0022-538X/10/$12.00 doi:10.1128/JVI.02323-09
Copyright © 2010, American Society for Microbiology. All Rights Reserved.
Jaagsiekte Sheep Retrovirus Transformation in Madin-Darby Canine
Kidney Epithelial Cell Three-Dimensional Culture
䌤
Chassidy Johnson, Kiah Sanders, and Hung Fan*
Department of Molecular Biology and Biochemistry and Cancer Research Institute, University of California, Irvine, California 92697
Received 3 November 2009/Accepted 27 February 2010
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of a contagious lung cancer in sheep that shares
similarities with human bronchioloalveolar carcinoma (BAC). JSRV is unique because the envelope gene (env)
is the oncogene, as it can transform cells in culture and induce tumors in animals. The phosphatidylinositol 3-kinase (PI3K)–Akt–mTOR and H/N-Ras–MEK–mitogen-activated protein kinase (MAPK) pathways have been shown to be critical for Env transformation. However, the question still remains of how disruption of these pathways relates to tumor formation. To address this, JSRV Env transformation was studied in the context of epithelial structure, using the polarized Madin-Darby canine kidney (MDCK) epithelial cell three-dimensional (3-D) culture system. The results indicated that JSRV Env-transformed MDCK cells were larger and had full or multiple lumens, in contrast to the single lumens observed in controls. The altered phenotype was largely mediated by an increase in proliferation, in addition to overcoming the proliferative suppression signal. JSRV Env was not found to disrupt polarity or tight junctions or to inhibit lumen apoptosis. The PI3K-Akt-mTOR pathway was important for Env transformation in MDCK cells, although the mechanisms of action differed in 3-D and monolayer cultures. PI3K-dependent signaling to mTOR occurred in monolayers, while PI3K-inde-pendent signaling to mTOR occurred in 3-D culture. In contrast, the H/N-Ras-MEK-MAPK pathway was found to be inhibitory to transformation in both normal and transformed MDCK cells in 3-D culture. However, in monolayer culture, inhibition of MEK reverted the transformed phenotype, suggesting a different mecha-nism(s) of action in monolayer versus 3-D culture.
Lung cancer is the leading cause of cancer deaths worldwide. While most cases are associated with cigarette smoking, 15% of cases in men and 53% in women occur in nonsmokers (39). Lung cancer in nonsmokers is most commonly diagnosed as adenocarcinoma (21). Jaagsiekte sheep retrovirus (JSRV) is the etiological agent of a contagious neoplasm in sheep termed ovine pulmonary adenocarcinoma (OPA) (35). OPA is derived from secretory epithelial cells, type II pneumocytes, and Clara cells and closely resembles bronchioloalveolar carcinoma (BAC) in humans (36, 41). The similarities between OPA and BAC make JSRV and OPA a useful animal model for studying lung carcinogenesis.
JSRV is unique because the envelope protein (Env, encoded by theenvgene) is the oncogene. Expression of Env can trans-form cells in culture (2, 30, 43) as well as induce tumors in animals (38, 51). Initial studies illustrated the importance of the phosphatidylinositol 3-kinase (PI3K)–Akt–mTOR pathway in JSRV Env transformation. It is now clear that there are PI3K-dependent and -independent mechanisms of Akt-mTOR signaling. PI3K-dependent signaling was evident because JSRV Env-transformed cell lines have constitutively active Akt and inhibiting PI3K prevented transformation and caused re-version of the transformed phenotype (24, 25, 37, 52). On the other hand, PI3K-independent Akt activation was later dem-onstrated, since cells in which PI3K was disabled could still be
transformed by JSRV and, under these conditions, there was still activation of Akt (29).
More recently, the H/N-Ras–MEK–mitogen-activated pro-tein kinase (MAPK) pathway was found to play a more impor-tant role in JSRV Env transformation, since inhibiting MEK1 and H/N-Ras prevented transformation in NIH 3T3 and RK3E cell monolayers (28). Note that p38 MAPK has a negative role in JSRV Env transformation, as inhibiting p38 MAPK in-creases transformation. The finding that JSRV Env does not induce transformation solely through the PI3K-Akt-mTOR pathway is supported by the fact that not all OPA tissues demonstrate activated Akt (46). Together, these results under-score the complexity of JSRV Env transformation and the need to further understand the signaling pathways involved in transformation.
Madin-Darby canine kidney (MDCK) epithelial cells grown in three-dimensional (3-D) culture by being embedded in En-gelbreth-Holm-Swarm tumor extract (Matrigel) or collagen readily form spherical structures termed “acini.” Acini grow as a single layer of polarized cells that are attached to the under-lying basement membrane and enclose a central hollow lumen (7, 26, 33, 34). Acinar structure and cell-cell and cell-environ-ment interactions mimic what occursin vivo. Maintenance of this highly ordered structure is critical for proper differentiated cell function, and when it is disrupted, malignancy can ensue. The events of MDCK acinus formation have been well defined and include cell polarization, secretion of basement mem-brane, formation of tight junctions, and responsiveness to apop-totic and proliferative suppressive signals.
To date, JSRV Env transformation has been studied in monolayer culture. While this has proven informative, there are limitations because monolayers do not recapitulate the
* Corresponding author. Mailing address: Cancer Research Insti-tute, Department of Molecular Biology and Biochemistry, University of California, 839 Medical Sciences Ct., Room 236, Irvine, CA 92697. Phone: (949) 824-5554. Fax: (949) 824-4023. E-mail: hyfan@uci.edu.
䌤Published ahead of print on 10 March 2010.
5379
on November 8, 2019 by guest
http://jvi.asm.org/
polarized epithelial structures of the lung. Since JSRV Env can transform MDCK cells and the mechanisms for acinus forma-tion in 3-D culture have been well defined, study of JSRV-transformed MDCK cells in 3-D cultures is a useful approach to studying JSRV transformation under conditions more closely resembling those of lung epithelial cellsin vivo. In these studies, we found that Env disrupts MDCK acinar structure, leading to large aberrant structures with multiple lumens. This disruption is largely mediated by an increase in proliferation and also by overcoming the proliferative suppression signal associated with lumen clearing. JSRV Env did not disrupt establishment of cell polarity, tight junctions, or apoptosis as-sociated with lumen clearing. Signaling through PI3K-Akt-mTOR and H/N-Ras-MEK-MAPK differed between mono-layer and 3-D cultures.
