JOURNALOFVIROLOGY,Feb. 1970, p. 109-113 Vol. 5,No. 2 Copyright ©1970 American Society for Microbiology Printedin U.S.A.
Acid-Soluble Material of Adenovirus
P. A. BOULANGER, F. JAUME, P. FLAMENCOURT, AND G. BISERTE UnitedeRecherches surla Biochimie des
ProtMines
del'INSERM, Lille,FranceReceivedforpublication 22 September 1969
Two
methods
aredescribed
for adenovirus capsiddisruption
and extraction of acid-solubleproteins
fromthe viral core. The acid-soluble material of adenovirusconsisted of three major
proteins, one of them being selectively extracted after milddisruption
of the virus particle. Some chemical properties of these proteins are reported.The presence of internal component(s) within the adenoviruscapsid, in
addition
to deoxyribo-nucleic acid (DNA), has beensuspected for a long time frommorphological considerations
(22, 24). The discrepancy observed between basic aminoacids
contentsof whole virion and
thecapsidsub-units (2,
12-14)
suggested the existence of
anarginine-rich
internalprotein, previously
postu-lated (18, 19). Evidence for
anewinternal
antigenhas been
given by serological
tests(20)
andcon-firmed by
acrylamide-gel electrophoresis
ofviral
nucleoprotein
cores obtained after disruption of theviruscapsid
(8).Recent
findings (10, 11) have
demonstrated
the
complexity
of the adenoviruscomposition
and specially of the inner core.Acrylamide-gel
elec-trophoresis of disrupted
virions
yielded
ninedistinct
polypeptides,
threeof them
being
asso-ciated
with the
DNA-containing viral
core. More-over, two acid-extracted basicproteins
werere-covered after
amild
sequential
disintegration
of type 5virions and characterized
immunologically
(15).
TheseDNA-associated proteins
werefound
tobe
relatively
rich inarginine (11,
15,
21). Thepresent report describes amethod fordis-ruption of virus particles and extraction of
several
(inner?) acid-soluble proteins from
twoadenoviruses belonging
to the sameimmuno-logical subgroups,
types 2and
5. Italso
presents somepreliminary
results
on thebiochemical
properties of
theseproteins.
MATERIALS AND METHODS
Preparationof the virions.Twoserotypesof human adenovirus were studied: types 2 and 5. The virus particleswerepurifiedfrom Freonextractsofinfected KB cellskindly suppliedbyPr. J. Samaille (Depart-mentofVirology,InstitutPasteurdeLille), byuseofa procedure previously described (2). The Freon
ex-tract wasfreed of cell debris by filtrationon
hyflo-supercel under vacuum, and the virusparticlesandthe soluble antigenswereprecipitatedbyammonium sul-fateat54%saturation (pH 7.0)
overnight
at4C. Theprecipitate formed was centrifuged at 2,500 X g for 30 min at 4 C. The sediment thus obtained was dis-solved in a minimum of 0.1 M tris(hydroxymethyl)-aminomethane-0.2 M NaCl buffer (pH 8.0) and chro-matographed on Sepharose 4B (Pharmacia Fine
Chemicals) equilibrated with the same buffer. The virions, excluded from the gel, eluted in the void vol-ume ofthe column. The purityofthis fraction was controlledimmunologically byusing arabbitanti-KB cell immune serum and a rabbit anti-equine serum
immuneserum, todetectpossible cellular orseric
con-taminantsfrom the culture medium.
Disruption of the virus particles. Thefractions cor-responding to virions werepooledandsubjected to a disrupting treatment. Two methods were usedfor dis-ruption of the virus capsids: amild procedure and a
drastic one.Formethod A, amilddisruption ofthe
capsids, the virus suspension was dialyzed against distilledwaterandlyophilized.Thistreatmentproved
tobe able to disrupt the virus capsids (2). For method B, a drastic disruption of thecapsids, thevirus
sus-pensionwascentrifugedat105,000X gfor 3hr, and thevirus sedimentthusobtainedwashomogenized in distilled water by means ofanUltra-Turrax
homog-enizerfor 2 min at4 C. The homogenate was then
lyophilized.
