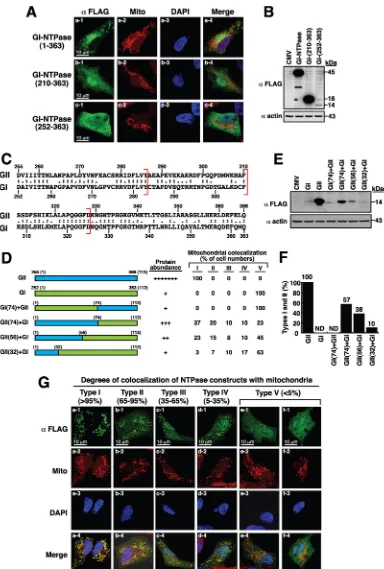
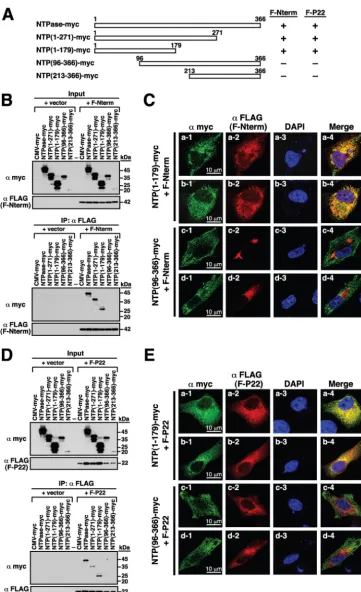
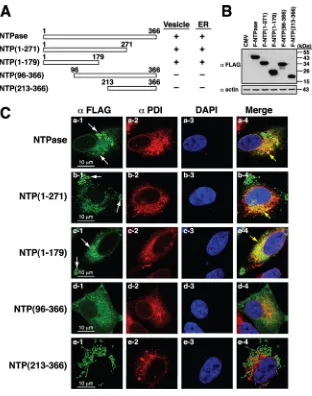
Subcellular Localization and Functional Characterization of GII.4 Norovirus-Encoded NTPase
Full text
Figure



Related documents
The aim of the present study was to further investigate the associations between sex, clinical data, type, severity and prevalence of cardinal symptoms suggestive of gas- troparesis
(2014), Accounts receivables management and financial performance of manufacturing firms in Nakuru County, Kenya , unpublished manuscript, school of business, university of
The evolution of initial rectangular pulses of dust sound waves in the cylindrical plasma waveguides with di- electrics has demonstrated the essential surface nonlinearity near
Brown dashed line – model output produced assuming initial fluxes at 50 % steady state values, variable pyrite burial, fixed weathering fluxes and 1 pyr... Sensitivity
How might the “ audio compression ” hypothesis be put to a rigorous test. First of all, it is imperative that a for- mal, exhaustive and statistically robust comparison of
This study aimed at identifying the level of depression and sense of insecurity among a sample of female refugee adolescents, and the impact of correcting cognitive distortions
There would be significant difference among students of VII, VIII and IX class students of selected Secondary schools of Kalluru mandal with regard to their Emotional
The primary objective of the BATTLE trial is to dem- onstrate the clinical superiority of primary stenting using the Zilver® PTX® stent system versus a latest generation bare