Vol. 34, No. 1 JOURNALOF VIROLOGY, Apr.1980, p. 280-284
0022-538X/80/04-0280/05$02.00/0
Cell-Free Translation of Avian
Erythroblastosis Virus RNA
TONY PAWSON* ANDG. STEVEN MARTIN
Department of Zoology, University ofCalifornia,Berkeley,California94720
Avianerythroblastosis virus (AEV) RNA rescued from nonproducer cells by
superinfection with a helpervirus is translated into three polypeptides in the
messenger-dependent
rabbitreticulocyte lysate. A 75,000 molecular weightpoly-peptide (P75A
V)
is synthesized from 28SRNA and is encoded by the5' sectionofthe AEVRNA, including gag-related andAEV-specificsequences. Thep75AEV synthesized in infected cellsand the
p75AEv
synthesized in the cell-free system areelectrophoretically identical. A44,000molecularweight polypeptide(p44'V)
is synthesized from 20-24S RNA, apparently from the3' section of the
AEV-specific RNAsequence. Aminor37,000 molecular weight polypeptide
(P37'V)
iS synthesized from 20S AEV RNA. A comparison is drawn between the cell-freeproducts of MC29 andAEVRNAs. The avianacuteleukemia virusesare agroup
ofhighly oncogenic, transforming RNA tumor
viruseswhichinduceleukemias, sarcomas, and
carcinomas ininfectedchickens (1,7, 12). They have been divided into three classesonthe basis of their oncogenic spectra (1, 2, 4, 6, 11; P. H. Duesberg, K.Bister, and C. Moscovici, Virology, in press). The prototypes of these groups are
avian myelocytomatosis virus (MC29), avian erythroblastosisvirus(AEV), and avian myelo-blastosis virus (AMV). Theyare allreplication defective, and a nondefective helper virus is
thereforerequired to rescue theleukemia viral RNAgenomefromtransformed cells (1,2, 5,11). Such superinfected cells release both virions containingacute leukemia virus RNA and
viri-ons containinghelper virus RNA (2, 11; Dues-berg et al., Virology, in press). Although the defectiveness of theacuteleukemia viruseshas
precludedadetailedgenetic analysis,a biochem-icalinvestigationofseveral avianacuteleukemia viruses has revealeda commongeneticstructure
(2, 4, 6, 11; Duesberget al., Virology, inpress). The genomic RNA measures 5 to 6 kilobases (kb) and contains threeregions. Group-specific regions are located at the 5' and 3' ends and
measure about 1 kb and 1.5 to 2.5
kb,
respec-tively.These sequencesarerelatedtothe repli-cativegenesof nondefective viruses of theavian group,andinthecaseofAEV,areisogenicwith
thenaturallyoccurring, nontransforming helper, avianerythroblastosis-associated virus(AEAV) (2). The 5' group-specific section contains gag
(group-specific antigen)generelatedsequences.
The 3'sectionprobablycontainsenv
(envelope)
gene related sequences and a C or modified C region. The thirdregion is an intemal
specific
sequence of2 to 3kb which isunrelated tothe
replicativesequences ofnondefectivehelper
vi-ruses, to the src (transforming) gene of Rous sarcoma virus (RSV), or to the specific se-quencesof otherclasses ofacuteleukemiavirus
(2,4, 6, 11, 16, 17; Duesbergetal., Virology,in
press). Becauseviruses withasimilaroncogenic spectrum have related specific sequences (1, 2, 4, 6, 7), these intemal specific sequences are
thoughttobelongtothetransforming (onc)gene
of the virus.
Nonproducer cells transformed by the avian
acute leukemia viruses contain virus-specific, nonvirion gag-related proteins (3, 4, 8, 9). A 110,000molecularweight polypeptide
(P11OMc)
can be detected in MC29-infected cells after immunoprecipitation with anti-gag serum (3,
11). This polypeptide contains MC29-specific peptidesequences,andalsop19 andpartofp27
(the twogroup-specific antigens located atthe N-terminus of Pr769`9[18], the primary product of the
gag
gene) (3, 11). We have shown that PlOMc is encoded by MC29 virion RNA and is translated from full-length 28S MC29 RNA, being initiatednearthe 5' end andsynthesized from thegag-related5'group-specific regionand the MC29-specific region (11). It is thereforeacandidate for theMC29-transforming protein.In
addition,a56,000 molecularweightpolypeptide (P56MC)wassynthesized frompolyadenylicacid [poly(A)]-containing 20-24S (3-kb) RNA,anda 37,000 molecular weight polypeptide (p37MC)
froma 15-18S (1.5 kb) RNA. p37MC isenv re-lated and is probably translated from the 3'
group-specificregion.