Extraction of acid-soluble proteins.The nucleopro-teincomplexesareinsoluble in 0.14 MNaCl,whereas the outer coatproteinsaresolubleunderthese condi-tions. Therefore, the first stepconsistedof
removing
the structural antigens released from the disrupted capsidsinto the 0.14 M NaCl supernatant fluid.
Method A. Lyophilized virus
particles
(100 mg)
weresuspended in 0.14 Msodiumchloridecontaining
0.01 M sodiumcitrate(pH 7.0) andstirredovernight at4 C. Thesuspensionwascentrifugedat 1,000X g for30min, and the supernatantfluidwasdiscarded. Thesedimentwassuccessivelywashed andcentrifuged
three timesin the sodium chloride-sodium citrate buf-fer, and the final sedimentwastwice washed withethyl
alcoholbeforebeingsubjectedtotheextraction proce-durefor histones (7).The
nucleoprotein
sedimentwas stirred with 15to20mlof 0.25NHCI for 10 min at 4C,and thesuspensionwascentrifuged
at1,100
X g for 30 min. The acid-soluble proteins wererecovered from the supernatant fluid by precipitation with 6109
on November 11, 2019 by guest
http://jvi.asm.org/
BOULANGER ET AL.
volumes of acetoneat -20Covernight. Thisfraction wascalledfraction AS1. The yield of fraction AS1was approximately 2mg.
Method B.Lyophilized virusparticles (100 mg) were
homogenized in 0.14 M NaCl-0.01 M sodium citrate buffer (pH7.0) bymeansofanUltra-Turrax
homoge-nizerfor 2 min, and the suspension wascentrifugedat 1,100 X g for 30min. Thesupernatant fluid was
dis-carded, and the sediment was further washed in the same buffer by magnetic stirring at 4 C overnight. After three additional rinses and centrifugations in the sodium chloride-sodium citrate buffer, the sedi-ment was washed with ethyl alcohol. This sediment wasfirst extracted with 0.25 NHCl for 15 min at 4 C andcentrifuged at 1,100X g for 30 min. The remain-ing sediment was then subjected to a further extrac-tion with 0.25 NHClfor12hrat 4C.Theacid-soluble proteins wererecovered from the two successiveHCI extractsby precipitationwith 6 volumesof acetone at -20 C overnight, and centrifugation at 2,500 X g for 15 min. The supernatant fractions werediscarded and the two sediments werekept separately for chemi-cal analysis. The first extract was chemi-called fraction AS2 and the second one-corresponding to what was ex-tracted after AS2-was called AS3. The yield of frac-tion AS2 was 6 to 7 mg, the yield of fracfrac-tion AS3 was about 5 mg.
Disc electrophoresis. The acid-extracted proteins obtainedfrom adenoviruses 2and5werecheckedby
analytical discelectrophoresis in 15% acrylamide gels containing 6 M ureaatpH4.3, byamodification (1) of themethod of Reisfeld et al. (16) adapted to histones. Antisera. Anti-equine serum, anti-adenovirus 2 hexon antigen, whole adenovirus 2, and anti-whole adenovirus 5 rabbit immune sera were used. These two latter immune sera were prepared by
in-jectinganimals with lyophilized virus particlespurified by gel filtrationchromatography.
Amino acid analysis. Amino acid analyses were
per-formedafter24 hrofhydrolysis in 5.6NHClat110C under vacuum, by using a Technicon autoanalyzer. The tryptophan content was determined spectro-photometrically (17).
N-terminal amino acid. TheN-terminal amino acid residue was determined by the dansyl-amino acid procedure (5) adapted to proteins (23).
RESULTS
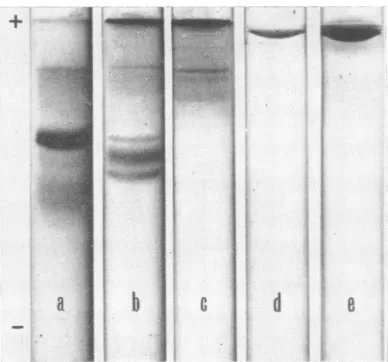
Adenovirus 5 acid-soluble components. The
acrylamide-gel
electrophoretic patterns of thedifferent acid extracts were
quite
different(Fig.
