Copyright©1969 American Societyfor Microbiology Printed in U.S.A.
Influence of
the
Rate of Cell
Growth and Cell
Density
on
Interferon Action
in
Chick
Embryo
Cells1
T. G. ROSSMAN2 AND J. VILCEK
Departmentof Microbiology,New York UniversityMedical Center, New York,New York 10016
Receivedforpublication15April 1969
Chick embryo cells becamemore sensitive to the action of interferonthelonger
they remained in culture. This phenomenonwas foundevenbefore confluency had
been reached. Therelative insensitivity of newly seededcells wasnot duetoa loss
ofreceptors.Cells synthesizing deoxyribonucleic acid (DNA)atahighratewereless
sensitivetointerferon action thancells synthesizing DNAatalowrate,butthe
in-hibition ofDNAsynthesis had no effect oninterferon action. An increase in the
number ofcellsused for seeding resulted inanearlierappearance ofincreased
sensi-tivityto interferon action. These resultsare discussed in relation to the induction
process inanimalcells.
It has previously been reported that the
capacitiestoproduceandtorespondtotheaction
of interferon areinfluencedbytheageof cells in
culture. Cantell and Paucker (2) found that
1-to3-day-old cultures ofaHeLacell lineproduce
less interferon andareless sensitivetoits action
than are 6- to 10-day-old cultures. In chick
embryo cell cultures, 6- to 8-day-old cells
pro-duce 5 to 18 times moreinterferon per cell than
1- to 2-day-old cells (8). Similar findings were
reported by Carver and Marcus (3), who
com-pared young and aged confluent monolayers of
chickembryo cells. Recently,Lockart(13)showed
that therate atwhich the antiviral effect of
inter-feron is conferred isfaster inagedchick embryo
cells.
Interferon seems to act indirectly by causing
thesynthesis ofnewribonucleic acid (RNA) and
protein (6, 21). Presumably, the new protein(s)
isresponsible for the antiviral effect, perhaps by
attaching toribosomes rendering themincapable
of forming a functional polysome with viral
RNA (12, 14). The production ofinterferon in
responsetoavirus alsorequiresnewRNA
synthe-sis (7). Thus, both the productionand the action
of interferon show a resemblance to enzyme
induction. Since both processes are less efficient
in young cells, it is possible that they are two
examples of a more general phenomenon; any
IThis work wastakeninpartfromadissertationbyT. G.
Rossman submitted to the facultyof New York Universityin
partial fulfillment of the requirementsfor the Ph.D.degree.
2Present address: DepartmentofPathology, NewYork
Uni-versity Medical Center, New York, N.Y.
inducible process may be hampered by cell growth.
Wehaveattemptedto definethe factors which
are responsible forthe increase in the sensitivity
ofchick embryo cells to interferon action with
aging. We were particularly interested in
ascer-taining whether cell growth or cell density is
involvedinthis phenomenon.
MATERIALS AND METHODS
Cell cultures. The methods, media, and solutions
used in the preparation and cultivation of primary
chick embryo cellshave been described (23). Plastic
flasks (250 ml; Falcon Plastic) were seeded with
20 X 106cells in25ml ofmedium. Secondary cultures
ofchickembryocellswereobtained bytrypsinization
of2- to 3-day old flasks of primary cultures, unless
otherwise stated. They were usuallyseeded at 2.5 X
106 cells per petri dish (60 mm; Falcon Plastic).
Theyreached a maximum density of approximately
6 X 106cells perpetri dish.
Viruses.Theorigin and method of plaque assay of
vesicular stomatitis virus (VSV) were the same as
indicated previously (23). Chikungunya virus was
obtained fromJ.Casals, Yale University, New Haven,
Conn. Stock 10% suspensions were prepared from
brains of suckling Swiss Webster mice inoculated intracerebrally. Chikungunya virus was titrated by
intracerebral inoculation ofsuckling mice. All virus
stockswere kept frozenat -70C.
Interferon. Interferon was produced in chick
embryo cell cultures grown in flasks. Five-to
7-day-old cultures wereinoculated withChikungunyavirus
at a multiplicity of about 0.5 LD5o per cell. After
inoculation, cells were maintained at 36C in Eagle
minimal essential medium (MEM) without serum.