MATERIALS AND METHODS
Cell lines and vectors. MDCK cells were grown at 37°C and 5% CO2in advanced minimal essential medium (MEM) supplemented with 10% fetal bo-vine serum, penicillin (100 U/ml), and streptomycin (100g/ml). The JSRV envelope-expressing vector pLXSNenvHA (EnvHA) was generated by cloning JSRV Env cDNA containing a C-terminal influenza virus hemagglutinin (HA) tag into the XhoI restriction site of pLXSN (Clontech) and was confirmed by DNA sequencing. The Phoenix-ampho packaging cell line was provided by G. R. Nolan (http://www.stanford.edu/group/nolan/retroviral_systems/phx.html), and vector production was performed as outlined by the supplier, with the indicated modifications. Following transfection of Phoenix-ampho cells with pLXSN or pLXSNenvHA, cells were incubated at 32°C for 48 h and vectors were collected from the supernatant and processed. MDCK cells were seeded at 1⫻106
cells in a 10-cm plate, and the following day, infection by the vectors was performed (5⫻104
CFU/ml) in the presence of 4g/ml Polybrene. Stably transduced cell lines were obtained by selection in 800g/ml G418 for 2 weeks, and mass cultures resistant to G418 were continuously maintained in 400g/ml G418. Stable expression of Env was confirmed by Western blotting using an antibody to HA (Sigma), as described below. The stable cell lines were used in experiments after 2 passages (approximately 15 days after transduction).
Morphogenesis assay.Three-dimensional cultures were performed as previ-ously described (9), with the indicated modifications. Specifically, single-cell suspensions were resuspended in a collagen-Matrigel (BD Bioscience) mixture (80:20) at 6⫻103 cells/ml, and 2⫻104cells/well were seeded into 4-well chamber slides. Culture medium was replaced every other day. Rat tail collagen was prepared from rat tails as previously described (45). Pharmacological inhib-itors were employed to evaluate the signaling pathways of JSRV Env transfor-mation in 3-D culture. Cells were cultured in PP2 (20M), SB203580 (5M), PD98059 (20M), FTI-277 (5g/ml), rapamycin (10 ng/ml; Calbiochem), far-nesyl thiosalicylic acid (100M; Cayman Chemical), or LY294002 (10M; Cell Signaling) and replaced during medium changes.
Cellular proliferation assays.Stably transduced MDCK cells were plated in 6-well plates at 5.0⫻104
cells/well in duplicate. The number of cells/well was determined daily by trypan blue exclusion, using a hemacytometer. For prolif-eration assays in the presence of rapamycin (10 ng/ml), LY294002 (10M), or PD98059 (20M), medium with the inhibitors was replaced every other day.
Transformation assays.Stably transduced MDCK cells were seeded at 1.0⫻ 106cells per 10-cm plate and monitored for focus formation. Dishes were ana-lyzed by phase-contrast microscopy at various times, and photographs were taken. To determine the effects of signaling pathways on Env transformation, cells were treated with rapamycin, LY294002, FTI-277, or PD98059 at the dos-ages mentioned above. Reversion of the transformed phenotype was performed by seeding stably transduced cells at 5⫻104
cells/well in a 6-well plate and serum starving them for 24 h. Pharmacological inhibitors were added, morphology was observed 48 h later by phase-contrast microscopy, and photographs were taken.
Immunofluorescence.The immunostaining of acinar structures was performed as previously described (9). Antibodies used in analysis included Ki-67 (1:200; Zymed), c-caspase-3 (1:300; Cell Signaling), GM130 (1:300; BD Biosciences), HA (1:200; Cell Signaling), and ZO-1 (20g/ml; Invitrogen). Acini or EnvHA structures were counterstained with phallotoxin-546 and Prolong antifade DAPI (4⬘,6-diamidino-2-phenylindole; Molecular Probes). Structures were analyzed by confocal microscopy, using a Zeiss Axiovert 200M microscope, and were ana-lyzed with Zeiss LSM 510 META multispectral analyzer software. Quantitative
data were obtained by analyzing more than 100 acini/structure/sample for the indicated variable. The results are represented as averages for three independent experiments performed in duplicate. Cell death by apoptosis was confirmed by ethidium bromide staining of acini as described previously (9). Specifically, samples were stained in 1M ethidium bromide in phosphate-buffered saline (PBS) for 15 min at 37°C. Samples were visualized by fluorescence microscopy.
RESULTS
MDCK cells stably transduced with JSRV Env are
trans-formed.It has been shown that MDCK cells grown in a
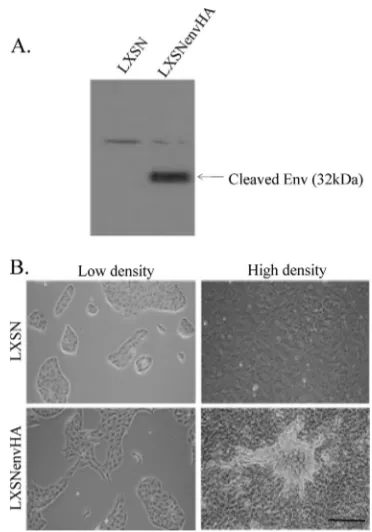
[image:2.585.326.512.66.332.2]mono-layer can be transformed by Env (25). To examine the impact of JSRV Env on MDCK cells grown in 3-D culture, MDCK cells were transduced with a murine leukemia virus-based ret-roviral vector encoding JSRV Env with a C-terminal influenza virus HA tag (LXSNenvHA). As a control, cells were also transduced with the backbone vector (LXSN). Transduced cells were selected with G418 and passaged two times before use. Stable expression of Env was confirmed by Western blot analysis of cell extracts, using an antibody for HA (Fig. 1A). The 32-kDa TM protein product containing the HA tag is indicated. Stable expression of Env resulted in morphological changes indicative of MDCK cell transformation (Fig. 1B). On plastic, EnvHA-transduced cells displayed an elongated shape at low density and foci formed at high density, in contrast to
FIG. 1. Stably transduced MDCK cells. MDCK cells were stably transduced with retroviral vector LXSN or LXSNenvHA and selected with G418 to create stably transduced lines. (A) SDS-PAGE and immunoblot analysis of cell lysates from MDCK cells stably transduced with LXSN or LXSNenvHA was performed using an antibody to the HA tag. The mobility of the posttranslationally cleaved Env protein (32 kDa) containing the HA tag is indicated. (B) The morphologies of stably transduced MDCK cell lines at low and high densities were examined by light microscopy. Photographs were taken 48 h (low density) and 20 days (high density) following plating. Magnification, ⫻100; bar, 200m.
5380 JOHNSON ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
the control LXSN-transduced cells, which maintained the cob-blestone growth pattern of normal MDCK cells (Fig. 1B). The finding that EnvHA cells displayed an elongated phenotype at low density and formed foci at high density indicated that LXSNEnvHA transforms MDCK cells, consistent with a pre-vious report (25). Based on transformed morphology, the transformation observed by Liu and Miller was higher than that observed in this study, which employed HA-tagged Env. JSRV Env proteins containing C-terminal epitope tags have a lower efficiency of transformation than does wild-type Env (17, 27, 28).
JSRV Env disrupts MDCK cell acinar size and structure.
