34% 1% -14% 97% 97% -20 % R e d u c tio n in R h a m n o lip id Co n g e n er Co m p o s it io n
PAO1 WT PAO1+ CA+SA
a
b
c
d
2.7 6 x10 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 0.7 0.6 0.50.5 1.01.52.0 2.53.0 3.54.0 4.5 5.05.56.0 6.5 7.0 7.58.0 8.5 9.0 Counts Vs. Acquisition Time (min)
1.65 6 x10 1.60 1.55 1.50 1.45 1.40 1.35 1.30 1.25 1.20 1.15 1.05 1.05 1.00 0.95 0.90 0.80 0.85 0.75 0.70 0.65 0.60 0.55 0.50 0.40 0.35
0.51.0 1.5 2.02.5 3.0 3.54.0 4.5 5.0 5.56.0 6.57.07.5 8.0 8.59.0 Counts Vs. Acquisition Time (min)
0.45
Fig. 6 QSIs significantly reducerhl-regulated rhamnolipid production
P. aeruginosa. a The QSIs reduces the rhamnolipid crude yield significantly compared to the untreated PAO1 cells.b% reduction in the two main RL congeners Rha-C10-C10and Rha-Rha-C10-C10relative
[image:12.595.198.504.52.446.2]bio-synthesis to S-adenosyl–L-methionine (SAM) (Churchill and Chen2011) which then undergoes lactonization to form the N-acyl–homoserine lactone. CA is predicated to bind in the SAM binding pocket of LasI, thus preventing SAM binding and subsequent 3-oxo-C12-HSL synthesis. In the absence of AHL, LasR will not dimerize and therefore cannot bind to DNA. These interactions could modulate the QS autoinducer levels. The LasI::3-oxo-C12-HSL complex regulates the expression of many downstream genes includinglasIandrhlI. Since we showed CA reduceslasIexpression and previously CA has shown to reduce signal molecule concentration (Chang et al.2014), we suggest that the intracellular concentration of autoinducer signal mole-cules was not sufficient to trigger the activation of genes involved with rhamnolipid and protease synthesis as shown in this study. To date, there is no crystal structure for RhlR, its inherent instability in vitro has proven intractable to crystallisation and biochemical characterisation. Based on similarity, mechanistic interpretations of LasR with AHL ligands and inhibitors are expected to extend to RhlR. However, RhlR remains a viable QS target for developing targeted inhibitors,lasRmutants are frequently isolated from cystic fibrosis patients suggesting the redundancy of LasR as a master regulatory in chronic CF infections (Feltner et al.2016).
The reductions inlasRIandrhlRIexpressions from CA treat-ments were correlated by assessing the activity oflas-regulated elastase and protease and rhl-regulated pyocyanin and rhamnolipid productions. CA at sub-inhibitory concentrations caused a significant decrease in elastase (22%) and protease (65%) activities. Pyocyanin, which is a good indicator ofrhlI
inhibition (Chang et al.2014), showed a decrease of 32% with CA. These reductions were comparable to other findings with cinnamaldehyde as QSI in the literature (Brackman et al.2008; Brackman et al.2011). Although CA was not able to abolish rhamnolipid production, the treatment caused a decrease in rhamnolipid yield with the two main di-rhamnolipid congeners Rha-Rha-C10−C10and Rha-Rha-C10-C12levels reduced by 59% and 34% respectively compared to that of untreated cells. The inhibition at post-translational levels of these virulence factors complemented the reverse transcription quantitative polymerase chain reaction (RT-qPCR) data from this study where we ob-served significant reductions inlasA,lasB,rhlA,rhlBandrhlC
expressions following CA treatment.