P56Mc
mustbetranslated eitherfromthe 3' endof the specific region, or theadjacent sectionofgroup-specific region,to its3'side.The genetic structure of AEV has recently
beendetermined,andit appearstodefinea new class of onc gene (2, 17). A 75,000 molecular 280
on November 10, 2019 by guest
http://jvi.asm.org/
weightpolypeptide (p75AEV) hasbeen identified
innonproducercellstransformedbyAEVafter immunoprec pitation with anti-gag serum (8). Like Pll0MC this polypeptide contains se-quencesin common withPr769'9(namely, p19), andspecificsequencesunrelatedtoany replica-tivegeneproduct (8). Herewewishedto deter-minewhetherp75AEV isdirectlytranslated from the AEV genome, the region from which it is translated, and other translation products of
AEVRNA.
The strategy employed was similar to that describedpreviouslyforMC29(11).We isolated
aclone of AEV-transformed nonproducer cells by infecting chick fibroblasts with strain ES4 AEV(RAV-1) athighdilution and thencloning the transformed cells in methylcellulose (7). Thisclone of AEV-transformed fibroblastswas
identified as a nonproducer by its inability to
release either infectious transforming virus, or
particles with reverse transcriptase activity. This nonproducer clone was labeled with [35S]methionine, lysed,andimmunoprecipitated with antiserum to disrupted AMV (directed mainly against the
gag
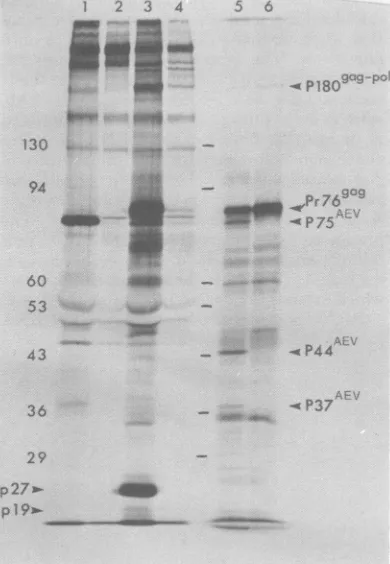
proteins) (13, 14). These cells synthesize p75A V but no viral structural proteins (Fig. 1,lane 1). We thensuperinfected thesenonproducercellswithring-neck pheasant virus (RPV) andrepeated the immunoprecipi-tationexperiment. RPV encodesaPr76'agwhichcan be electrophoretically separated from p75AEV.Superinfection with RPV resulted in the synthesis ofPr769'9,theappearanceof p27 and p19, andareduction in thesynthesis ofp75AEV (Fig. 1, lane 3). Medium was harvested from parallel cultures of RPV-superinfected
AEV-transformed cells, the virionswerepurified, and poly(A)-containing,denatured 50-70S RNAwas
isolated and translated in thereticulocyte lysate (15). Thecell-free products of AEV(RPV) RNA (Fig. 1, lane5) were thencompared with those of RPV alone (Fig. 1, lane 6). Although both AEV(RPV) and RPV RNAsaretranslated into Pr769'9,
P180V'-i",
and other products (11, 14),three polypeptides are synthesized solely from AEV(RPV) RNA and must therefore be
en-codedbyAEV RNA. Thelargest AEV-specific product has a molecular weight of75,000 and
comigrates with
p75AEV
identifiedinAEVnon-producercells(Fig. 1). ThesecondAEV-specific polypeptide has a molecular weight of 44,000
(P44AE
), and the third has amolecularweightof 37,000(P37
AEV)
The AEV(RPV) virion RNA was then
frac-tionatedby centrifugation througha 15to30% glycerol gradienttoidentifythesize of the RNA
species fromwhich each ofthethree AEV-spe-cificpolypeptidesissynthesized (11). The RNA
281
wasrecovered from eachfraction and translated in the cell-freesystem, and theproducts were
analyzed by sodium dodecyl
sulfate-polyacryl-amidegel electrophoresis (Fig. 2). P75 EV is
syn-1 2 3 4
*.
5 6
130 94
60 53
v.._;'
Om
.:wf
WI
43
36
Pr76
a9
-W Aiv
-4P75
_ - P44
- P37 F..