Tissue culture fluidswerecollected24hrafter
inocula-7
on November 11, 2019 by guest
http://jvi.asm.org/
tion andwerestoredat4C until used. Infectious virus
wasinactivated by heatingthe interferon at60 C for
1hr. The interferonwastitrated by the plaque
inhibi-tion method (24).
Virus yieldassay.After removal of the fluids from
the monolayer,VSVwasaddedatamultiplicityofat
least 3plaque-forming units (PFU)/cell in 0.5 mlof
MEM. The cultures were incubated for 1 hr and
thenwerewashed four times with Earle balanced salt solution (Grand Island Biological Co., Grand Island,
N.Y.). Then 4 ml of MEM containing 2% gamma
globulin-free calfserum was added, and the cultures
were incubated at 36 C. Duplicate samples of the
fluidsweretaken at9to 16hrand stored at -25 C until titrated.
Deoxyribonucleic acid (DNA) synthesis. DNA
synthesis was determined by the cover slip method
(1). Glass petri dishes (60 mm) containing 3 cover
slips (9 by22mm;BellcoGlass, Inc., Vineland, N.J.)
were seeded with secondary chick embryo cells.
DNA synthesis was measured by a 1-hr pulse with
3H-thymidine (Nuclear-Chicago Corp., Des Plaines,
Ill., 5,000 mc/mM), adding 0.2 ml of a 20 ,ic/ml
solution to 2 ml of medium.At the end of thepulse, the medium wasremoved and cultures were flooded with ice-cold acetic acid-ethyl alcohol (1:3). After
pouring off the acetic acid-ethyl alcohol, 70% ethyl
alcohol wasadded and remained on thecells for at
least30min.Thecoverslipswerethenputon aChen
type Bstaining rack (Arthur H.ThomasCo.,
Phila-delphia, Pa.) andplaced inice-cold 0.5 Mperchloric
acid or 5% trichloroacetic acid for 20 to 30 min. Theywerewashedtwotimes with distilled waterand
were dipped briefly in absolute ethyl alcohol-ether
(1:1) and then in ether. After the ether had
evapo-rated,thecover slips wereplacedincounting vials, a
toluene base scintillation fluid was added, and the
samples were counted in a Mark I Nuclear-Chicago
scintillationcounter.
Chemicals. Trypsin (1: 250) was obtained from Difco. It was routinely used at a concentration of
0.25% in removing a monolayer of chick embryo
cells from the surface ofa vessel. Sterile ethylene-diaminetetraacetic acid (EDTA) solution (1: 5,000)
was obtained from Grand Island Biological Co.
Hydroxyureawasagiftfrom E. R. SquibbandSons,
Inc., New Brunswick, N.J. Powdered Eagle MEM,
fetal calfserum, and gammaglobulin-free fetal calf
serumwere purchasedfromGrand Island Biological
Co.
RESULTS
Action of interferon in cells at different times
afterseeding. Secondarychick embryocells were
seeded inpetridishes (2 X 106cells perdish) and
received either MEM or 6unitsof interferon 17,
41, or65 hrlater. Aftera4-hr incubation period
at36C, the cells were challenged with VSVata
multiplicityof 3PFU/cellandayield experiment
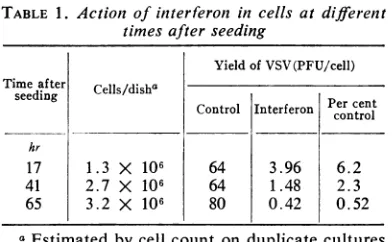
wascarriedout. Table 1 shows that thecells
be-came more sensitive to the action of interferon
[image:2.491.264.460.70.191.2]thelonger theyremained in culture. This"aging"
TABLE 1. Action of interferon in cells at different
timesafter seeding
Yield of VSV(PFU/cell) Time after Cells/disha
seeding Percent
Control Interferon control hr
17 1.3 X10i 64 3.96 6.2
41 2.7 X 106 64 1.48 2.3
65 3.2 X 10o 80 0.42 0.52
aEstimated by cell count on duplicate cultures
atthe time ofaddition ofinterferon.
effect occurred even though the cells had not
reached confluency by 65 hr.