1). The results depended on the extraction pro-cedure employed. The mild disruption of virus capsidsfollowed byashortHCIextractionyielded
a major protein band (fraction
AS1,
Fig. la),slightly
contaminatedby threeorfourminorcom-ponents. The drastic
disruption
of viruscapsids
followed
by
a shortHCI extractionyielded
three major components(fraction
AS2, Fig. lb); the slowestmigrating
one seemingly corresponds to fraction AS1 obtained after milddisruption.
Anextended HCI treatment of the
remaining
sedi-+
b
_.
.
.';:;
oxR
E>q
|'%
P_
...; -'9.S.-.' ....
.E.
[image:2.496.268.462.64.245.2]b
j.
...'.,.,
.>::.::.:....-::.:z..'.:....f,,,,,,O,.n
;n.o...
Z
,*
C
U
d
FIG. 1. Acrylamide-gel electrophoretic patternz of the
differenzt
acid-soluble fractions obtainied from adenovirus 5. The gel was formed of15% acrylamide in 0.5Mpotassiumi acetate(pH
4.3) containing 6 M urea. (a) Fractioni AS1 extractedby 0.25 N HC after mild disruption of the virus capsid; (b) fraction AS2 extracted by draistic disrutptioni of the virus capsid, followed by a short HCI treatment; (c) fractionz AS3 obtained from the remaininlg sediment of the AS2 extraction, by ani extendedstirrinigin 0.25NHCI; (d)purified hexoii antigeni riunl iunzder the same electro-phoretic coniditionis; (e) hexonz, pentton, anid fiber anti-genis mixture. AS1 conitainis onie major proteiln; AS2 con1sists of three major proteinis, anid the slowvest one corresponids to the
mjcjor
componenit of AS1; AS3 con-tainis several slow-migrating componients. Adenovirus 5 structural anttigents were Iot detected in the acid-solublceextracts.mentafterextraction ofAS2yieldedseveral
slow-migrating components (fraction AS3, Fig. lc) which were also present in the other extracts as
minor contaminants.
These three extracts were tested against anti-equine serum,anti-hexon antigen,andanti-whole adenovirus 5 immune sera. Anti-horse, rabbit-immune serum was used to detect possible
con-tamination from cell culture medium. No reac-tionwasobservedwiththese antiserafor fractions
AS1 and AS2. However, fraction AS3 gave a faintprecipitation line with the anti-whole
adeno-virus 5 immune serum,
showing
apatternofpar-partial identity with hexon
antigen (Fig.
2).The amino acidcompositions ofthe three
dif-ferent acidextracts fromadenovirus 5
(AS1,
one major protein; AS2, three major proteins; and AS3, severalslow-migrating
components)
are presented inTable 1. The general characteristics are ahigh contentof acidic aminoacids;
a highcontentofglycine, alanine,and
leucine;
lowvalues110
J. VIROL.on November 11, 2019 by guest
http://jvi.asm.org/
ACID-SOLUBLE MATERIAL OF ADENOVIRUS
[image:3.496.102.377.67.193.2]FIG. 2.