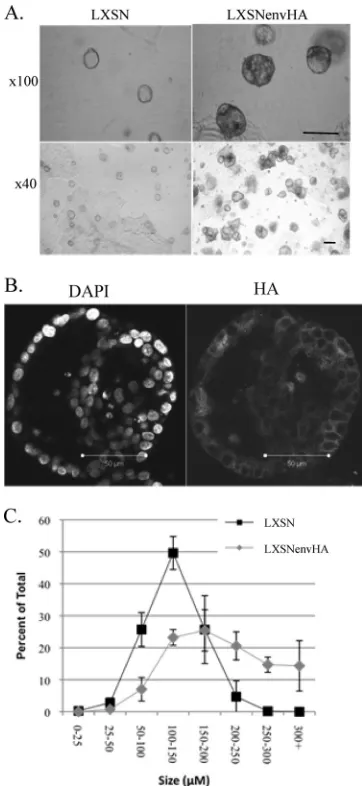
MDCK cell 3-D cultures were established by embedding a single-cell suspension of MDCK cells in a mixture of collagen type I and Matrigel. Control LXSN-transduced MDCK cells formed spherical structures termed acini in 3-D culture, and these were characterized by a single-cell ring of polarized cells enclosing a hollow lumen, as described previously (49, 50). In contrast, Env-expressing MDCK cells grown in 3-D culture resulted in aberrant acini (Env structures) that were larger and lacked the characteristic ring structure (Fig. 2A). Due to the fact that EnvHA-expressing MDCK cells do not form normal acini, they are referred to here as EnvHA structures. EnvHA was uniformly expressed in the structures, as determined by immunostaining with an antibody to HA (Fig. 2B). Control acini and EnvHA structures were scored by size at days 5, 10, and 20, and the percentages of the total for each size group were calculated (Fig. 2C and data not shown). As time pro-gressed, the EnvHA structures and control acini continued to increase in size, indicating that the cells were actively dividing. However, EnvHA structures showed more extensive increases in size. The most drastic difference in size was observed at day 20, when 49.6% of total EnvHA structures were 200 m or larger, in contrast to 4.9% for control acini (Fig. 2C). These results indicated that JSRV Env perturbed the 3-D structure and increased the size of MDCK acini.
The changes in EnvHA structures were further examined by staining with phallotoxin-546 (for actin) and DAPI (for nuclear DNA), and cross sections through the center of acini were visualized by confocal microscopy at days 5, 10, and 20 (Fig. 3A). LXSN-transduced cells formed well-organized acini con-taining a single layer of polarized cells (see below) surrounding a hollow lumen. In contrast, the EnvHA-transduced cells formed aberrant structures, with differences already evident by day 5. The aberrant morphology of EnvHA structures was most profound at day 20, when they contained multiple lumens (subacini) as well as dense areas of cells within the structure. Hollowness was used as an indicator of disrupted structure. A defect in hollow lumen formation was observed as early as day 5, with 58%⫾ 11% of control acini and 22.9%⫾ 6.2% of EnvHA structures containing hollow lumens (Fig. 3B). At day 20, 92%⫾ 5.7% of control acini were hollow, compared to 3.8%⫾ 2.5% of EnvHA structures. The EnvHA structures were characterized predominantly by multiple or partial lu-mens rather than a single hollow lumen. In some cases, pro-truding areas comprised of individual lumens were present, resulting in loss of the overall spherical shape.
Cell polarization is an early event in 3-D structure formation for epithelial cells and is a prerequisite for lumen formation (8). This raised the possibility that multiple lumens in EnvHA
[image:3.585.329.510.68.461.2]structures reflected a relative defect in polarization. Actin lo-calization is used as a marker for polarization because it local-izes to microvilli at the apical region of polarized epithelial cells (48). Control and EnvHA structures were polarized by days 5, 10, and 20, with the apical surfaces in the interior of the acini. However, in EnvHA structures, apical polarization was detected only in subregions where lumens had formed (Fig. 3A). Localization of thecis-Golgi protein GM130 is another marker of apical polarization. Consistent with actin staining, GM130 localized toward the lumen in control acini and EnvHA structures (Fig. 3C). In EnvHA structures, polariza-tion toward the lumen was observed by GM130 staining in the subacini as well (Fig. 3C). In EnvHA structures, polarization
FIG. 2. Effect of JSRV Env on acinus size. LXSN- and LXSNenvHA-transduced MDCK cells were grown in 3-D culture. (A) Light microscopy photographs of acini at day 20 of culture. Bar, 200M. (B) Acini were stained with DAPI (left) and anti-HA (right) at day 20 of culture and then examined by confocal microscopy. Magnification,⫻630. (C) The diame-ters through the cendiame-ters of acini were measured at 20 days, grouped into the indicated size categories, and represented as percentages of the total (means⫾standard errors). The results are representative of three inde-pendent experiments performed in triplicate, and more than 100 acini were counted.
on November 8, 2019 by guest
http://jvi.asm.org/
FIG. 3. Effect of JSRV Env on acinus structure and polarization. (A) LXSN- and LXSNenvHA-transduced MDCK cells were grown in 3-D culture and counterstained with DAPI (nuclei; blue) and phalloidin (actin; red) at the indicated time points. Shown are confocal cross sections through the center of the structures. (B) Acini were analyzed for hollowness at days 5 and 20, and the results are represented as percentages of total acini analyzed (ⱖ100) that were hollow. The experiment was performed in duplicate, and the means (⫾standard errors) from 3 independent experiments are shown. Statistical significance was determined by Student’sttest.*,P⫽0.001;**,P⫽0.00007. (C) Acini were immunostained with anti-GM130 (green) and DAPI (blue) at days 10 and 20 of culture and then analyzed by confocal microscopy. Higher-magnification images of the indicated areas (boxes) are shown in the insets. (D) Transduced MDCK cells were seeded in collagen (bottom) or Matrigel (top), immunostained with anti-GM130 (green), phalloidin (red), and DAPI (blue) at days 1, 2, and 5, and examined by confocal microscopy. Magnification,⫻400; bars, 50m.
5382 JOHNSON ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
was not dependent on cell contact with the extracellular matrix (ECM) of the Matrigel, since internal subacini also showed polarization. These results indicated that JSRV Env did not interfere with the intrinsic ability of MDCK cells to polarize. Next, earlier time points, when polarization is being estab-lished (days 1, 2, and 5), were examined to determine if there was a delay in establishment of polarity in EnvHA structures. Polarization was monitored in matrices that mediate rapid polarization (Matrigel) or delayed polarization (collagen). Us-ing actin and GM130 localization as indicators of polarity, no difference in kinetics of polarization was observed between the EnvHA- and control-transduced cells (Fig. 3D). Specifically, polarization was observed after 1 day in Matrigel culture and after 5 days in collagen culture. EnvHA structures grown in Matrigel displayed complete lumen formation on day 2 and multiple lumens on day 5. Thus, EnvHA structures polarize efficiently and can form proper lumens. The full- or multiple-lumen phenotype of JSRV-transformed MDCK cells was not due to a defect in establishing polarization; another mecha-nism(s) is involved. There was a considerable difference be-tween the sizes of EnvHA and control structures on day 2 (8 to 11 cells for EnvHA versus 2 to 4 cells for control structures) (Fig. 3D). This suggested that the abnormal behavior of JSRV EnvHA-transformed MDCK cells in 3-D culture might be at-tributed to increased proliferation.