29
[image:2.508.255.450.132.414.2]p27x _
FIG. 1.AEVpolypeptides synthesized in cells and invitro.Cells fromaclone ofAEV-transformed non-producer(NP)chickfibroblasts (see text) were labeled with 100uCi of[35S]methionine in methionine-free medium ina 10-cmdish (14). Cells were lysed and immunoprecipitated by using anti-AMV serum or normal goatserum asdescribed previously (13, 14). Lane 1, AEV-NP fibroblasts, anti-AMV; lane 2, AEV-NP fibroblasts, normal serum. Nonproducer cells weresuperinfectedwithRPV, and the immuno-precipitations were repeated: lane 3, AEV(RPV) fi-broblasts, anti-AMV. Lane 4,AEV(RPV) fibroblasts, control serum. Poly(A)-selected, denatured 50-70S RNA isolated from virions released from the AEV(RPV) infected fibroblasts was translated in the messenger-dependent rabbit reticulocyte lysate as de-scribed (11, 15), as was RNA isolated from RPV virions alone.Lane 5, AEV(RPV) RNA cell-free prod-ucts.Lane 6, RPV RNA cell-free products. Polypep-tideswereanalyzed by electrophoresis on a7.5% SDS-polyacrylamide slab gel (11) followed by fluorogra-phy. Coomassie brilliant blue-stained molecular weight markers (11)areindicated by horizontal bars between lanes 4 and 5, with themolecular weight (x10-3) written to the left of lane 1. Polypeptides discussed in thetextareindicated by arrows.
-aP180gclg-po.
on November 10, 2019 by guest
http://jvi.asm.org/
282 NOTES
thesizedpredominantly from 28SRNA (fraction
11),which representsthefull-length5.5-kb AEV
genomic RNA (2). In contrast, the RPV-encoded Pr769'9andP180VG'-PO aresynthesizedprimarily
from34SRNA (fractions 7, 8). Immunoprecipi-tation of fraction11 with asuccessionofantisera
raised againstproducts of thereplicativegenes
(Fig. 3) showsthatp75AEViSrecognizedby anti-gag serum. The synthesis of p75AEV from28S RNA suggests that itissynthesizedfrom the 5'
section of theAEV RNAbetween5.5 and 3 kb,
since in eucaryotesproteinsynthesisisinitiated at or nearthe 5' end of the mRNA (10). This
conclusion is also supportedby thepresenceof
gag-relatedpeptidesinp75AEV(8),and ofa
gag-relatedoligonucleotide in the 1-kb group-specific sequence at the 5' end of AEV RNA (2). The presence ofspecific peptides in the p75AEV
pro-tein (2;unpublished data) suggeststhatits cod-ingregion extends into the AEV-specificsection,
which extends from4.5 to 1.5kb from the 3' end.
p44AEV
is
synthesized from arange of RNAsize classes,butprimarilyfrom20-24S
(approx-imately
3kb)sizeRNA (Fig. 2,fractions15, 16).Ifp44
AEV
is initiatednearthe5' end of this 3-kbAEV-specificRNA, it must beencodedbythe 3'
half of the AEV-specific region between 3 and 1.5kb from the 3' end. This is consistent with
the observation that
p44AEV
synthesized from 20S (fraction 16) RNA is not immunoprecipi-tated by antiseraraisedagainst the gag,pol,or envgeneproducts, suggestingthat it isunrelatedtoanyreplicativegeneproduct (Fig. 3). This is
alsotrueof
p44AEV
synthesizedfromAEV(RAV-2) RNA(data notshown).
Tryptic
peptide
map-ping showsthat
p75AEV
andp44AEV
arelargely unrelated (datanotshown).p37AEV is synthesized primarily from 20S AEV-specific RNA (Fig. 2). Its identity is not yetclear, butpreliminaryevidence suggests that itmaybeenvrelated.
These experiments have been repeated with
AEVrescued by RAV-2orby AEAV (data not
shown).
In each casep44AEV
andp37AEV
are28S 185
+ 4
4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
P180
_9 ..
Pr7690
P75AE'V ._I.I...w
..mN
...~. %. ..*4" - 'I'li...~- l' ...s
[image:3.508.148.387.316.588.2]... _ ..