SA unlike CA did not produce the same level of inhibition on the transcriptional profiles of thelasRIandrhlRIIgenes with 2–4-fold reduction in mRNA levels being observed in the treated samples compared to untreated controls. The binding affinity of SA to the LasR protein (Yang et al.2009) possibly promoted conformational changes in the LasR-(3-oxo-C12 -HSL) complex thereby causing a reduced expression of down-stream genes. Due to the QS hierarchical arrangement,rhlR
expression can be regulated bylasR; hence, the highest inhi-bition in QS regulatory expression with SA was seen inrhlR. This was in agreement with a previously reported study where
SA was shown to reduce rhlRexpression in P. aeruginosa
(Yang et al.2009). The decreased expression inlasQS genes consequently repressed lasAand lasBlevels supporting the findings of Prithiviraj et al. (2005) using SA. Since SA did not lead to an inhibitory effect on the overall rhlregulon, significant downregulation was not observed in therhlBand
rhlCgenes. El-Mowafy et al. (2014) reported SA rich aspirin could cause significant downregulation in thelasRIandrhlRI
expressions. The findings however do not fully agree with the results from this study. The study with aspirin (El-Mowafy et al. 2014) used only one reference gene, rpoD, for data normalisation along with a higher concentration of the inhib-itor thereby giving slightly different results. Although SA did not show a profound effect at the transcriptional level, it seemed to be effective at the translational level. This can be hypothesised from this study considering higher reductions in virulence proteins elastase and protease were observed in the semi-quantitative assays following SA treatment. Reduction of these proteases when P. aeruginosa were supplemented with SA had been previously reported in a couple of studies with inhibition ranging between 40 and 80% (Prithiviraj et al. 2005; El-Mowafy et al. 2014). The choice of semi-quantitative assay and the selection of growth medium were perhaps responsible for the large inhibitory range being ob-served within the results published in the literature (Duan and Surette2007). The choice of media is very important as the production of secondary metabolites can be influenced by growth limiting factors present in the medium. However, SA had a negligible effect on therhl-controlled rhamnolipid pro-duction with HPLC results being similar to the untreated sam-ple. This complemented the qPCR findings where minimal reduction was seen in the rhamnolipid biosynthesis gene ex-pressions. Moreover, the unavailability of the RhlR crystal structure makes it difficult to predict the possible interaction sites for these inhibitors.
The combination treatment of CA and SA does not
show significant inhibitory effect on QS gene
expressions
HPLC-MS/MS analysis showed negligible presence of rhamnolipids strengthening the idea that the effect of the combi-nation treatment was strongly at the translational level. A com-putational model study of LuxI/LuxR QS suggested that LuxR competitive inhibitor, unlike LuxR non-competitive inhibitor, can display antagonistic effects when used in combination with a LuxI inhibitor (Anand et al.2013). Therefore, if an analogy is drawn with SA targeting LasR through competitive inhibition, then some of the inhibitory potential of LuxI-type inhibitor CA can be attenuated. However, the mechanism by which this could happen is not known and was beyond the scope of current work. A better understanding on how the inhibitors bind to the target proteins will help to elucidate the lower inhibitory effects ob-served at expression levels with the combination treatment espe-cially when we consider that significant downregulation was observed with CA alone.
With antibiotics fast losing their efficacy, alternative strategies are imperative. The sole use of QS inhibitors is unlikely to completely eradicate the bacterial infection and there would be legitimate concerns around potential toxicity of high concentra-tions of cinnamaldehyde where maximum permissible levels in foodstuffs have already been determined (Shreaz et al.2016). However, since the inhibitors reduce the virulence phenotypes and weaken the bacterial biofilms, this would allow the host innate immunity and externally administered antimicrobial com-pounds to function more effectively. Synergistic enhancement of antibiotics by administration of sub-inhibitory quorum quenching compounds is a potentially exciting future develop-ment but little is known about such effects at the molecular level. Our system provides a suitable model system for future studies aimed at elucidating these mechanisms and should contribute to extending the useable life span of current drugs.
Funding This work was supported by Ulster University, Northern
Ireland through a Vice Chancellor’s Research Scholarship studentship to SAKS Ahmed.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of
interest.
Ethical approval This article does not contain any studies with human
participants or animals performed by any of the authors.
Open Access This article is distributed under the terms of the Creative C o m m o n s A t t r i b u t i o n 4 . 0 I n t e r n a t i o n a l L i c e n s e ( h t t p : / / creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appro-priate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s noteSpringer Nature remains neutral with regard to jurisdic-tional claims in published maps and institujurisdic-tional affiliations.