JSRV Env increases proliferation and overcomes
prolifera-tive suppression signals.To determine if increased
prolifera-tion was the cause for larger and abnormal EnvHA structures, samples were immunostained with an antibody to Ki-67, a marker for cycling cells, and analyzed by confocal microscopy. As expected, no Ki-67-positive cells in control MDCK acini were observed at days 10 and 20 (Fig. 4A). In contrast, a notable number of cells in EnvHA structures were Ki-67 pos-itive. These results indicate that the disrupted structure and increased size of the EnvHA structures were at least partially attributable to an increase in proliferation. There was no de-finitive correlation between Ki-67 expression and localization of cells in the periphery versus the lumen. The fact that the Ki-67-positive cells were located in both the lumen and periph-ery indicates that interaction with ECM is not required to maintain proliferation of Env-expressing cells. Furthermore, the Env-mediated increase in proliferation was not biased to polarized or nonpolarized cells in the acini. These findings are consistent with the behavior of c-Myc-transformed MDCK cell acini, where c-Myc-induced proliferation and morphological alterations affect all compartments of the acini (40).
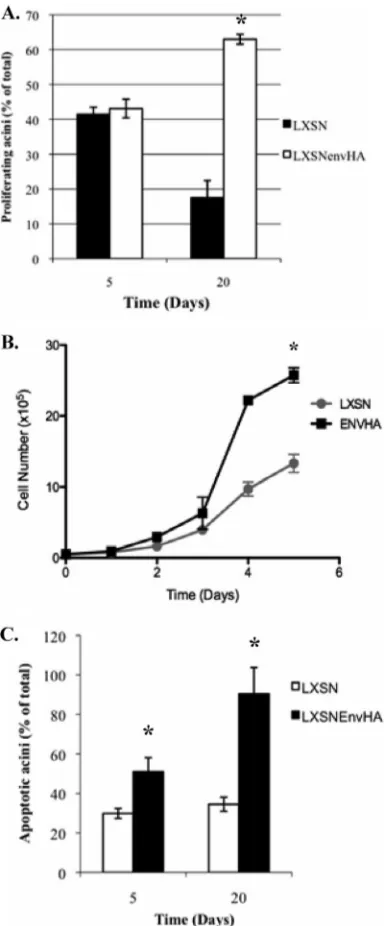
Epithelial cell acinar formation is characterized by two phases: a developmental stage when the cells proliferate followed by a maturation stage when proliferation is suppressed. Specifically, developing acini undergo proliferative suppression around day 10, and the structure of the acinus is maintained at this point (8, 40). To determine if Env-transformed MDCK cells can overcome the proliferative suppression signal in 3-D culture, the percentage of acini containing one or more cells expressing Ki-67 was determined on days 5 and 20 (Fig. 5A). There was no difference in the percentages of Ki-67-positive acini on day 5 (46% in LXSN-transduced cells versus 48% in LXSNenvHA-transduced cells) (Fig. 5A), although there were noticeably more Ki-67-positive cells per acinus in EnvHA structures than in the control acini (data not shown). In contrast, more
Ki-67-positive EnvHA structures than Ki-67-Ki-67-positive control acini were observed at day 20 (23% for LXSN-transduced cells ver-sus 63% for LXSNenvHA-transduced cells) (Fig. 5A). These results indicate that EnvHA-transformed cells were resistant to proliferative suppression signals in 3-D culture. To determine if the increase in growth mediated by Env is limited to 3-D culture, cell growth was also monitored in monolayer cultures on plastic. The results indicated that MDCK cells expressing EnvHA proliferated faster than vector control cells (Fig. 5B). Together, these findings indicate that JSRV Env is a potent inducer of proliferation in MDCK cells grown in 3-D and monolayer cultures.
Env structures are hyperapoptotic. Cells in the center of
acini undergo anoikis, a form of apoptosis in response to ma-trix detachment (4, 11). Responsiveness to this apoptotic signal and to proliferative suppression signals is critical for formation and maintenance of acinar structure. Expression of oncogenes can result in aberrant structures by mediating resistance to the apoptotic and proliferative suppression signals. In mammary epithelial cells transformed by the c-Myc oncogene, resistance to apoptosis is required for the transformed phenotype where cells remain in the interiors of acini grown in 3-D culture (8). Therefore, it was possible that JSRV Env mediated resistance to apoptosis, resulting in the full- or multiple-lumen pheno-type. To test this possibility, apoptosis was monitored at days 5 and 20 by staining for expression of cleaved caspase-3 (c-caspase-3), an effector caspase in apoptosis. High levels of apoptosis in EnvHA structures were detected at all time points analyzed and were most profound at day 20 (Fig. 4B). Specif-ically, ⬃90% of EnvHA structures contained one or more apoptotic cells at day 20, compared to⬃34% of control acini (Fig. 5C). The high levels of apoptosis in EnvHA structures were confirmed by staining acini with ethidium bromide, which stains DNA of apoptotic and dead cells. The results confirmed an overall high level of apoptosis and cell death in EnvHA structures compared to that in controls (Fig. 4C).
During MDCK cell acinus development, the apoptotic stim-ulus leading to lumen clearing does not occur until around day 10 in culture (8). Therefore, it was noteworthy that an increase in apoptosis was observed in EnvHA structures at day 5 (Fig. 5C). When localization of apoptotic cells in EnvHA structures was analyzed, it was found that apoptotic cells were localized to the periphery at day 5 and to both the periphery and interior at day 20 (Fig. 4B). It was also striking that in EnvHA structures, apoptotic cells were found at the periphery, whereas apoptosis in normal acinus formation is in the interior, leading to lumen clearing. Thus, the apoptosis observed in EnvHA structures is not due solely to the stimulus for lumen clearing, and another mechanism(s) is involved. Furthermore, the results indicate that Env does not mediate resistance to apoptosis and that lumen clearing still occurs, albeit incompletely—hence the subacini in the EnvHA structures. This is in contrast to the case for expression of oncogenes such as c-Myc, where resistance to apoptosis is observed and full lumens result.
Disruption of cell-cell interactions is observed early in de-velopment of carcinomas, rendering the disorganized acini hy-persensitive to apoptotic stimuli (3, 8, 13, 20, 40). To test the possibility that the hyperapoptotic state of EnvHA structures was due to a disruption in cell-cell interactions, the presence of tight junctions was monitored by immunostaining for ZO-1.
on November 8, 2019 by guest
http://jvi.asm.org/
FIG. 4. EnvHA structures are defective in responses to growth regulatory signals. (A) Confocal cross sections through the center of acini stained with phalloidin (red) and immunostained with anti-Ki-67 (green) at 10 and 20 days in culture. Magnification,⫻400; bar, 50 m. (B) Confocal cross sections through the center of acini stained with DAPI (blue) and phalloidin (red) and immunostained with anti-c-caspase-3 (green) at 5 and 20 days in culture. Magnification,⫻400; bar, 50m.*, c-caspase-3-positive cells. (C) Cell death in acini was monitored by ethidium bromide (EtBr) staining (1M) at 15 days of culture. Acini were analyzed by fluorescence microscopy; representative images are shown. Magnification, ⫻200; bar, 100 m. (D) Confocal cross sections of acini that were immunostained with an antibody to ZO-1 (green) and counterstained with DAPI (blue) are depicted. Magnification,⫻400; bars, 50m.