FIG. 2. TranslationoffractionatedAEV(RPV)virionRNA. A25-pgamountofpoly(A)-selected,denatured
50-70SAEV(RPV)virionRNAwasboiled in 10 mMTris-hydrochloride(pH7.5)-0.1%sodiumdodecyl sulfate
andsedimentedona12-ml,15to 30%glycerol gradientasdescribedpreviously (11, 14).Atotalof25fractions
werecollected,and the viralRNAwasrecoveredaftertheadditionofcarrieryeasttRNA. 10%oftheRNAin eachfractionwasthentranslated in 10
IlI
ofmessenger-dependentrabbitreticulocyte lysate,and theproductswereanalyzed by electrophoresison a7.5%sodiumdodecylsulfate-polyacrylamidegelandautoradiography.
The numberofeachfraction fromwhich the translated RNAwasrecovered isindicated above eachlane,as arethepositions of 18Sand28SRNA markerssedimented inparalleL
AE%.
P44
P37ALE
a'
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
Fraction 11 (283)
A B C D
Fraction 16(20S)
A B C D
130-
94.-VW gW
60
.-53
M.
AE'V
21!-P44 _
43-AEV P37
--
29-FIG. 3. Immunological analysis ofAEV-specific, cell-free products. AEV(RPV)RNAfrom gradient frac-tions 8(34S),11(28S),and 16(20S) (see Fig. 2)wastranslatedin thereticulocyte lysate.A10-,lIamountofthe products ofeachfractionwasanalyzedwithoutfurthertreatmentona7.5% sodiumdodecyl sulfate-polyacryl-amidegel (lanes A).Afurther15
IlI
wasimmunoprecipitatedwithasuccessionofantisera inexcess(14),thesupernatants from the first precipitation being immunoprecipitated with the second antiserum and the supernatantsfromthe secondprecipitation beingimmunoprecipitated bythe thirdantiserum,in thefollowing order: 1, anti-gagserum (lanes B); 2,anti-reversetranscriptaseserum (containing anti-gag activity) (lanes
C);3, anti-RSVPR-Cgp85serum(lanes D).The resultsareshownfortheproducts of28Sand20SAEV(RPV) RNA. Forfraction8(34S), onlytheanti-gagimmunoprecipitateis shown. The mobilitiesofmolecularweight markersaredisplayedasdescribed inFig. 1.
clearly translated specifically from AEV RNA and not from the helper RNA, and from the
sameRNA sizes after RNA fractionationasfrom
AEV (RPV) RNA. The Pr769'9 product of the RAV-2orAEAV RNAs issosimilar in
electro-phoretic mobilitytop75AEV that thetwo cannot be wellseparated. However,whenAEV(AEAV) isfractionated,p75AEVcanbe identifiedasbeing
synthesized from 28S RNA. In the case of
AEV(RAV-2), RNA isolated from virions
re-leased from fibroblasts or erythroblasts was
translated into identical products. Thus, the three cell-free products ofAEV RNAare
syn-thesizedindependentlyofthehelper virusorcell
typeoforigin.
Both p75AEV and p44AEv appear to contain regions translatedfromtheAEV-specific region.
Thus far, only p75AEV has been identified in AEV-transformed cells, and it is not clear if p44AEV is also present in infected cells, or if it
represents anin vitroartifact. Thus,wedonot know yetwhether p75AEVis the sole transform-ing protein of AEV. We are subjecting the
smaller cell-free products of AEV and MC29to
a moredetailedanalysis and investigating their
possible synthesisininfectedcells.
It is striking how similar are the products
synthesized from MC29 and AEVvirion RNAs invitro. Bothcode foralarge gag-related
poly-peptide synthesized from the 5' section of the RNA andincluding specificsequences(P110MC,
p75AEV);a smallerpolypeptide synthesized from
the middle ofthe RNA (P56Mc,p44AEV), proba-bly fromspecific sequences, though thisis less Fr.8
B
P180
130-
UI~60-
53.-
43-
36- 29-VOL. 34,1980
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.508.133.374.73.378.2]284 NOTES
clear for P56MC; and a small, apparently
env-related polypeptide synthesized from the 3'
group-specificregion
(p37Mc,
p37AEV).Thus, al-though theoncgenesof these virusesappear,at least inpart, tobeunrelated, theoverallgeneticstructure and mode ofgene expression of AEV
and MC29seemverysimilar.
Wethank Masae Namba and Louise Roberts fortechnical assistance, P. Duesberg for encouragement anda stock of AEVstrain ES4(AEAV), andT.Grafforagift of AEV strain
ES4 (RAV-1). Anti-PR-C gp85 serum was a gift from D.
Bolognesi, antireverse transcriptaseserum wasfromH.
Op-permann,and inactivatedStaphyloccusaureus werefromL.