Interferon action and DNA synthesis at different
times after seeding. Secondary chick embryo
cells werefoundtoattach tothebottom ofapetri
dishby 3.5 hr afterseeding,enablingus toinclude
very youngcells in these experiments. Interferon
actionwasstudiedasabove,exceptthat12.5units
of interferon was used. DNA synthesis was
measured as described above.
The 66-hr-old cultures werefullyconfluent, as
canbe seenbythehighcell countandbythe very
low rateofDNAsynthesis (Table 2). (The
differ-ences in cell densities in the results shown in
Tables 1and 2 canprobably be explainedin terms of variations in plating efficiency.) Both the
5-and 17-hr-old cells synthesized DNA at a high
rate and both were less sensitive to interferon
action than the 66-hr-old cells.
Effect of hydroxyurea on interferon action.
Hydroxyurea is able to inhibit DNA synthesis,
probably by inhibiting ribonucleoside
diphos-phatereductase (15, 26). Secondarychick embryo
cells were preincubated for 1 hr in 1,250 .tg of
hydroxyurea per ml or in MEM before receiving
either 2 units of interferon or MEM. The
hy-droxyurea-treated cells were also incontact with
the drug during the subsequent incubation with
interferonor MEM.Thecells hadbeen seeded at
a slightly lower density (2 X 106cells per dish)
so that complete confluency would not be
ob-tained untilseveraldays after seeding.
Table 3 showsthat, atboth 23 and70 hr, the
cellcounts werelow and the rate of DNA
synthe-siswashigh,indicatingthatinneither case had the
cells beenconfluent.Thegrowthrate(as indicated
by the rate of DNA synthesis) was somewhat
lower in the 70-hr-old cultures.
Thehydroxyurea inhibitedatleast 75% of the
DNA synthesis, but the efficiency of interferon
actionwasnotaffected bythe drug. There was an
increase in the yield of VSV from the
hydroxy-urea-treated cells (inbothcontrol and
on November 11, 2019 by guest
http://jvi.asm.org/
TABLE 2. Interferon action and DNA synthesis at different times after seeding
YieldofVSV (PFU/cell) 'H-thymidineincorporatien Time after Cells/disha
seeding Per cent Counts per Normalized
Control Interferon control mnper counts per mm
coverslip per coverslipb
hr
5 2.2 X 10O 70 0.18 0.26 1,597 725
17 3.7 X 106 96 0.16 0.17 2,980 780
66 5.7 X 106 134 0.059 0.044 236 41
SeeTable 1.
bCounts per min percoverslip/cellcount X 106.
TABLE 3. Effect ofhydroxyurea on interferon action
Yield of VSV(PFU/cell) 'H-thymidine
Time incorporation
after Cells/disha Treatment (normalized
seeding Percent countsper min
Control Interferon control percoverslip) hr
23 0.96 X 106 None 46.0 2.68 5.8 1,640
Hydroxyureac 54.3 3.28 6.1 406
70 1.94 X 106 None 47.0 0.268 0.57 1,345
Hydroxyureac 75.8 0.433 0.57 158
aSeeTable 1.
b SeeTable 2.
-Incubated with 1 ,250jg ofhydroxyureapermlfor1hr before, and during, treatment with interferon.
treated
cells).
This mayreflect the greateravail-ability of ribonucleotide diphosphates in these
cells.
Actionofinterferon in secondary cellsobtained
fromtrypsinizedEDTA-treated cultures. It is
con-ceivablethatthelow
efficiency
ofinterferonactionin young cells is due to
trypsin
digestion ofre-ceptor sites for interferon. EDTA is a
chelating
agent which removes cells from a surface by
removing theions needed for attachment andis
therefore lesslikely to cause permanent
destruc-tiontoreceptor sites. Whenasolutionof
0.25%
trypsinwasaddedto amonolayer ofchickembryo
cells, the cells came off the surface of the flask
and also
separated
from each other to form auniform
suspension.