References
Aghazadeh M, Hojabri Z, Mahdian R, Nahaei MR, Rahmati M, Hojabri T, Pirzadeh T, Pajand O (2014) Role of efflux pumps: MexAB-OprM and MexXY(-OprA), AmpC cephalosporinase and OprD porin in non-metallo-β-lactamase producingPseudomonas aeruginosaisolated from cystic fibrosis and burn patients. Infect Genet Evol 24:187–192 Anand R, Rai N, Thattai M (2013) Interactions among quorum sensing
inhibitors. PLoS One 8:e2254
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250 Anwar H, Costerton JW (1990) Enhanced activity of combination of
tobramycin and piperacillin for eradication of sessile biofilm cells ofPseudomonas aeruginosa. Antimicrob Agents Chemother 34: 1666–1671
Bottomley MJ, Muraglia E, Bazzo R, Carfì A (2007) Molecular insights into quorum sensing in the human pathogenPseudomonas aeruginosafrom the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem 282:13592–13600
Brackman G, Defoirdt T, Miyamoto C, Bossier P, Van Calenbergh S, Nelis H, Coenye T (2008) Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence inVibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol 8:149–162
Brackman G, Celen S, Hillaert U, van Calenbergh S, Cos P, Maes L, Nelis HJ, Coenye T (2011) Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence ofVibrio spp. PLoS One 6:e16084 Brown MRW, Allison DG, Gilbert P (1988) Resistance of bacterial
biofilms to antibiotics a growth-rate related effect? J Antimicrob Chemother 22:777–780
Chang CY, Krishnan T, Wang H, Chen Y, Yin WF, Chong YM, Tan LY, Chong TM, Chan KG (2014) Non-antibiotic quorum sensing inhib-itors acting against N-acyl homoserine lactone synthase as druggable target. Sci Rep 4
Chen ML, Penfold J, Thomas RK, Smyth TJP, Perfumo A, Marchant R, Banat IM, Stevenson P, Parry A, Tucker I, Grillo I (2010) Solution self-assembly and adsorption at the air-water interface of the monorhamnose and dirhamnose rhamnolipids and their mixtures. Langmuir 26:18281–18292
Chen M, Dong C, Penfold J, Thomas RK, Smyth TJP, Perfumo A, Marchant R, Banat IM, Stevenson P, Parry A, Tucker I, Grillo I (2013) Influence of calcium ions on rhamnolipid and rhamnolipid/anionic surfactant ad-sorption and self-assembly. Langmuir 29:3912–3923
Churchill MEA, Chen LL (2011) Structural basis of acyl-homoserine lactone-dependent signalling. Chem Reviews 111:68–85
Duan K, Surette MG (2007) Environmental regulationof Pseudomonas aeruginosaPAO1 Las and Rhl quorum-sensing systems. J Bacteriol 189:4827–4836
El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shaaban MI (2014) Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins inPseudomonas aeruginosa. Microb Pathog 74:25–32 Elshikh M, Ahmed S, Funston S, Dunlop P, McGaw M, Marchant R,
Banat IM (2016) Resazurin-based 96-well plate microdilution meth-od for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett 38:1015–1019
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in
Pseudomonas aeruginosa: interchangeability of the two anthranilate synthase and evolutionary implications. J Bacteriol 172:884–900 Feltner JB, Wolter DJ, Pope CE, Groleau MC, Smalley NE, Greenberg
quorum-sensing hierarchy in pseudomonas aeruginosa. MBIO 7: e01513–16
Fu W, Xie W, Zhang Z, Wang S, Wu Q, Liu Y, Zhou X, Zhou X, Zhang Y (2013) Exploring valid reference genes for quantitative real-time PCR analysis inPlutella xylostella(Lepidoptera: Plutellidae). Int J Biol Sci 9:792–802
Gellatly SL, Hancock REW (2013)Pseudomonas aeruginosa: new in-sights into pathogenesis and host defenses. Pathog Dis 67:159–173 Gökalsın B, Aksoydan B, Erman B, Sesal NC (2017) Reducing virulence