Gel-diffusion
precipitation tests. Left:immunodiffusion
withthe AS3 fraction in thecenitral well; (a) anti-Ad5lyophilized virions; (b) anti-hexon antigeni; (c) anti-horse serum. Right: IS, anti-Ad5 virionis in the cen-tralwell;Iand 4, AS3; 2, AS1; 3 and 6;htexon antigent; 5, AS2.Areactionz of partialidentity forms between AS3 andhexon.TABLE 1. Aminioacidcompositionisof threedifferenzt
HCI extractsfrom adenioviruses 2and 5
Aminoacida ASP THR SER GLU PRO GLY ALA CYS VAL MET ILE LEU TYR PHE LYS HIS ARG TRP A B B/A LYS/ARG Adenovirus5 ASi 8.1 5.9 11.3 10.6 5.6 14.5 12.1 0.0 5.6 0.3 2.9 4.6 2.3 1.6 3.7 1.8 9.1 NDc 18.7 14.6 0.78 0.40 AS2 9.7 5.5 6.4 11.6 5.2 8.6 7.8 1.0 6.3 1.8 4.8 7.9 3.2 3.4 6.9 2.5 6.3 1.1 21.3 15.7 0.74 l 1.10
5b_
AS3 10.3 5.6 6.6 11.3 5.0 9.8 8.2 0.4 5.7 1.1 4.9 7.9 3.0 4.0 7.6 2.3 5.1 1.2 21.6 15.0 0.69 1.49 Adenovirus2b AS1 7.8 7.2 13.7 6.5 4.7 11.7 12.4 0.0 5.8 0.3 2.1 3.6 1.6 3.2 4.4 3.2 11.7 ND 14.3 19.3 1.35 0.38 AS2 10.0 5.9 6.1 10.0 6.0 8.2 8.8 1.1 6.1 1.9 4.6 7.9 3.1 3.5 5.9 2.2 7.5 1.2 20.0 15.6 0.78 0.79 AS3 12.0 5.2 6.4 10.9 5.0 10.2 9.3 0.0 6.3 0.0 4.8 8.1 2.0 3.7 8.1 2.0 4.5 1.5 22.9 14.6 0.64 1.80a ASP, aspartic acid; THR, threonine; SER,
serine; GLU, glutamic acid; PRO, proline; GLY, glycine; ALA, alanine; CYS, cysteine; VAL, valine; MET, methionine; ILE, isoleucine; LEU, leucine; TYR, tyrosine, PHE, phenylalanine; LYS, lysine; HIS, histidine; ARG, arginine; TRP,tryptophan;A, acidic;B, basic.
bAS1 correspondsto onemajorprotein, AS2to
three major proteins, and AS3 to several slow-migrating components. The amino acid contents
are expressed as moles/100 moles of all amino acids found.
cND, not determined.
for
sulfur-containing aniino acids, and
thepres-ence
of
tryptophan. The mostremarkablepoint is
the
composition
of thefraction
AS1containing
one
major protein;
this protein hasahighglycine,alanine, serine,
arginine, andglutamic acid
con-tent
and
isrelatively
leucine-
andlysine-poor.
Three
N-terminal amino
acids were foundin
fraction
AS2: glycine, alanine, andthreonine;
traces of
dansyl-proline
were also found, but probablycorrespond to some contaminant.Frac-tion
AS1 possessesglycine
astheN-terminalresi-due.
Adenovirus2acid-soluble components.The
acid
extracts
from
adenovirus2virions
arequite
simi-lar to
adenovirus
5 extracts(Fig.
3). Aftermild
disruption of
theadenovirus
2capsids,
HC1ex-traction
yielded
onemajor
band(fraction AS1,
Fig. 3a), which corresponds to the slowest
mi-grating
bandof
the three componentsextracted
after
drastic disruption
and short HC1 treatment(fraction
AS2,Fig. 3b).
Extended HCIextraction
yielded
slow-migrating
components, asforadeno-virus5
(fraction
AS3,Fig. 3c).
Thislatterextractfaintly
reacts with anti-adenovirus 2 immuneserum,
with
apattern
ofpartial
antigenic
identity
with
hexon
antigen.
Theother
acid extractsdo
not reactwith the different antisera tested. The
amino
acidcompositions
of theadenovirus
2 acid-extracted
proteins
obtained
withthe
dif-ferent
procedures
arepresented
inTable
1. The general chemical characteristics are the same asfor the adenovirus 5 acid-extracted
proteins.
Ahigh
glycine, alanine, serine,
andarginine
con-tent wasalso found in AS1 fraction from adeno-virus 2, but some differences wereobserved.
Adenovirus2AS1 hasahigher basic amino acids
contentand a lower
glutamic
acid content. The highglutamic
acid content ofadenovirus
5AS1
VOL.