5384 JOHNSON ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
Tight junctions detected by ZO-1 are present at apical cell-cell junctions. Tight junctions were present in EnvHA structures, although only where polarization had occurred (Fig. 4D). These findings underscore the connection between cell-cell junctions and polarity (33). Together, the results indicate that apoptosis in EnvHA structures is driven by the stimulus for lumen clearing and by a second unknown mechanism(s), likely the high levels of proliferation. Evidence for this has been observed in c-Myc-expressing MCF-10A cells in 3-D culture, where hyperproliferation was responsible for the morphologi-cal alterations and spontaneous apoptosis that were observed (40).
Signaling pathways in JSRV Env transformation.Previous
reports of monolayer transformation assays indicate that JSRV Env signals through the PI3K-Akt-mTOR and H/N-Ras-MEK-MAPK pathways, with the latter playing a more critical role (28). MDCK cells transformed with JSRV Env were found to have PI3K-dependent constitutive activation of Akt (25). How-ever, the role of the PI3K-Akt-mTOR pathway on transforma-tion was not assessed. Additransforma-tionally, Src has been reported to play a role in JSRV Env transformation in other cell lines (17, 47). To further evaluate the role(s) of these pathways in trans-formation of MDCK cells, transtrans-formation was monitored in the presence of pharmacological inhibitors. Reversion of the transformed phenotype of EnvHA MDCK cells grown in the presence of pharmacological inhibitors was observed (Fig. 6 and data not shown). LY294002 is a potent inhibitor of PI3K, and rapamycin inhibits the downstream effector kinase, mTOR. As expected, dimethyl sulfoxide (DMSO)-treated EnvHA cells cultured at low density displayed a transformed phenotype compared to the cobblestone morphology of LXSN-transduced cells. At high density, many regions of over-growth were observed. Rapamycin-treated EnvHA cells cul-tured at low density were less spindle shaped, showing a de-crease in transformation. Inhibition of PI3K (LY294002) resulted in EnvHA cells that were morphologically similar to LXSN-transduced cells. The transformed phenotype of EnvHA cells at high density was decreased when they were treated with LY294002 and rapamycin (data not shown). Thus, inhibiting PI3K had a stronger effect on reversion of the trans-formed phenotype than inhibiting mTOR. The finding that inhibition of PI3K is more potent than that of mTOR at re-version of transformation is consistent with PI3K signaling upstream of mTOR. Together, these results indicate that the PI3K-Akt-mTOR pathway is important for Env transformation in MDCK cells grown in a monolayer.
Next, the role of the H/N-Ras-MEK-MAPK pathway in JSRV Env transformation in a monolayer was assessed. Inhi-bition of MEK (PD98059) caused reversion of the transformed phenotype of EnvHA cells at low and high densities (Fig. 6 and data not shown). Thus, signaling through MEK is important for Env transformation of MDCK cells in a monolayer. In contrast, inhibition of H/N-Ras with FTI-277 increased the transformed phenotype of LXSN- and EnvHA-transduced MDCK cells at low and high densities (Fig. 6 and data not shown). Therefore, H/N-Ras has an inhibitory effect on trans-formation in normal and Env-transformed MDCK cells.
[image:7.585.67.259.82.545.2]We next examined the roles of the PI3K-Akt-mTOR, Ras-MEK-MAPK, and Src pathways in Env transformation of MDCK cells in 3-D culture. LXSN acini or EnvHA structures
FIG. 5. EnvHA structures are hyperapoptotic and resistant to pro-liferative suppression signals. (A) Stably transduced MDCK cells were grown in 3-D culture and analyzed for Ki-67 expression at days 5 and 20, and the results are represented as percentages of the total acini analyzed (ⱖ100) that were Ki-67 positive. The experiment was per-formed in duplicate, and data represent the means (⫾standard errors) for 3 independent experiments. Statistical significance was determined by Student’sttest.*,P⫽0.006. (B) Cell growth curve for transduced MDCK cells. A total of 1⫻105cells were seeded in a 6-well plate in
duplicate, and the number of viable cells was determined by trypan blue exclusion over a period of 4 days. The results are representative of three independent experiments.*,P ⬍ 0.0001. (C) Transduced MDCK cells were grown in 3-D culture, and acini were analyzed for c-caspase-3 expression at days 5 and 20 of culture. The results are represented as percentages of the total acini analyzed (ⱖ100) that were positive. The experiment was performed in duplicate, and data represent the means (⫾standard errors) for 3 independent experi-ments.*,P⬍0.05.
on November 8, 2019 by guest
http://jvi.asm.org/
grown in the presence of pharmacological inhibitors were stained with phallotoxin (actin) and DAPI (nuclei), and the sizes and structures were assessed by confocal microscopy. Treatment with the MEK inhibitor (PD98059) actually en-hanced the transformed phenotype of EnvHA structures, as they were larger (64.15%⫾2.9% of acini wereⱖ150m with DMSO versus 86.75%⫾7.7% with PD98059) (Table 1) and more full lumens were observed than multiple lumens (Fig. 7). Interestingly, treatment of control acini with PD98059 also
[image:8.585.133.450.63.570.2]FIG. 6. Pathways of JSRV Env transformation. Stably transduced MDCK cells were serum starved for 24 h and then treated with DMSO, rapamycin (10 ng/ml), LY294002 (10M), PD98059 (20M), or FTI-277 (5g/ml) for 48 h, and light microscopy photographs were taken. Magnification,⫻100; bar, 200m.
TABLE 1. Effects of inhibitors on acinus size
Inhibitor
% Acini ofⱖ150ma
LXSN LXSNenvHA
DMSO 20.1⫾0.99 64.15⫾2.9
LY294002 2.2⫾1.3 38.4⫾11.34
Rapamycin 2.6⫾0.57 12.36⫾6.39
FTI-277 16.75⫾0.92 63.1⫾0.14
PD98059 75⫾6.9 86.75⫾7.7
a
Data are means⫾standard deviations.