Crawford.
This workwassupported by Public Health Servicegrant
NIH CA17542. T.P.wassupported byPublicHealth Service
training grant CA 09041 from the National Institutes of Health.
LITERATURE CITED
1. Beug, H.,A.vonKirchbach,G.Doderlein,J.-F. Con-science,and T. Graf. 1979. Chickenhaematopoietic cells transformed bysevenstrainsofdefectiveavian leukemia viruses displaythreedistinctphenotypes of differentiation.Cell18:375-390.
2. Bister, K., and P. H.Duesberg. 1979. Structure and specificsequencesofavianerythroblastosisvirusRNA: evidence for multiple classes of transforming genes
among avian tumor viruses. Proc. Natl. Acad. Sci.
U.S.A. 76:5023-5027.
3. Bister, K.,M.J.Hayman, andP. K.Vogt.1977. De-fectiveness of avianmyelocytomatosisvirusMC29: iso-lationoflong-termnonproducerculturesandanalysis ofvirus-specific polypeptide synthesis. Virology 82:431-448.
4. Bister,K.,H.-C.Loliger,and P. H. Duesberg. 1979. Oligoribonucleotidemapandproteinof CMII:detection of conserved and nonconserved genetic elements in avianacuteleukemiavirusesCMII, MC29,and MH2. J.Virol. 32:208-219.
5. Bister,K., andP. K.Vogt.1978.Geneticanalysisof the defectiveness in strain MC29 avian leukosis virus. Vi-rology88:213-221.
6. Duesberg,P. H.,and P. K.Vogt. 1979. Avian acute
leukemiavirusesMC29 andMH2 share specific RNA
sequences:evidence forasecond classof transforming
genes.Proc. Natl.Acad. Sci.U.S.A. 76:1633-1637.
7. Graf, T., B.Royer-Pokora, G. Schubert, andH. Beug. 1976.Evidencefor the multiple oncogenic potentialof
cloned leukemiavirus: in vitro and in vivo studieswith
avianerythroblastosis virus.Virology 71:423-433. 8. Hayman,M.J.,B.Royer-Pokora, and T. Graf.1979.
Defectiveness of avianerythroblastosis virus: synthesis ofa75Kgag-related protein.Virology 92:31-45. 9. Hu,S.S.F.,C.Moscovici,and P. K.Vogt.1978.The
defectiveness of Mill Hill2,acarcinoma-inducing avian oncovirus.Virology 89:162-178.
10. Kozak, M. 1978. Howdo eucaryotic ribosomes select initiation regions in messenger RNA? Cell 15:1109-1123.
11. Mellon,P.,A.Pawson,K.Bister,G.S.Martin,and P. H.Duesberg.1978.Specific RNAsequencesandgene
products ofMC29 avian acute leukemia virus. Proc. Natl.Acad.Sci.U.S.A.75:5874-5878.
12. Mladenov, Z.,U.Heine,D.Beard,and J.W. Beard. 1967.Strain MC29avianleukosisvirus. Myelocytoma, endothelioma andrenal growths: pathomorphological andultrastructural aspects.J. Natl.CancerInst. 38: 251.
13. Oppermann, H.,J. M.Bishop,H. E.Varmus,andL. Leevintow. 1977.Ajointproduct ofthegenesgagand pol of avian sarcoma virus: a possible precursor of
reversetranscriptase. Cell12:993-1005.
14. Pawson,T.,P.Mellon,P.Duesberg,and G.S. Martin. 1980.envgeneof Roussarcomavirus:identification of thegeneproduct by cell-free translation. J. Virol.33: 993-1003.
15.Pelham,H. R.B.,andR.J.Jackson.1976.Anefficient mRNA-dependent translationsystemfromreticulocyte lysates.Eur. J. Biochem. 67:247-256.
16. Sheiness, D.,L. Fanshier, and J. M. Bishop. 1978.
Identificationofnucleotidesequences whichmay
en-code theoncogenic capacityof avian retrovirusMC29. J.Virol.28:600-610.
17. Stehelin, D.,and T. Graf. 1978.Avianmyelocytomatosis anderythroblastosisviruses lack thetransforminggene srcof aviansarcomaviruses. Cell 13:745-750.
18. Vogt,V.M., R.Eisenmann, andH.Digelmann.1975. Generation of avian myeloblastosis virus structural polypeptides by proteolytic cleavage of a precursor
polypeptide.J. Mol. Biol. 96:471-493.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/