5,
1970III
on November 11, 2019 by guest
http://jvi.asm.org/
[image:3.496.43.233.277.537.2]a
b
c
FIG. 3. Acrylamide-gel electrophoresis of the acid-extractedproteins from adenovirus2. (a) AS1; (b) AS2; (c) AS3. The HCIextractions werecarriedout as
de-scribedinthe legend of Fig.1.Theelectrophoretic pat-terns are quite similar to those of adenovirus S acid
extracts.
could be due to some acidic contaminating
ma-terial.
The same three N-terminal amino acids, gly-cine,
alanine,
andthreonine, werefound infrac-tion AS2; fraction AS1 has glycine asthe N-ter-minalresidue.
DISCUSSION
Two simple procedures-mild and drastic-havebeendescribed fordisruption of the adeno-virus capsid and extraction of acid-soluble
pro-teins
from
theviralcore,afterremoving
the solu-bleantigensinthe 0.14MNaClsupernatantfrac-tion. Thenucleoprotein complexesareinsoluble in
0.14 M NaCl (unlike the
adenovirus
structuralantigens), and the acid-soluble proteinsare
there-fore extracted from theviralnucleoprotein core.
Our mild disruption procedure followed by a
shortHCI extraction yieldsonemajorcomponent (AS1); a drastic
disruption
with a short HCItreatment yields three major proteins (AS2), among which the slowest migrating one
corre-spondsto componentAS1.No structural antigens
werefound in theAS1 andAS2extracts,asshown
by
immunological
control and analytical acryl-amide-gel electrophoresis. However, it is likely that the 0.14 MNaCl sediment contains, besidesnucleoprotein,
somemorphological
components
belonging
toincompletely disrupted capsids;
anextended HC1 treatment
is ableto partially
hydro-lyze these capsid morphological subunits. This
can
explain
thepresence,
inAS3
fraction (ob-tainedby
aprolonged stirring
in 0.25 NHCI
after drasticcapsid disintegration),
of acomponent
sharing antigenic determinant
with hexonantigen.
The
acid-extracted proteins
arequite different
from adenovirus structural
antigensin
physical
and
chemical
properties. Ouranalytical
discelectrophoreses
were runby
theprocedure
usually
employed for
histones,
with15% acrylamide
gel
containing
6M
ureaatlow pH
value.Under
theseconditions,
theadenovirus structural
antigens-hexon,
penton,
andfiber-of
high molecular
weights (300,000
to60,000) hardly
enter thegel and remain
nearthe
origin
(Fig. ld and le),
whereas the
migration of the
acid-soluble
pro-teins
corresponds
tomolecules of
approximately
10,000
to50,000.
Thisrange
ofmolecular sizes
is inagreement
with theresults of Maizel
etal.
(10)
for the
internal components
V, VI,
and VII(44,000, 24,000, and
24,000,
respectively).
Theprevious
investigators who studied the adenovirus
core
(8, 15, 21) employed
5%acrylamide gels
foranalytical electrophoresis, but this acrylamide
concentration is
notsuitable for
agood
fractiona-tion
of
low-molecular-weight
proteins
ashistones.
It is
therefore impossible
to assertwhether
thefractions
wehave
obtained correspond
topre-viously found proteins.
The
amino acid
composition
ofthe
whole
acid-soluble material
(fraction AS,) differs from that
of
structural antigens (2),
especially in
basicamino acids
content(Table 1). More significant
is the
analysis of
theAS1 fraction from
adeno-viruses
2and
5,because of
its
high degree of purity
(Fig.
laand
3a). This chemical
composition is
characterized
by
ahigh
glycine,
alanine,
serine,
and
arginine content, which has
neverbeen
de-scribed
for
anyadenovirus
protein. Such
ahigh
alanine and serine
contentis
found in F2b histone
from calf
thymus (4), but this histone has
ahigher
lysine
contentand isrelatively
arginine-
andgly-cine-poor in
comparison with AS1. An important
result is the
presenceof tryptophan
infractions
AS2 and AS3,
whichconfirms the
findings by
Maizel
etal.(10,
11) of
afair
amount ottrypto-phan in
the
coreproteins.