and biofilm ofPseudomonas aeruginosaby potential quorum sens-ing inhibitor carotenoid: zeaxanthin. Microb Ecol 74:466–473 Gould TA, Schweizer HP, Churchill MEA (2004) Structure of the
Pseudomonas aeruginosaacyl-homoserinelactone synthase LasI. Mol Microbiol 53:1135–1146
Gupta P, Chhibber S, Harjai K (2015) Efficacy of purified lactonase and ciprofloxacin in preventing systemic spread of Pseudomonas aeruginosain murine burn wound model. Burns 41:153–162 Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N,
Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Høiby N, Givskov M (2003) Attenuation ofPseudomonas aeruginosa vir-ulence by quorum sensing inhibitors. EMBO J 22:3803–3815 Hossain MA, Lee SJ, Park NH, Mechesso AF, Birhanu BT, Kang J, Reza
MA, Suh JW, Park SC (2017) Impact of phenolic compounds in the acyl homoserine lactone-mediated quorum sensing regulatory path-ways. Sci Rep 7:10618
Jayelakshmi H, Omanakuttan A, Pandurangan N, Vargis VS, Maneesh M, Nair BG, Kumar GB (2016) Clove bud oil reduces kynurenine and inhibits pqsA gene expression inP. aeruginosa. Appl Microbiol Biotechnol 100:3681–3692
Jefferson KK, Goldmann DA, Pier GB (2005) Use of confocal micros-copy to analyze the rate of vancomycin penetration through
Staphylococcus aureusbiofilms. Antimicrob Agents Chemother 49:2467–2473
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 32:224–245
Kim HS, Lee SH, Byun Y, Park HD (2015) 6-Gingerol reduces
Pseudomonas aeruginosabiofilm formation and virulence via quo-rum sensing inhibition. Sci Rep 5
Kisch JM a, Utpatel C, Hilterhaus L, Streit WR, Liese A (2014)
Pseudomonas aeruginosabiofilm growth inhibition on medical plastic materials by immobilized esterases and acylase. Chembiochem 15:1911–1919
Lee J, Zhang L (2014) The hierarchy quorum sensing network in
Pseudomonas aeruginosa. Protein Cell 6:26–41
López-Causapé C, Sommer LM, Cabot G, Rubio R, Ocampo-Sosa AA, Johansen HK, Figuerola J, Cantón R, Kidd TJ, Molin S, Oliver A (2017) Evolution of thePseudomonas aeruginosamutational resistome in an international cystic fibrosis clone. Sci Rep 7:5555 Luna RA, Millecker LA, Webb CR, Mason SK, Whaley EM, Starke JR,
Hiatt PW, Versalovic J (2013) Molecular epidemiological surveil-lance of multidrug-resistantPseudomonas aeruginosain a pediatric population of patients with cystic fibrosis and determination of risk factors for infection with the Houston-1 strain. J Clin Microbiol 51: 1237–1240
Luo J, Dong B, Wang K, Cai S, Liu T, Cheng X, Lei D, Chen Y, Li Y, Kong J, Chen Y (2017) Baicalin inhibits biofilm formation, attenu-ates the quorum sensing-controlled virulence and enhances
Pseudomonas aeruginosaclearance in a mouse peritoneal implant infection model. PLoS One 12:e0176883
Morohoshi T, Someya N, Ikeda T (2009) Novel N-acylhomoserine lac-tone-degrading bacteria isolated from the leaf surface ofSolanum tuberosum and their quorum-quenching properties. Biosci Biotechnol Biochem 73:2124–2127
Musthafa KS, Ravi AV, Annapoorani A, Packiavathy ISV, Pandian SK (2010) Evaluation of anti-quorum-sensing activity of edible plants
and fruits through inhibition of the n-acyl-homoserine lactone sys-tem inChromobacterium violaceumandPseudomonas aeruginosa. Chemotherapy 56:333–339