ButwhenanEDTAsolution(1:5,000) wasadded, the cellscameoff theflask
in one continuous sheet. Rapid
pipetting
wasnecessary to break apart the clumps of cells.
Trypsinized cells in theexperiment weretreated
in a similar manner as the control. Even after
rapidpipetting,the EDTA-treated cells contained
someclumps,making cell countsunreliable.
Either MEM or 6.4 units ofinterferon wasin
contact with the cells for 3 hr before challenge
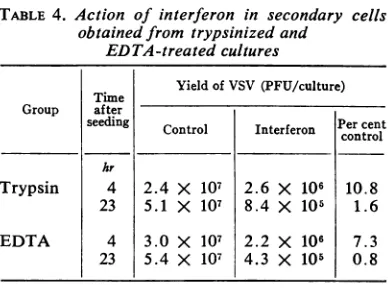
with VSV. Table 4 shows that both trypsinized
and EDTA-treated cells became more sensitive
TABLE 4. Action ofinterferon in secondary cells
obtained from trypsinized and
EDTA-treatedcultures
Timne Yield of VSV(PFU/culture)
Group after
seeding Control Interferon Per cent Control Iterferon control hr
Trypsin 4 2.4 X 107 2.6 X 101 10.8
23 5.1 X 107 8.4 X 105 1.6
EDTA 4 3.0 X 107 2.2 X 106 7.3
23 5.4 X 107 4.3 X 105 0.8
to interferon action with age. The increase in
sensitivity is quite similar inboth groups ofcells.
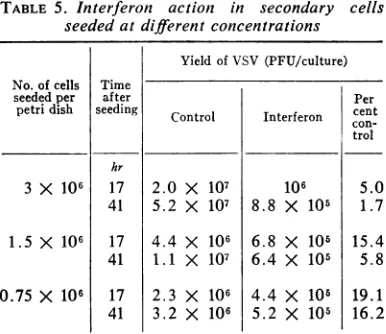
Interferon action and cell density. To test the
effect of cell density on interferon action, petri
plates were seeded with 0.75, 1.5, or 3.0 X 106
secondary chick embryo cells. At 17 and 41 hr
afterseeding, thecells were treated for 4 hr with
4units of interferonorMEM,after whichayield
experiment was carried out (Table 5). It is clear
that an increase in cell density resulted in
in-creased sensitivity to the action of interferon.
Within anyonegroupseededatacertaindensity,
VOL.4,1969
9
on November 11, 2019 by guest
http://jvi.asm.org/
[image:3.491.246.440.378.522.2]TABLE 5. Interferon action in secondary cells
seededatdifferent concentrations
Yield of VSV (PFU/culture) No.of cells Time
seeded per after Per
petri dish seeding Control Interferon cent
con-trol hr
3 X 106 17 2.0 X 107 106 5.0
41 5.2 X 107 8.8 X 105 1.7
1.5 X 106 17 4.4 X 106 6.8 X 105 15.4 41 1.1 X 107 6.4 X 105 5.8
0.75 X 106 17 2.3 X 106 4.4 X 105 19.1
41 3.2 X 106 5.2 X 105 16.2
the sensitivity of cells to interferon action was
increased with their time in culture.
DISCUSSION
Carver and Marcus (3) postulated that some
time-dependent process alters cultured cells so
that they become more susceptible to interferon
action. Butthey felt that this agingprocess may
be initiated only after a critical cell density is
reached. In their work, even after the cells were
fully confluent, further aging increased their
sensitivity to interferon action. Because this was
truefor interferonproductionaswellasfor
inter-feron action, it was suggested that the aging
process may represent a general phenomenon common toall inducible processes.
Our data show that, even before the cells
be-came confluent, the amount of time in culture
influenced the sensitivity of cells to interferon
action. Therefore, itseemsunlikely thatacritical
densityisnecessaryfor theagingprocess. On the
other hand,celldensityisanimportantfactor in
determining the sensitivity ofcells to interferon
action. Itmayeven beamajorcomponentofthis
phenomenon.
As cells become confluent, there is an overall
decrease in the rate of macromolecularsynthesis
(11). Increasing the rate ofprotein synthesis by
various methods has been shown to result in a
decrease in the production of interferon (5).