Itwould be
of greatinterest
to carry outthis tryptophan
determina-tion
on eachacid-soluble
protein,
afterfurther
fractionation and purification.
The
N-terminal
amino acid analyses confirm
the
previous
findings by
Laver etal.
(9) for
theadenovirus
core,by
anothertechnique.
These
authors found
glycine
andalanine
asmajor
N-terminal
residues,
and in somepreparations,
VIROL.Im
on November 11, 2019 by guest
http://jvi.asm.org/
[image:4.496.67.260.71.312.2]ACID-SOLUBLE MATERIAL OF ADENOVIRUS
threonine,
but thislatter amino
acid
wascon-sidered
as acontaminant.
Our resultsimply
that oneof
the two mostrapid migrating
componentsof
AS2
possesses threonineas N-terminal aminoacid, since
AS1,
whichcorresponds
to theslowest
one, has
glycine
asN-terminal.Are these
proteins histones? They
cannot be properlycalled histones since
they contain
trypto-phan and
ahigh
amountof acidic amino acids
(Table 1).
However,these
proteins
wereextracted
by
theprocedure for
theisolation
of
histones orhistone-like
proteins (or
both).
Ashistones,
they
canbe
easily dissolved
indistilled
water, they aredeeply stained
by amido black, and
they
have an
electrophoretic
migration
similar tobasic
proteins. Their acidic character
canbe
tentatively
explained
inseveral
ways.(i)
Asalready
suggested
(21), it
maybe that
anacidic internal
componentis rendered
acid-soluble by its close
association
with
amorebasic
component.(ii)
"High
aspartic-glutamic histones" have been
previously
reported
(6), and
especially
in
metabolically
active tissues
(3). (iii) Regarding
the HCIsolubility of these
adenovirus internal
pioteins
and their
cathodic
migration when
run inacrylamide
gel
atlow
pH
values, it
canbe
postulated
that
they
have
anexcess
of basic
charges,
owing
toamidification of
their acidic
groups.Only
determination of
theamide
groups canelucidate
this
point.
Without
being "histones,"
aschemically defined,
theacid-extracted
proteins
of
adenovirus have
perhaps
anhistone-like role,
asDNA-associated
proteins.
If
this
werethe
case,these internal
proteins
would
have
amajor
function in adenovirus
replication.
ACKNOWLEDGMENT
This investigation was supported by a Convention de
Re-cherchen°67-00-537 from theDelegation Generaleala Recherche ScientifiqueetTechnique.
ADDENDUM IN PROOF
After submission of this report, wedetermined the amide content of the AS2 fraction extracted from
adenovirus 2 by amino acid
analysis
after totalen-zymatic hydrolysis (by using Pronase and
leucine-aminopeptidase,
successively); 60%
of the freecar-boxylic groups of
aspartic
andglutamic
acids werefoundto beamidified. This result thus confirms that
AS2 contains an
important
excess of basiccharges,
whichexplainsitsphysicalandchemicalproperties.
LITERATURE ClIED
1. Bonner, J.,G. R.Chalkley,M.Dahmus,D.Fambrough, F.
Fujimura, R. C. Huang, J. Huberman, R. Jensen, K.
Marushige, H. Ohlenbusch, B. M. Olivera, and J.
Wid-holm. 1968. Isolation and characterization of
chromo-somal nucleoproteins, p. 3-65. In L. Grossman and K.
Moldave (ed.),Methods inenzymology,vol. XIIB. Aca-demic PressInc.,NewYork.
2. Boulanger, P. A., P. Flamencourt, and G. Biserte. 1969. Isolation and comparative chemical study of structural proteinsof theadenoviruses 2 and 5: hexon and fiber
anti-gens. Eur. J. Biochem. 10:116-131.
3.Boulanger, P. A., F.Jaume,Y. Moschetto, andG. Biserte. 1969. Isolation of histones from virus-induced tumors.
Fed. Eur. Biochem. Soc. Letters 4: 291-294.