5386 JOHNSON ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
resulted in abnormalities in growth, with the appearance of subacini (Fig. 7) and increased size (20.1%⫾0.99% of acini were ⱖ150 m with DMSO versus 75% ⫾ 6.9% with PD98059) (Table 1). Thus, negative regulation of cell growth by MEK may be a feature of both normal and transformed
[image:9.585.110.476.67.607.2]MDCK cells in 3-D culture. Treatment with an H/N-Ras in-hibitor (FTI-277) enhanced the transformed phenotype of EnvHA structures, as there was a further loss in the spherical structure, with filling of the lumens, and many areas no longer contained an outer ring of polarized cells (Fig. 7). Additionally,
FIG. 7. Pathways of Env transformation in 3-D culture. LXSN- and LXSNenvHA-transduced MDCK cells were grown in 3-D culture in the presence of the indicated inhibitors. Day 10 acini were counterstained with phalloidin (actin; red) and DAPI (nuclei; blue). Shown are confocal cross sections through the center of the structures.
on November 8, 2019 by guest
http://jvi.asm.org/
control acini treated with FTI-277 no longer displayed the spherical structure, and cells extended at the edges into the ECM, although there was no change in overall acinus size (64.15%⫾2.9% of acini were ⱖ150m with DMSO versus 63.1% ⫾ 0.14% with FTI-277) (Table 1). Inhibiting K-Ras (FTS) and p38 (SB203580) did not significantly change the size or structure of EnvHA structures (Fig. 7). Thus, as with mono-layer transformation, H/N-Ras has an inhibitory effect on transformation of normal and EnvHA-transformed MDCK cells, perhaps by signaling to MEK. Lastly, inhibition of Src (PP2) did not restore the transformed phenotype of Env acini (Fig. 7).
In contrast to the Ras-Raf-MEK-MAPK pathway, the PI3K-Akt-mTOR pathway played a major role in Env transforma-tion in 3-D culture. Inhibitransforma-tion of PI3K (LY294002) decreased EnvHA structure size (64.15%⫾2.9% of acini wereⱖ150m with DMSO versus 38.4%⫾11.34% with LY294002) (Table 1), but it did not restore the architecture (Fig. 7). In contrast, inhibition of mTOR (rapamycin) decreased acinar size more effectively (64.15% ⫾ 2.9% of acini were ⱖ150 m with DMSO versus 12.36%⫾6.39% with rapamycin) (Table 1) and largely restored the architecture, as many acini had hollow lumens (Fig. 7). To determine if the increase in proliferation in 3-D culture was due to activated PI3K-Akt-mTor signaling, proliferation in the presence of the inhibitors was monitored by staining for Ki-67. Overall, there was less Ki-67 expression per acinus when the cultures were treated with rapamycin and LY294002 than that with DMSO treatment. Although an over-all decrease in Ki-67 expression was observed, the percentage of proliferating acini in the presence of the inhibitors did not change compared to that with DMSO treatment (data not shown). These results indicate that the PI3K-Akt-mTOR path-way plays a critical role in Env transformation in 3-D culture and suggests that mTOR signaling is more critical than PI3K signaling in Env transformation in 3-D culture.
To determine if Env-enhanced cell proliferation through the PI3K-Akt-mTOR pathway is specific to 3-D culture, prolifer-ation in monolayers was monitored in the presence of rapa-mycin and LY294002. Proliferation of EnvHA-transformed MDCK cells in a monolayer was substantially decreased in the presence of LY294002 and rapamycin compared to that with DMSO treatment (Fig. 8A and B). However, treatment with PD98059 did not significantly affect proliferation of EnvHA-transformed MDCK cells compared to DMSO treatment (Fig. 8C). The finding that inhibition of MEK did not inhibit pro-liferation was striking.
DISCUSSION
In this report, we studied JSRV transformation in the con-text of epithelial structure by using the MDCK 3-D cell culture system. Results from this study indicated that Env expression greatly increases the size and disrupts the structure of MDCK acini. In particular, the EnvHA structures did not show the single hollow lumens typical of control MDCK cells and other epithelial cells grown in 3-D culture; multiple lumens or com-pletely filled structures were observed instead. Two EnvHA-induced changes were increased proliferation and a lack of the proliferative suppression that leads to lumen formation in con-trol MDCK acini.
[image:10.585.324.517.66.494.2]Establishment of epithelial cell polarization is critical for formation of acini with single lumens, and disruption in polar-ization results in acini with multiple lumens (32). However, in EnvHA structures, the ability to polarize was not disrupted, although subtle effects cannot be ruled out. For example, a defect in detection of hollow lumen space could result in mul-tiple lumens. In MDCK cells, overexpression of the polarity regulator PAR1 kinase blocks free-surface detection, resulting in multiple lumens (6). Other oncogenic viruses disrupt polar-ization pathways. For example, human papillomavirus (HPV) E6, human T-cell leukemia virus (HTLV) Tax, and arenavirus 9 ORF1 proteins interact with and degrade the polarity protein
FIG. 8. Pathways of JSRV Env proliferation. Cell growth curves are shown for transduced MDCK cells cultured in the presence of rapamycin (A), LY294002 (B), or PD98059 (C). A total of 1⫻105cells
were seeded in a 6-well plate in duplicate, and the number of viable cells was determined by trypan blue exclusion over a period of 4 days. The results are representative of three independent experiments.*, P⬍0.05.
5388 JOHNSON ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
discs large (Dlg), contributing to transformation (12, 14, 19, 22, 42). It is possible that JSRV Env alters one of the polarity pathways involved in sensing of apical space, resulting in mul-tiple lumens. Alternatively, mulmul-tiple lumens could result from high levels of proliferation and resistance to the proliferative suppression signals and/or apoptosis (see below).
In EnvHA structures, multiple lumens followed formation of a normal acinus that contained a single lumen (Fig. 3D). The fact that normal acini formed first, followed by multiple-lumen formation, suggests that excessive proliferation in Env-express-ing MDCK cells could be responsible for the observed pheno-type. Indeed, more cells were present in EnvHA structures than in control MDCK acini at all time points, and EnvHA structures were highly proliferative, as evident from Ki-67 ex-pression at all times. The proliferative effect of Env on MDCK cells was not limited to 3-D culture, as cells in a monolayer also displayed increased proliferation. OPA-derived type II pneu-mocytes also display high levels of proliferation in culture (46). In normal MDCK cell 3-D culture, proliferative suppression occurs around day 10, following lumen formation. Since Ki-67 expression was detected at days 10 and 20, EnvHA-trans-formed MDCK cells were resistant to proliferation suppres-sion signals. The fact that the Env structures had high levels of proliferation and were resistant to proliferative suppression indicates that JSRV Env disrupts proliferation control during transformation.
In addition to proliferative suppression, apoptosis of inner mass cells is critical for the maturation of normal MDCK cell acini with hollow lumens. Acini with increased size and full lumens can result from increased proliferation as well as from inhibition of proliferative suppression and apoptotic stimuli. Multiple changes are required to give abnormal structures with multiple lumens. For example, overexpression of cyclin D1 or HPV E7 in MCF-10A cells resulted in acini that were larger but had normal hollow structures because the transformed cells, while having increased proliferation, were not resistant to apoptosis (8). It seemed possible that EnvHA-transduced MDCK cells were resistant to apoptotic stimuli, resulting in a lack of lumen clearing. However, EnvHA structures were ac-tually hyperapoptotic, with high levels of apoptosis detected at all time points analyzed. Apoptosis was observed at day 5 and along the periphery of the structures, which is different from the apoptosis responsible for lumen clearing. These results suggest that some of the apoptosis observed in the EnvHA structures is distinct from apoptosis involved in lumen clearing. On the other hand, EnvHA structures contained polarized subacini with hollow lumens, indicating that apoptosis to clear lumens still occurs. One possible explanation for the high apop-totic rate and incomplete lumen clearing could be that Env mediates high levels of proliferation with compensatory in-creases in apoptosis. However, it is possible that Env mediates some resistance to anoikis, since proliferating cells were evi-dent in the interiors of EnvHA structures.