Previously we reported a decrease in sensitivity
tointerferon in cells treated withserum,which is
able to stimulate cellular RNA and protein
synthesis (25). It has been suggested (5) that
cells synthesizing protein at a high rate also
synthesize more ofa repressorandare therefore
more difficult to induce. Young, nonconfluent
cell cultures(according tothisargument) areless
sensitivetointerferon action becausethey
synthe-size protein (repressor) at a higher rate than older
confluent monolayers.
The phenomenon of increased sensitivity to
interferon action in older cells shows some
re-semblance toobservationsin the rat liver (17, 18).
Inthe mitoticallyinactiveadult liver, the levels of
tryptophan pyrrolase synthesized in response to
hydrocortisone are much higher than in the
dividing cells of the regenerating liver. More
specifically, it is during the period after partial
hepatectomy when DNA synthesis is known to
occur that theloss ofinducibilityis the greatest.
It was thus suggested that there may be an
in-compatibility between DNA synthesis and the
transcription ofmessengerRNA. Ourdatacannot
beexplainedonthis basis.Theinhibition ofDNA
synthesis in growing cells did not increase the
sensitivityof the cells tointerferon action.
More-over, other workers have shown that RNA
syn-thesis is inhibited only where the chromosomes
are condensed during replication,but that RNA
continues tobesynthesizedin thenonreplicating
regions (16,20). On the other hand,itseemsquite
reasonable to assume that induction should be
inhibited duringmitosis, sincethereislittleor no
RNA synthesis in animal cells during that time
(6,9,10,16,22).Infact, colchicinehas been found
to inhibit both the production and the action of
interferon (19). Althoughtheincreasedsensitivity of aged cellscannotbe
explained solely
interms ofadecreased mitotic rate,it is conceivable thatthisis a contributing factor.
Out data also show that the decreased
sensi-tivity in newly seeded cells is not the result of trypsindigestion of receptor sitesfor interferon.
Cantell and Paucker (2) also reported that
re-moval of themonolayer of HeLacellsbyscraping
orby EDTAgives similar resultsinaging
experi-ments. Our finding that the sensitivity of cells
tointerferon actionvarieswiththe number ofcells
seededper petri dish also indicatesthat destruc-tion ofreceptorsites isnot alikely explanation for the decreased sensitivity of newly seeded cells.
Thus, although thereasonsforthe increase in
sensitivity to the action of interferon with the
"aging" of chickembryocells in vitro couldnot
be fully explained, twofactors wereruled outas
the underlying cause ofthis phenomenon. They
are(i) therateofcellularDNAsynthesis and(ii)
theregeneration ofa cell receptor for interferon
with aging. Cell density was definitely found to
play a role, but since the growth rate decreases
with increasedcell densityand sinceRNA
synthe-sis is inhibited during mitosis, it is difficult to
separate effects resulting from increased density
from effects resulting from a decreased mitotic
rate.
on November 11, 2019 by guest
http://jvi.asm.org/
ACKNOWLEDGMENTS
This investigation wassupported by Public Health Service
grantsAl07057,GM01290, and1-KO4-Al-38784.
We thank Fermina Varacalli for experttechnical assistance andGeraldine Hodgson for the preparation of this manuscript.
LITERATURE CITED
1. Baltimore, D., and R. M. Franklin. 1963. Effects of
puro-mycinandp-fluorophenylalanineonmengovirusribonucleic acid andproteinsynthesis.Biochim.Biophys.Acta 76:431-441.
2.Cantell, K.,and K. Paucker. 1963. Studies onviral inter-ference intwolines ofHeLacells.Virology19:81-87. 3. Carver,D.H.,andP. L.Marcus.1967.Enhancedinterferon
productionfrom chickembryocellsagedinvitro.Virology
32:247-257.
4.Feinendegen, L.E.,V. P.Bond,W.W.Shreeve, andR. B. Painter. 1960. RNA and DNAmetabolism in human tissue culturecellsstudied with tritiatedcytidine. Exp.CellRes. 19:443-459.