4. Butler,J. A. V., E. W.Johns,and D. M. P.Phillips. 1968.
p.221. In J. A.V. Butler and D. Noble (ed.),Progressin biophysics and molecular biology, vol. 7. Pergamon
Press,Oxford.
5. Gray,W.R.,andB.S.Hartley.1963.Afluorescentend-group
reagent forproteinsandpeptides.Biochem. J.89:59 P. 6. Johns,E.W.1964.p.52.InJ.Bonner and P.0.P.Ts'O(ed.),
The nucleohistones.HoldenDay,Inc.,SanFrancisco. 7. Johns,E.W.,D.M.P.Phillips,P.Simson,andJ. A.V.
But-ler. 1961. Theelectrophoresisofhistones and histones frac-tionsonstarchgel.Biochem. J.80:189-193.
8. Laver,W.G.,H. G.Pereira,W.C.Russel,andR.C. Valen-tine.1968.Isolationofaninternal component from aden-ovirustype5. J. Mol. B;ol. .37:379-386.
9. Laver,W.G.,J. R.Suriano,and M.Green. 1967. Adenovius
proteins. II. N-terminal amino acid analysis. J. Virol. 1:723-728.
10.Maizel,J.V., Jr.,D.0.White,andM. D.Scharff.1968. The
polypeptidesof adenovirus. I. Evidence formultiple protein
components in the virion andacomparisonof types2, 7A,
and 12. Virology 36:115-125.
11. Maizel,J.V., Jr.,D.0.White,andM. D.Scharff. 1968. The
polypeptidesofadenovirus.II. Solubleproteins,cores,top
components andthestructure ofthe virion. Virology 36: 126-136.
12. Pettersson,U.,L.Philipson,and S.Hoglund.1967. Structural proteinsofadenoviruses. I. Purification and characterization of the adenovirustype2 hexonantigen. Virology 33:575-590.
13.Pettersson,U.,L.Philipson,andS.Hoglund.1968.Structural
proteinsofadenoviruses. II. Purification and characteriza-tion of theadenovirus type2fiberantigen.Virology 35:204-215.
14.Polasa, H., and M. Green. 1967. Adenovirus proteins. I. Aminoacidcomposition ofoncogenicandnononcogenic
humanadenovirus.Virology31:565-567.
15.Prage,L.,U.Pettersson,andL.Philipson.1968. Internalbasic
proteinsinadenovirus.Virology 36:508-511.
16. Reisfeld,R.A.,V.J.Lewis,andD. E.Williams. 1962.Disk
electrophoresis of basic proteins and peptides on
poly-acrylamide gels. Nature 195:281-283.
17. Spies,J.R.,and D.C. Chambers.1949.Chemical determina-tionoftryptophaneinproteins.Anal. Chem.21:1249-1266. 18. Rouse, H. C., and R. W. Schlesinger. 1967. An
arginine-dependent step in thematuration of type 2 adenovirus. Virology 33:513-522.
19. Russel,W.C.,andY. Becker. 1968.Amaturation factorfor adenovirus. Virology 35:18-27.
20. Russel,W. C.,and B. E. Knight. 1967. Evidence foranew
antigen within the adenovirus capsid. J. Gen. Vircl. 1:
523-528.
21. Russel, W. C., W. G. Laver, and P. J. Sanderson. 1968. Internalcomponents of adenovirus. Nature219:1127-1130. 22. Russel,W.C.,R.C.Valentine,and H. G. Pereira. 1967. The effect of heaton the anatomy ofthe adenovirus. J. Gen. Virol. 1:509-522.
23. Schmer,G. 1967. Quantitative Bestimmungvon10-4
Atmole
N-terminalen Aminosauiren beiImmunoglobulinen durch Markierung mit 1-Dimethyl-amino-naphthalene-5-sul-fonyl-chlorid. Z.Physiol. Chem. 348:199-204.
24.Valentine,R.C.,andH.G.Pereira.1965.Antigensand struc-tureofthe adenovirus.J. Mol. Biol.13:13-20.