O’Reilly MC, Dong SH, Rossi FM, Karlen KM, Kumar RS, Nair SK, Blackwell HE (2018) Structural and biochemical studies of non-native sgonists of the lasR quorum-sensing receptor Reveal an L3 loopBOut^conformation for lasR. Cell Chem Biol 25:1128–1139 O’Toole GA (2011) Microtiter dish biofilm formation assay. J Vis Exp 47:
e2437
Ouyang J, Sun F, Feng W, Sun Y, Qiu X, Xiong L, Liu Y, Chen Y (2016) Quercetin is an effective inhibitor of quorum sensing, biofilm for-mation and virulence factors inPseudomonas aeruginosa. J Appl Microbiol 120:966–974
Papenfort K, Bassler BL (2016) Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol 14:576–588 Patel SJ, Oliveira AP, Zhou JJ, Alba L, Furuya EY, Weisenberg SA, Jia H,
Clock SA, Kubin CJ, Jenkins SG, Schuetz AN, Behta M, Della-Latta P, Whittier S, Rhee K, Saiman L (2014) Risk factors and outcomes of infections caused by extremely drug-resistant gram-negative bacilli in patients hospitalized in intensive care units. Am J Infect Control 42:626–631
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Prithiviraj B, Bais HP, Weir T, Suresh B, Najarro EH, Dayakar BV, Schweizer HP, Vivanco JM (2005) Down regulation of virulence factors ofPseudomonas aeruginosaby salicylic acid attenuates its virulence onArabidopsis thaliana andCaenorhabditis elegans. Infect Immun 73:5319–5328
Rasmussen R (2001) Quantification on the LightCycler. In: Rapid cycle real-time PCR. p. 21–34
Scott M, Hwa T (2011) Bacterial growth laws and their applications. Curr Opin Biotechnol 22:559–565
Shreaz S, Wani WA, Behbehani JM, Raja V, Irshad M, Karched M, Ali I, Siddiqi WA, Hun LT (2016) Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 112:116–131 Skindersoe ME, Alhede M, Phipps R, Yang L, Jensen PO, Rasmussen
TB, Bjarnsholt T, Tolker-Nielsen T, Høiby N, Givskov M (2008) Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:3648–3663 Smyth TJP, Perfumo A, Marchant R, Banat IM (2010) Isolation and
analysis of low molecular weight microbial glycolipids. In: Handbook of hydrocarbon and lipid microbiology. p. 3706–3723 Sousa A, Pereira M (2014)Pseudomonas aeruginosadiversification
dur-ing infection development in cystic fibrosis lungs—a review. Pathogens 3:680–703
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FSL, Hufnagle WO, Kowallk DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GKS, Wu Z, Paulsen IT, Relzer J, Saler MH, Hancock REW, Lory S, Olson MV (2000) Complete genome se-quence ofPseudomonas aeruginosaPAO1, an opportunistic patho-gen. Nature 406:959–964
Valderrey AD, Pozuelo MJ, Jimenez PA, Macia MD, Oliver A, Rotger R (2010) Chronic colonization byPseudomonas aeruginosaof patients with obstructive lung diseases: cystic fibrosis, bronchiectasis, and chronic obstructive pulmonary disease. Diagn Microbiol Infect Dis 68:20–27 Vandesompele J, De Preter K, ilip P, Poppe B, Van Roy N, De Paepe A,
rank S (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:34–31
regulons: effects of growth phase and environment. J Bacteriol 185: 2080–2095
Werner E, Roe F, Bugnicourt A, Franklin MJ, Heydorn A, Molin S, Pitts B, Stewart PS (2004) Stratified growth inPseudomonas aeruginosa
biofilms. Appl Environ Microbiol 70:6188–6196
Wu H, Song Z, Hentzer M, Andersen JB, Molin S, Givskov M, Høiby N (2004) Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance inPseudomonas aeruginosalung infection in mice. J Antimicrob Chemother 53:1054–1061
Yang L, Rybtke MT, Jakobsen TH, Hentzer M, Bjarnsholt T, Givskov M, Tolker-Nielsen T (2009) Computer-aided identification of recog-nized drugs asPseudomonas aeruginosaquorum-sensing inhibitors. Antimicrob Agents Chemother 53:2432–2443