Previous studies of monolayer cultures reported that JSRV Env transforms cells predominantly through the PI3K-Akt-mTOR and H/N-Ras-MEK-MAPK pathways (1, 2, 5, 24, 25, 28, 29, 37, 52). Different signaling requirements for transfor-mation have been reported, likely due to the cell type and/or species specificities. Since different signaling events occur in the context of epithelial structure, we hypothesized that
sig-naling in monolayer and 3-D cultures might differ. Sensitivity of Env-transformed MDCK cells to PI3K and mTOR inhibi-tors in monolayer culture confirmed the role of the PI3K-Akt-mTOR pathway in morphological transformation (Fig. 6). In-hibiting PI3K (with LY294002) reverted the transformed phenotype more efficiently than did inhibiting mTOR (with rapamycin). A possible explanation for this observation is “Akt rebound,” which accounts for the limited effects of mTOR inhibition in cancer therapy (16, 31). When mTOR is inhibited, negative feedback to PI3K no longer occurs, resulting in accu-mulation of p-Akt. Thus, inhibition of mTOR would partially revert the transformed phenotype compared to inhibition of PI3K. Alternatively, other downstream targets of PI3K besides AKT/mTOR may be involved in JSRV transformation.
In 3-D culture, EnvHA structures treated with rapamycin and LY294002 were decreased in size compared to those un-dergoing DMSO treatment, indicating a role of this pathway in Env-induced cell division and transformation in 3-D culture. Treatment of EnvHA structures with rapamycin had a stronger effect on reverting the transformed phenotype to structures with single lumens than did treatment with LY294002. Thus, PI3K-independent signaling to mTOR appears to occur during Env-mediated MDCK cell transformation in 3-D culture. This would be consistent with the previous finding in monolayers of JSRV Env signaling to mTOR through PI3K-dependent and PI3K-independent mechanisms (17, 28).
Treatment with PD98059 (MEK inhibitor) inhibited Env transformation in monolayer cultures, suggesting that Env sig-nals through MEK during MDCK cell transformation. In con-trast, inhibition of H/N-Ras (with FTI-277) enhanced transfor-mation of control and Env-expressing MDCK cells, indicating that H/N-Ras has a negative role in transformation in mono-layers. These findings suggest that H/N-Ras has additional or alternative downstream targets to MEK in monolayers, some of which inhibit transformation. In 3-D culture, inhibition of MEK (PD98059) and H/N-Ras (FTI-277) enhanced both transformation and the size of control acini and EnvHA struc-tures. These results indicate a general inhibition of MDCK cell growth in 3-D culture by the H/N-Ras-MEK pathway, in con-trast to a positive role for MEK in transformation in a mono-layer.
The results from this study indicate that Env is a potent inducer of proliferation in monolayer and 3-D cultures. As expected, inhibition of PI3K (LY294002) and mTOR (rapa-mycin) decreased proliferation and caused reversion of the transformed phenotype in monolayers. However, while inhibi-tion of MEK (PD98059) caused reversion of the transformed phenotype in monolayers, it did not decrease proliferation. Together, these results suggest that Env transformation of MDCK cells in monolayers involves both PI3K-Akt-mTOR-mediated proliferative and MEK-PI3K-Akt-mTOR-mediated nonproliferative mechanisms.
ACKNOWLEDGMENTS
This work was supported by NIH grant R01 CA94188. C.J. was supported by a postdoctoral fellowship from the George F. Hewitt Foundation and by NIH training grant T32 CA0009054. K.S. was also supported by an NIH training grant. The support of the Optical Biol-ogy Shared Resource of the UCI Chao Family Comprehensive Cancer Center is acknowledged, as is the support of the UCI Cancer Research Institute.
on November 8, 2019 by guest
http://jvi.asm.org/
REFERENCES
1.Alberti, A., C. Murgia, S. L. Liu, M. Mura, C. Cousens, M. Sharp, A. D. Miller, and M. Palmarini.2002. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol.76:5387–5394.
2.Allen, T. E., K. J. Sherrill, S. M. Crispell, M. R. Perrott, J. O. Carlson, and J. C. DeMartini.2002. The Jaagsiekte sheep retrovirus envelope gene in-duces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol.83:2733–2742. 3.Bates, R. C., A. Buret, D. F. van Helden, M. A. Horton, and G. F. Burns.
1994. Apoptosis induced by inhibition of intercellular contact. J. Cell Biol.
125:403–415.
4.Boudreau, N., C. J. Sympson, Z. Werb, and M. J. Bissell.1995. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science267:891–893.
5.Chow, Y. H., A. Alberti, M. Mura, C. Pretto, P. Murcia, L. M. Albritton, and M. Palmarini.2003. Transformation of rodent fibroblasts by the Jaagsiekte sheep retrovirus envelope is receptor independent and does not require the surface domain. J. Virol.77:6341–6350.
6.Cohen, D., P. J. Brennwald, E. Rodriguez-Boulan, and A. Musch.2004. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol.164:717–727.
7.Debnath, J., and J. S. Brugge.2005. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer5:675–688.
8.Debnath, J., K. R. Mills, N. L. Collins, M. J. Reginato, S. K. Muthuswamy, and J. S. Brugge.2002. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell
111:29–40.
9.Debnath, J., S. K. Muthuswamy, and J. S. Brugge.2003. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimen-sional basement membrane cultures. Methods30:256–268.
10. Reference deleted.
11.Frisch, S. M., K. Vuori, E. Ruoslahti, and P. Y. Chan-Hui.1996. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol.134:
793–799.
12.Gardiol, D., C. Kuhne, B. Glaunsinger, S. S. Lee, R. Javier, and L. Banks.
1999. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 18:
5487–5496.
13.Hermiston, M. L., and J. I. Gordon.1995. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J. Cell Biol.129:489–506.
14.Hirata, A., M. Higuchi, A. Niinuma, M. Ohashi, M. Fukushi, M. Oie, T. Akiyama, Y. Tanaka, F. Gejyo, and M. Fujii.2004. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology318:327–336. 15. Reference deleted.
16.Huang, J., and B. D. Manning.2009. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans.37:217–222. 17.Hull, S., and H. Fan.2006. Mutational analysis of the cytoplasmic tail of
Jaagsiekte sheep retrovirus envelope protein. J. Virol.80:8069–8080. 18.Juin, P., A. O. Hueber, T. Littlewood, and G. Evan.1999. c-Myc-induced
sensitization to apoptosis is mediated through cytochrome c release. Genes Dev.13:1367–1381.