5.Friedman, R. M. 1966. Interferon production and protein synthesisin chickcells.J.Bacteriol.91:1224-1229. 6. Friedman,R.M.,andJ. A.Sonnabend. 1964.Inhibitionof
interferonactionbyp-fluorophenylalanine.Nature 203:366-367.
7.Heller,E. 1963. EnhancementofChikungunyavirus replica-tion and inhibireplica-tionof interferonproduction byactinomycin D. Virology21:652-656.
8.Henslova, E., and H. Libikova. 1966. Optimal conditions
for interferon formation in chick embryo cell cultures
infected with tick-borne encephalitis virus. Acta Virol. 10:475-479.
9.King,D.W.,and M. L.Barnhisel. 1967.SynthesisofRNA
inmammaliancellsduringmitosis andinterphase. J.Cell Biol. 33:265-272.
10. Konrad, C. G. 1963. Protein synthesisand RNAsynthesis duringmitosisinanimal cells. J.CellBiol.19:267-277.
11. Levine,E.M.,Y.Becker,C. W.Boone,and H.Eagle.1965.
Contact inhibition, macromolecular synthesis and poly-ribosomes in cultured human diploid fibroblasts. Proc.
Nat.Acad. Sci. U.S.A. 53:350-356.
12. Levy,H.B.,and W. A. Carter.1968. Themechanism of action
ofinterferon,p.95-110. In G. Rita(ed.),The
interferons-an international symposium. Academic Press Inc., New York.
13.Lockart, R. Z., Jr. 1968.Viralinterference in agedcultures of chick embryocels, p.45-55.InM. SandersandE. H. Lennette (ed.), Medicalandappliedvirology. Warren H. Green, Inc., St. Louis.
14. Marcus,P. I.,andJ. M. Salb. 1968. On the translation in-hibitory protein ofinterferon action, p. 111-127.In G. Rita (ed.), Theinterferons-an international symposium. AcademicPressInc., NewYork.
15.Neuhard, J. 1967. Studiesontheacid-soluble nucleotidepool in Fscherkhia coli. IV. Effectsofhydroxyurea. Biochim. Biophys. Acta 145:1-6.
16.Prescott, D. M., and M. A. Bender. 1962. Synthesisof RNA and protein during mitosis in mammalian tissueculture
cells.Exp.CellRes. 26:260-268.
17.Seidman,I.,G.W. Teebor,andF.F.Becker. 1966.
Depres-sion oftryptophanpyrrolaseinduction in regeneratingrat
liver. Proc. Soc.Exp.Biol.Med.123:274-276.
18.Seidman,1.,G. W. Teebor, and F. F. Becker. 1967.Hormonal
andsubstrateinduction of tryptophan pyrrolase in
regen-eratingratliver.Cancer Res. 27:1620-1625.
19.Solovyov, V. D.,andL. M.Mentkevich. 1965. The effectof colchicineon viralinterferenceandinterferon formation. ActaVirol. 9:308-312.
20. Taylor, H. J. 1960. Nucleic acid synthesisinrelationtothe cell divisioncycle.Ann. N.Y.Acad.Sci. 90:409-421. 21.Taylor,J. 1965. Studiesonthemechanismof action of
inter-feron. I. Interferon action and RNA synthesis in chick
embryo fibroblasts infected with Semliki Forest virus.
Virology 25:340-349.
22.Terasima, T., and L. J. Toimach. 1963. Growthandnucleic acid synthesis in synchronously dividing populations of HeLacells. Exp. CellRes.30:344-362.
23. Vilcek, J., and J. H.Freer. 1966. Inhibition of Sindbis virus plaque formation byextracts ofEscherichia coli. J. Bac-teriol.92:1716-1722.
24. Vilcek, J.,and D.Lowy.1967. Interaction ofinterferon wth chickembryocells. Arch.Ges.Virusforsch. 21:254-264. i 25.Vilcek, J., M. H. Ng,andT.G. Rossman. 1968. Studieson
the action of interferonin cellular and cell-free systems.
InG. Rita (ed.), Theinterferons-an international
sym-posium.AcademicPressInc.,New York.
26. Young, C., and S. Hodas. 1964. Hydroxyurea: inhibitory effectnnDNAmetabolism. Science146:1172-1174.