19.Kanamori, M., P. Sandy, S. Marzinotto, R. Benetti, C. Kai, Y. Hayashizaki, C. Schneider, and H. Suzuki.2003. The PDZ protein tax-interacting pro-tein-1 inhibits beta-catenin transcriptional activity and growth of colorectal cancer cells. J. Biol. Chem.278:38758–38764.
20.Kirshner, J., C. J. Chen, P. Liu, J. Huang, and J. E. Shively. 2003. CEACAM1-4S, a cell-cell adhesion molecule, mediates apoptosis and re-verts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc. Natl. Acad. Sci. U. S. A.100:521–526.
21.Koo, L. C., and J. H. Ho.1990. Worldwide epidemiological patterns of lung cancer in nonsmokers. Int. J. Epidemiol.19(Suppl. 1):S14–S23.
22.Lee, J. H., H. Koh, M. Kim, Y. Kim, S. Y. Lee, R. E. Karess, S. H. Lee, M. Shong, J. M. Kim, J. Kim, and J. Chung.2007. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature447:1017–1020. 23. Reference deleted.
24.Liu, S. L., M. I. Lerman, and A. D. Miller.2003. Putative phosphatidylino-sitol 3-kinase (PI3K) binding motifs in ovine betaretrovirus Env proteins are not essential for rodent fibroblast transformation and PI3K/Akt activation. J. Virol.77:7924–7935.
25.Liu, S. L., and A. D. Miller.2005. Transformation of Madin-Darby canine kidney epithelial cells by sheep retrovirus envelope proteins. J. Virol.79:
927–933.
26.Lubarsky, B., and M. A. Krasnow.2003. Tube morphogenesis: making and shaping biological tubes. Cell112:19–28.
27.Maeda, N., and H. Fan.2008. Signal transduction pathways utilized by enzootic nasal tumor virus (ENTV-1) envelope protein in transformation of
rat epithelial cells resemble those used by Jaagsiekte sheep retrovirus. Virus Genes36:147–155.
28.Maeda, N., W. Fu, A. Ortin, M. de las Heras, and H. Fan.2005. Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-ki-nase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transfor-mation of rodent fibroblast and epithelial cell lines. J. Virol.79:4440–4450. 29.Maeda, N., Y. Inoshima, D. A. Fruman, S. M. Brachmann, and H. Fan.2003. Transformation of mouse fibroblasts by Jaagsiekte sheep retrovirus envelope does not require phosphatidylinositol 3-kinase. J. Virol.77:9951–9959. 30.Maeda, N., M. Palmarini, C. Murgia, and H. Fan.2001. Direct
transforma-tion of rodent fibroblasts by Jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. U. S. A.98:4449–4454.
31.Manning, B. D.2004. Balancing Akt with S6K: implications for both meta-bolic diseases and tumorigenesis. J. Cell Biol.167:399–403.
32.Martin-Belmonte, F., W. Yu, A. E. Rodriguez-Fraticelli, A. J. Ewald, Z. Werb, M. A. Alonso, and K. Mostov.2008. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr. Biol.
18:507–513.
33.O’Brien, L. E., T. S. Jou, A. L. Pollack, Q. Zhang, S. H. Hansen, P. Yurch-enco, and K. E. Mostov.2001. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol.3:831–838. 34.O’Brien, L. E., M. M. Zegers, and K. E. Mostov.2002. Opinion: building
epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol.3:531–537.
35.Palmarini, M., and H. Fan.2001. Retrovirus-induced ovine pulmonary ad-enocarcinoma, an animal model for lung cancer. J. Natl. Cancer Inst.93:
1603–1614.
36.Palmarini, M., H. Fan, and J. M. Sharp.1997. Sheep pulmonary adenoma-tosis: a unique model of retrovirus-associated lung cancer. Trends Microbiol.
5:478–483.
37.Palmarini, M., N. Maeda, C. Murgia, C. De-Fraja, A. Hofacre, and H. Fan.
2001. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for en-velope-induced transformation of NIH 3T3 cells. J. Virol.75:11002–11009. 38.Palmarini, M., J. M. Sharp, M. de las Heras, and H. Fan.1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol.73:6964–6972.
39.Parkin, D. M., F. Bray, J. Ferlay, and P. Pisani.2005. Global cancer statis-tics, 2002. CA Cancer J. Clin.55:74–108.
40.Partanen, J. I., A. I. Nieminen, T. P. Makela, and J. Klefstrom.2007. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc. Natl. Acad. Sci. U. S. A.104:14694–14699.
41.Perk, K., and I. Hod.1982. Sheep lung carcinoma: an endemic analogue of a sporadic human neoplasm. J. Natl. Cancer Inst.69:747–749.
42.Pim, D., M. Thomas, R. Javier, D. Gardiol, and L. Banks.2000. HPV E6 targeted degradation of the discs large protein: evidence for the involvement of a novel ubiquitin ligase. Oncogene19:719–725.
43.Rai, S. K., F. M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller.2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for Jaag-siekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. U. S. A.98:4443–4448.
44. Reference deleted.
45.Shannon, J. M., R. J. Mason, and S. D. Jennings.1987. Functional differ-entiation of alveolar type II epithelial cells in vitro: effects of cell shape, cell-matrix interactions and cell-cell interactions. Biochim. Biophys. Acta
931:143–156.
46.Suau, F., V. Cottin, F. Archer, S. Croze, J. Chastang, G. Cordier, F. Thivolet-Bejui, J. F. Mornex, and C. Leroux.2006. Telomerase activation in a model of lung adenocarcinoma. Eur. Respir. J.27:1175–1182.
47.Varela, M., M. Golder, F. Archer, M. de las Heras, C. Leroux, and M. Palmarini.2008. A large animal model to evaluate the effects of Hsp90 inhibitors for the treatment of lung adenocarcinoma. Virology371:206–215. 48.Vieira, O. V., K. Gaus, P. Verkade, J. Fullekrug, W. L. Vaz, and K. Simons.
2006. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. U. S. A.103:18556–18561.
49.Wang, A. Z., G. K. Ojakian, and W. J. Nelson.1990. Steps in the morpho-genesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multi-cellular epithelial (MDCK) cysts. J. Cell Sci.95:137–151.
50.Wang, A. Z., G. K. Ojakian, and W. J. Nelson.1990. Steps in the morpho-genesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J. Cell Sci.95:153–165.
51.Wootton, S. K., C. L. Halbert, and A. D. Miller.2005. Sheep retrovirus structural protein induces lung tumours. Nature434:904–907.
52.Zavala, G., C. Pretto, Y. H. Chow, L. Jones, A. Alberti, E. Grego, M. De las Heras, and M. Palmarini.2003. Relevance of Akt phosphorylation in cell transformation induced by Jaagsiekte sheep retrovirus. Virology312:95–105.
5390 JOHNSON ET AL. J. VIROL.